Abstract
Somatostatin and cortistatin are neuromodulators with divergent expression patterns and biological roles. Whereas expression and function of genes encoding somatostatin (PSS1) and the related peptide cortistatin (PSS2) have been studied in detail for the central nervous system (CNS) and immune system, relatively little is known about their expression patterns in the peripheral nervous system (PNS). We compare the expression patterns of PSS1 and PSS2 in chicken embryos. At E14, PSS1 is higher in the CNS versus PNS, whereas PSS2 is higher in the PNS. During early development, PSS1 is transiently expressed in lumbar sympathetic ganglia and is detectable at low levels throughout the development of dorsal root and ciliary ganglia. In contrast, PSS2 expression increases as development progresses in sympathetic and dorsal root ganglia, whereas levels in ciliary ganglia by E8 are more than 100-fold higher than in sympathetic ganglia. Activin, which induces somatostatin-like immunoreactivity in ciliary ganglion neurons in vivo and in vitro, controls PSS2 expression by stabilizing PSS2 but not PSS1 mRNA. We conclude that much of the somatostatin-like immunoreactivity in the developing avian peripheral nervous system is actually cortistatin, the PSS2 product, as opposed to true somatostatin, which is the PSS1 product. