Abstract
The largest superfamily of membrane proteins that translate extracellular signals into intracellular messages are the 7-transmembrane-spanning (7TM) G protein-coupled receptors (GPCR). One of the ways in which their activity is controlled is by the process of desensitization and endocytosis, whereby agonist-activated receptors are rapidly and often reversibly silenced through removal from the cell surface. Indeed, following endocytosis, individual receptors can be sorted differentially between recycling endosomes and lysosomes, which controls the reversibility of the silencing. Thus, endocytosis can either serve as a mechanism for receptor resensitization by delivering receptors back to the plasma membrane or facilitate receptor downregulation by serving as the first step towards targeting the receptors to lysosomes for degradation. The sorting of receptors to the lysosomal pathway can be facilitated by interaction with an array of accessory proteins. One of these proteins is the GPCR-associated sorting protein 1 (GASP-1), which specifically targets several 7TM-GPCR to the lysosomal pathway after endocytosis. Furthermore, GASP-1 was recently found to directly affect the signaling capacity of a 7TM-GPCR. Importantly, the in vivo relevance of GASP-1-dependent receptor sorting has also begun to be verified in animal models. Here, we summarize the recent advances in elucidating GASP-1-dependent receptor sorting functions and their potential implications in vivo.
Key Words: G protein-coupled receptor-associated sorting protein 1; G protein-coupled receptor-associated sorting protein 2; Postendocytic receptor sorting; Seven-transmembrane domain G protein-coupled receptor; Trafficking; Degradation; Receptor, downregulation; Adaptor protein
Introduction
Endocytosis of 7-Transmembrane-Spanning G Protein-Coupled Receptors
Seven-transmembrane-spanning G protein-coupled receptors (7TM-GPCRs) comprise the largest superfamily of membrane proteins that translate extracellular signals into intracellular messages and can be activated by a wide range of compounds including odorants, photons, hormones and neurotransmitters [1,2]. Due, likely, to their involvement in a variety of physiological and pathophysiological processes, the activity of 7TM-GPCRs is extensively regulated. One of the ways in which receptor-mediated signaling is modulated is by the process of endocytosis, whereby agonist-activated cell surface receptors are rapidly removed from the plasma membrane (fig. 1). Typically, receptor endocytosis is facilitated by phosphorylation of the receptor by GPCR kinases and the subsequent interaction with nonvisual (β)-arrestins, both of which ‘desensitize’ signal transduction between receptor and G protein. In general, receptors can be endocytosed via clathrin-coated pits, caveolae or uncoated pits [3]. However, most cell surface receptors are internalized by clathrin-dependent mechanisms, where the invagination and pit assembly are supported by clathrin and adaptor proteins such as the adaptor protein 2 [4,5].
Fig. 1.
β-Arrestin/clathrin-dependent endocytosis of 7TM-GPCRs. Agonist binding to 7TM-GPCRs leads to the activation of heterotrimeric G proteins and initiation of a signal transduction cascade (1). Desensitization of receptors is facilitated by phosphorylation through GPCR kinases (GRK) (2) and subsequent recruitment of β-arrestins (3). The endocytosis of the receptors is finally mediated by clathrin and adaptor protein 2 (AP-2) (4). The vesicle abscission is facilitated by dynamin and leads to the formation of cargo-containing endocytic vesicles (5).
Postendocytic Sorting of 7TM-GPCRs
Following endocytosis, individual receptors can be sorted between recycling and degradative pathways (fig. 2) [6,7]. Thus, the postendocytic fate of a 7TM-GPCR determines the role of endocytosis in signal transduction. For a recycling receptor, endocytosis can serve as a mechanism for receptor resensitization by delivering internalized receptors to endosomes, from where they can be recycled to the plasma membrane in a fully active state. Alternatively, for receptors that are degraded, rapid endocytosis can serve as a first step towards receptor downregulation by delivering the receptors to endosomes, from which they are further targeted to the lysosomes. The cascade of events for each receptor, globally referred to as ‘receptor trafficking’, is thought to be initiated already at the plasma membrane, where receptors are ‘tagged’ – e.g. phosphorylated or ubiquitinated – to determine their endocytic and postendocytic fates.
Fig. 2.
Postendocytic sorting of 7TM-GPCRs. Following internalization, receptors can be differentially sorted between recycling and degradative fates. Recycling of receptors can be mediated by interaction with postsynaptic density 95/disk large/zonula occludens-1 (PDZ)-domain-containing proteins such as Na+/H+ exchanger regulatory factor (NHERF)/ezrinradixin-moesin (ERM)-binding phosphoprotein 50 (EBP50). Interaction with the endosomal sorting complex required for transport (ESCRT) complexes, sorting nexin-1 (SNX-1), dysbindin or GPCR-associated sorting protein 1 (GASP-1) leads to targeting of 7TM-GPCRs to the lysosomal pathway for degradation.
Receptor phosphorylation can occur in response to activation by an agonist and is necessary for receptor endocytosis [8,9,10]. However, it has been suggested that receptor phosphorylation not only influences endocytosis, but also influences the postendocytic fate of a receptor by regulating interactions with sorting proteins or by inducing other posttranslational modifications [11,12]. Indeed, ubiquitination of 7TM-GPCRs, which involves the covalent attachment of ubiquitin to lysine residues of the cytoplasmic tail, has in some cases been shown to act as a ‘sorting signal’ that promotes endocytosis and lysosomal targeting [13,14,15]. In addition, several proteins have been identified that specifically target 7TM-GPCRs to either recycling [11,16,17,18,19] or degradative pathways [20,21].
Recycling of 7TM-GPCRs
Traditionally, recycling of receptors was thought to occur by default. This was based on the observations that (i) the membrane itself is continuously recycled, and (ii) disruption of lysosomal sorting often results in enhanced recycling of 7TM-GPCRs [22,23,24]. Intriguingly, recent findings support a model of regulated recycling of some 7TM-GPCRs that is mediated by specific recycling sequences contained in their cytoplasmic tails [16,25,26,27]. One example of sequence-directed recycling involves the β2-adrenergic receptor (β2-AR). β2-AR contains a distal sequence (DSLL) that has been shown to be important for its recycling to the cell surface after endocytosis via interactions with postsynaptic density 95/disk large/zonula occludens-1 (PDZ)-domain-containing proteins of the Na+/H+ exchanger regulatory factor (NHERF)/ezrinradixin-moesin (ERM)-binding phosphoprotein 50 (EBP50) family [16,28]. Deletion of this domain, or phosphorylation of the serine in this domain, disrupts the interaction of the β2-AR with NHERF/EBP50, prevents receptor recycling [11] and promotes receptor degradation [29].
Lysosomal Sorting of 7TM-GPCRs
The sorting of receptors to the lysosomal pathway does not occur by default, but instead appears to be facilitated by interaction with one or more of an array of sorting proteins. For ubiquitinated proteins, the highly conserved endosomal sorting complex required for transport (ESCRT) machinery directs the transport of ubiquitinated cargo for degradation via a compartment termed the multivesicular body [25]. The sorting process is initiated by a protein complex (often referred to as ESCRT-0) consisting of the hepatocyte-growth-factor-regulated tyrosine kinase substrate (Hrs) and the signal transduction adapter molecule, which recognize ubiquitinated proteins with their ubiquitin-interaction motifs. By interaction of Hrs with the ubiquitin E2 variant domain of the tumor suppressor gene 101, the binding of ESCRT-I complex is initiated. Subsequently, ESCRT-II and -III complexes are recruited to the endosomal membrane, which deliver the cargo to the lysosomes for degradation [30,31,32]. However, the ESCRT machinery appears to be required for degradation of membrane proteins, also including some 7TM-GPCRs that are not ubiquitinated. For example, the δ-opioid receptor (DOR) is ubiquitinated and targeted for degradation following its endocytosis. However, ubiquitination of DOR is not required for either ligand-induced endocytosis or postendocytic degradation of DOR [26]. Together these data suggest that the ESCRT machinery in some way can recognize and select nonubiquitinated receptors and transport them for degradation. However, in this case it is unclear what ‘tags’ these nonubiquitinated proteins for degradation.
One possibility is the existence of accessory sorting proteins that serve as linkers between membrane proteins and the ESCRT machinery. Sorting nexin-1 (SNX-1) is one non-ESCRT protein that is involved in targeting 7TM-GPCRs to the lysosomal pathway. Initially, SNX-1 was shown to bind to Hrs, and to promote the targeting of the non-GPCR epidermal growth factor receptor to the degradative pathway [33]. Subsequently, SNX-1 was shown to effect the downregulation of the protease-activated receptor-1 [34] 7TM-GPCR, and is reported to bind, at least in vitro, to the C termini of several 7TM-GPCR such as the virally encoded chemokine receptor US28 [35] and the DOR [35]. However, not all 7TM-GPCRs that bind SNX-1 are targeted for degradation; for instance, the muscarinic receptors M1 and M4 both interact with SNX-1 [35], but are still known to be efficiently recycled after agonist stimulation [36,37]. More recently, dysbindin – a cytoplasmic protein encoded by DTNBP1 [38] – was implicated in the postendocytic sorting of the dopamine D2 receptor and DOR to the degradative pathway and was shown to coimmunoprecipitate with both Hrs proteins and another sorting protein, the GPCR-associated sorting protein 1 (GASP-1) [39].
The GPCR-Associated Sorting Proteins
GASP-1 was discovered by Whistler et al. [7] in 2002 in a yeast two-hybrid screen with the carboxyl terminus of the DOR and has since been reported to specifically target several diverse 7TM-GPCRs to the lysosomal/degradative pathway [7,29,40,41,42,43,44,45] (table 1). Although 9 additional family members of GASP-1 (GASP-2 to GASP-10) have been suggested [47], there is no functional evidence to date that any of these other GASP family members are involved in the sorting of 7TM-GPCRs.
Table 1.
GASP-1 interaction partners and their associated functions
| Interaction partners | Interaction |
Function |
Ref. No. | ||
|---|---|---|---|---|---|
| GST | CoIP | in vitro | in vivo | ||
| Angiotensin II receptor type 1 (ATI) | yes | n.d. | 35 | ||
| Bradykinin 1 receptor (B1R) | yes | yes | 46 | ||
| β1-AR | yes | n.d. | 47 | ||
| β2-AR | yes | no | competition with recycling proteins | 29 | |
| B-alaR (mutant β2-AR) | yes | yes | targeted degradation | 29 | |
| Calcitonin receptor (CALCR) | yes | n.d. | 47 | ||
| Cannabinoid 1 receptor (CB1R) | yes | yes | targeted degradation | agonist-induced receptor downreg-ulation in the spinal cord in mice and development of antinociceptive tolerance | 42, 43, 48 |
| Dopamine D2 receptor (D2R) | yes | yes | targeted degradation; reduced resensitization in rat neurons | increased receptor downregulation after chronic cocaine administration in GASP-1 KO mice | 7, 29, 40, 41, 45 |
| DOR | yes | yes | targeted degradation | 7 | |
| Dysbindin | n.d. | yes | 39 | ||
| Histamine H2 receptor (H2R)1 | yes | n.d. | 47 | ||
| 5-hydroxytryptamine 7 receptor (5-HT7) | yes | n.d. | 47 | ||
| κ-Opioid receptor (KOR) | yes | n.d. | 47 | ||
| μ-Opioid receptor truncated (MOR-TR) | yes | yes | targeted degradation | 29 | |
| M1 | yes | n.d. | increased receptor downregulation after chronic cocaine administration in GASP-1 KO mice | 41,47 | |
| M21 | yes | n.d. | increased receptor downregulation after chronic cocaine administration in GASP-1 KO mice | 41,47 | |
| Opioid receptor-like 1 (ORL1) | yes | n.d. | 47 | ||
| ORF74 | yes | n.d. | 35 | ||
| Tachikinin NK1 receptor (NK1) | yes | n.d. | 35 | ||
| Thromboxane A2α receptor (TXA2α) | yes | n.d. | 47 | ||
| US28 | yes | yes | targeted degradation; enhanced signaling | 35,44 | |
GST = In vitro binding to glutathione S-transferase fusion proteins; CoIP = coimmunoprecipitation; n.d. = not determined.
Low-affinity binding.
GASP-1 is a large acidic protein of 1,394 amino acids that is highly expressed in brain and in a few additional tissues [49], encoded by a gene on the X chromosome in both humans and mice [47]. The 497-amino-acid COOH terminal of GASP-1 (cGASP-1) can disrupt the interaction of GASP-1 with 7TM-GPCRs [7,40,42] and, when overexpressed, cGASP-1 can function as a ‘dominant negative competitor’ for GASP. For instance, the DOR-GASP-1 interaction is disrupted by overexpression of dominant negative cGASP-1 [7], which inhibits the trafficking of DOR to lysosomes and promotes its recycling back to the cell surface [7].
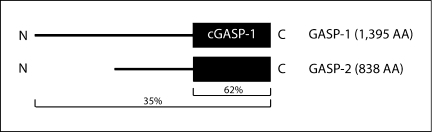
GASP-2, with the closest homology to GASP-1, has been shown to directly bind the D2 dopamine receptor (D2R) and the β2-AR [29], the viral chemokine receptor US28 [44] as well as huntingtin (Htt), a protein playing a role in the neurodegenerative disorder Huntington's disease [50] (table 2). However, to date no functional consequence has been reported for protein interactions with GASP-2. Nevertheless, cGASP-1 shares 62% sequence identity with the C-terminal region of GASP-2 (fig. 3). Hence, it is possible that if GASP-1 and GASP-2 have similar functions, cGASP-1 will act as a dominant negative for both.
Table 2.
GASP-2 interaction partners and their associated functions
| Interaction partners | Interaction |
Function |
Ref. No. | ||
|---|---|---|---|---|---|
| GST | CoIP | in vitro | in vivo | ||
| β1-AR1 | yes | n.d. | 47 | ||
| β2-AR | yes | yes | 29 | ||
| B-alaR (mutant p2-AR) | yes | yes | 29 | ||
| Calcitonin receptor (CALCR) | yes | n.d. | 47 | ||
| Dopamine D2 receptor (D2R) | yes | n.d. | 29 | ||
| Htt | n.d. | yes | colocalization in SH-SY5Y cells | 50 | |
| Histamine H2 receptor (H2R)1 | yes | n.d. | 47 | ||
| 5-hydroxytryptamine 7 receptor (5-HT7)1 | yes | n.d. | 47 | ||
| μ-Opioid receptor truncated (MOR-TR) | yes | yes | 29 | ||
| M1 | yes | n.d. | 47 | ||
| M21 | yes | n.d. | 47 | ||
| US28 | yes | yes | 44 | ||
GST = In vitro binding to glutathione S-transferase fusion proteins; CoIP = coimmunoprecipitation; n.d. = not determined.
Low-affinity binding.
Fig. 3.
Schematic representation of the sequence alignment of GASP-1 and GASP-2 proteins. GASP-1 and GASP-2 show a total amino acid identity of 35%. cGASP-1 and GASP-2 display a 62% amino acid similarity.
GASP-1-Dependent Sorting of 7TM-GPCRs
Intriguingly, GASP-1 seems to show selectivity for individual members of 7TM-GPCR subfamilies. For example, the DOR, bradykinin 1 receptor and the D2R are targeted for degradation by GASP-1, while their respective family members μ-opioid receptor, bradykinin 2 receptor and dopamine D1 receptor (D1R) do not interact with GASP-1 and are recycled to the plasma membrane rather than targeted for degradation [7,40,46].
A study by Thompson et al. [29] showed that the relative affinity of individual receptors for GASP-1 and other sorting proteins can regulate their postendocytic sorting capacities. For instance, the C terminus of wild-type β2-AR binds to GASP-1 in glutathione S-transferase pull-down assays. However, no direct protein-protein interaction of β2-AR with GASP-1 was detected in a HEK293 cell model, resulting in an efficient recycling of the β2-AR. Hence, it seems that recycling proteins such as NHERF/EBP50 and N-ethylmaleimide-sensitive factor ‘win’ the competition with GASP-1 for binding to this receptor. Indeed, when the PDZ domain of β2-AR is disrupted and, thus, β2-AR cannot bind the recycling proteins, this mutant β2-AR associates with GASP-1 and is degraded [29]. Another example is the μ-opioid receptor, where structural sequence determinants within the C tail of the receptor seem to prevent the binding to GASP-1. Here, the truncation of the last 17 amino acids of the receptor [26] was shown to enhance its interaction with GASP-1 and lead to lysosomal targeting of the mutant receptor [29]. In contrast, the deletion of a small motif within the cytoplasmic tail of D1R blocked the recycling of the receptor, but had no effect on the affinity of D1R for GASP-1. As a result, this mutant D1R was neither recycled nor degraded. Hence, preventing the interaction of 7TM-GPCRs with recycling proteins alone does not promote receptor degradation, unless the receptor shows an affinity for degrading sorting proteins such as GASP-1 [29].
GASP-1: Regulator of Signal Transduction?
The sorting of individual receptors between recycling and degradation is a tightly controlled process that is of fundamental importance for the regulation of 7TM-GPCR signaling. Until recently, the only function reported for GASP-1 was the targeting of a variety of 7TM-GPCRs to the degradative/lysosomal pathway.
Intriguingly, we have recently demonstrated that GASP-1 is directly involved in modulating the signaling capacity of a 7TM-GPCR. GASP-1 targets the virally encoded 7TM-GPCR US28 to late endosomes/lysosomes [44], a place that has been suggested to be crucial for the envelopment of viruses. In addition, we observed that the overexpression of GASP-1 in HEK293 cells significantly enhanced the Gαq/phospholipase C/inositol phosphate turnover by US28, whereas shRNA silencing of GASP-1 dramatically reduced inositol phosphate formation in these cells [44]. Moreover, the activation of the transcription factors nuclear factor-κB and cyclic-AMP-responsive element-binding protein via US28 were also either enhanced or prevented by overexpression or shRNA silencing of GASP-1, respectively [44]. It is not clear yet whether the enhanced/decreased signaling capacity of US28 in the presence/absence of GASP-1 is caused by (i) a more active conformational state of the receptor upon GASP-1 binding, or (ii) its relocalization into a particular endosomal compartment that is important for signal transduction [51,52]. However, the capacity of GASP-1 not only to modulate the postendocytic sorting, but also to directly influence the signaling activity of 7TM-GPCRs might point towards an important role of GASP-1 in connecting both processes.
In vivo Relevance of GASP-1-Dependent Sorting
The first evidence suggesting that GASP could have an in vivo function was provided by experiments examining D2R function in the rat ventral tegmental area. Ventral tegmental area slices pretreated with D2R agonist failed to recover from receptor desensitization consistent with the ability of D2R to degrade after endocytosis. However, disrupting the D2R-GASP-1 interaction using an inhibitory antibody allowed the recovery of functional D2R responses [40]. Shortly thereafter, observations that the CB1 cannabinoid receptor was sorted to lysosomes by GASP in vitro[42] led to experiments assessing the in vivo relevance of this finding.
Tappe-Theodor et al. [43] linked the development of analgesic tolerance to cannabinoids to the postendocytic trafficking of the cannabinoid receptor CB1[43]. In this study, mice were treated with an adenovirus expressing dominant negative cGASP-1 and were chronically treated with a cannabinoid drug. The mice receiving the control virus showed tolerance to the analgesic effects, while the mice receiving dominant negative cGASP-1 showed a significantly reduced tolerance [43].
Based on the strength of these initial studies, mice with a disruption of GASP-1 were generated. Using these mice, Martini et al. [48] demonstrated that the development of tolerance to many of the physiological effects of cannabinoids were significantly reduced in GASP-1 knockout mice. Importantly, CB1 receptor levels in GASP-1 knockout mice were not altered in the spinal cord and cerebellum after repeated cannabinoid administration, whereas receptor levels were significantly decreased in the wild-type littermates.
Also using these mice, Thompson et al. [45] have shown that repeated treatment of wild-type but not GASP-1 knockout mice with cocaine leads to a downregulation of D2R in mouse striatum. In addition, disruption of GASP-1 has been shown to alter behavioral responses to cocaine. For example, GASP-1 knockout mice show a reduced locomotor sensitization to cocaine and reduced acquisition of cocaine self-administration [41,45].
Taken together, these early studies begin to reveal the in vivo relevance of GASP-1. The in vivo relevance of GASP-1-dependent sorting of 7TM-GPCRs including the development of tolerance or the sensitization/responsiveness to drugs might help guide new therapeutic approaches. This is especially important since the postendocytic fate of a 7TM-GPCR target in response to drugs has rarely been evaluated during preclinical development. Thus, more studies are clearly needed to unravel the complex processes and the proteins and mechanisms involved in controlling the sorting of 7TM-GPCRs.
Conclusion
Accumulating evidence suggests an important role for GASP-1 in the sorting of many 7TM-GPCRs to the degradative pathway. Several studies have now shown that lysosomal targeting involves a direct interaction of GASP-1 with the cytoplasmic tails of the receptors, and that these C termini contain specific ‘target’ sequences that may facilitate the interaction with GASP-1. Recently, GASP-1 has also been found to be a crucial determent in directly regulating the signaling capacity of a 7TM-GPCR, e.g. the viral receptor US28. Whether GASP-1 directly favors a more active conformation of the receptor or rather targets it to a ‘signalosome’ as yet remains unclear.
Importantly, the in vitro findings were also translated into animal models, verifying that GASP-1-dependent sorting of 7TM-GPCRs has functional consequences in vivo. The first in vivo studies identified the involvement of GASP-1 in the development of tolerance and sensitization to drugs of abuse and pain, including cocaine and cannabinoids. However, there is a plethora of GPCR drugs in which the trafficking fate of the receptor remains unstudied. Hence, the identification of GASP-1 as a key player in receptor downregulation might be a step towards a more accurate understanding of the molecular mechanisms underlying 7TM-GPCR drug efficacy, side effects and tolerance.
Acknowledgements
This study was supported by grants from the Austrian Science Fund (P18723 to M.W.), the Jubiläumsfonds of the Austrian National Bank and the Lanyar Stiftung Graz (both to M.W.), the Molecular Medicine Ph.D. Program of the Medical University of Graz, Austria (E.M., J.K., P.T.), the BaCaVisiting Scientists program (P.T.) and by funds provided by the State of California for medical research through the University of California San Francisco and the National Institute of Mental Health Grant R01 MH68442 (J.L.W.).
References
- 1.Klabunde T, Hessler G. Drug design strategies for targeting G protein-coupled receptors. Chembiochem. 2002;3:928–944. doi: 10.1002/1439-7633(20021004)3:10<928::AID-CBIC928>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 3.Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichsen L, Meyerholz A, Groos S, Ungewickell EJ. Bending a membrane: how clathrin affects budding. Proc Natl Acad Sci USA. 2006;103:8715–8720. doi: 10.1073/pnas.0600312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G protein-coupled receptors. Trends Pharmacol Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- 7.Whistler JL, Enquist J, Marley A, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 8.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 9.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson SS, Zhang J, Barak LS, Caron MG. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 1998;62:1561–1565. doi: 10.1016/s0024-3205(98)00107-6. [DOI] [PubMed] [Google Scholar]
- 11.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 12.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 14.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120(pt 4):543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- 15.Levkowitz G, Waterman H, Zamir E, et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- 17.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the β2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282:3095–3104. doi: 10.1074/jbc.M605398200. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Lauffer B, von Zastrow M, Kobilka B, Xiang Y. NSF regulates β2-adrenoceptors trafficking and signaling in cardiomyocytes. Mol Pharmacol. 2007;72:429–439. doi: 10.1124/mol.107.037747. [DOI] [PubMed] [Google Scholar]
- 20.Brady AE, Limbird LE. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal. 2002;14:297–309. doi: 10.1016/s0898-6568(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 21.Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol. 2002;4:534–539. doi: 10.1038/ncb815. [DOI] [PubMed] [Google Scholar]
- 23.Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 24.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 25.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 26.Tanowitz M, von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J Biol Chem. 2002;277:50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- 27.He J, Bellini M, Inuzuka H, et al. Proteomic analysis of β1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem. 2006;281:2820–2827. doi: 10.1074/jbc.M509503200. [DOI] [PubMed] [Google Scholar]
- 28.Hall RA, Premont RT, Chow CW, et al. The β2-adrenergic receptor interacts with the Na+/H+ exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 29.Thompson D, Pusch M, Whistler JL. Changes in G protein-coupled receptor sorting protein affinity regulate postendocytic targeting of G protein-coupled receptors. J Biol Chem. 2007;282:29178–29185. doi: 10.1074/jbc.M704014200. [DOI] [PubMed] [Google Scholar]
- 30.Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slagsvold T, Pattni K, Maler⊘d L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Chin LS, Raynor MC, Wei X, Chen HQ, Li L. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J Biol Chem. 2001;276:7069–7078. doi: 10.1074/jbc.M004129200. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Zhou Y, Szabo K, Haft CR, Trejo J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell. 2002;13:1965–1976. doi: 10.1091/mbc.E01-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heydorn A, S⊘ndergaard BP, Ersb⊘ll B, et al. A library of 7TM receptor C-terminal tails: interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 36.van Koppen CJ. Multiple pathways for the dynamin-regulated internalization of muscarinic acetylcholine receptors. Biochem Soc Trans. 2001;29(pt 4):505–508. doi: 10.1042/bst0290505. [DOI] [PubMed] [Google Scholar]
- 37.Krudewig R, Langer B, Vogler O, et al. Distinct internalization of M2 muscarinic acetylcholine receptors confers selective and long-lasting desensitization of signaling to phospholipase C. J Neurochem. 2000;74:1721–1730. doi: 10.1046/j.1471-4159.2000.0741721.x. [DOI] [PubMed] [Google Scholar]
- 38.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- 39.Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PLoS One. 2010;5:e9325. doi: 10.1371/journal.pone.0009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartlett SE, Enquist J, Hopf FW, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci USA. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boeuf J, Trigo JM, Moreau PH, et al. Attenuated behavioural responses to acute and chronic cocaine in GASP-1-deficient mice. Eur J Neurosci. 2009;30:860–868. doi: 10.1111/j.1460-9568.2009.06865.x. [DOI] [PubMed] [Google Scholar]
- 42.Martini L, Waldhoer M, Pusch M, et al. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 43.Tappe-Theodor A, Agarwal N, Katona I, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschische P, Moser E, Thompson D, et al. The G protein-coupled receptor-associated sorting protein GASP-1 regulates the signaling and trafficking of the viral chemokine receptor US28. Traffic. 2010;11:660–674. doi: 10.1111/j.1600-0854.2010.1045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson D, Martini L, Whistler JL: Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PLoS One, in press. [DOI] [PMC free article] [PubMed]
- 46.Enquist J, Skröder C, Whistler JL, Leeb-Lundberg LM. Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol Pharmacol. 2007;71:494–507. doi: 10.1124/mol.106.030858. [DOI] [PubMed] [Google Scholar]
- 47.Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor-associated sorting proteins. J Neurochem. 2004;89:766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 48.Martini L, Thompson D, Kharazia V, Whistler JL. Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology. 2010;35:1363–1373. doi: 10.1038/npp.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suyama M, Nagase T, Ohara O. HUGE: a database for human large proteins identified by Kazusa cDNA sequencing project. Nucleic Acids Res. 1999;27:338–339. doi: 10.1093/nar/27.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horn SC, Lalowski M, Goehler H, Droge A, Wanker EE, Stelzl U. Huntingtin interacts with the receptor sorting family protein GASP2. J Neural Transm. 2006;113:1081–1090. doi: 10.1007/s00702-006-0514-6. [DOI] [PubMed] [Google Scholar]
- 51.Waldhoer M, Casarosa P, Rosenkilde MM, et al. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- 52.Fraile-Ramos A, Kledal TN, Pelchen-Matthews A, Bowers K, Schwartz TW, Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol Biol Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]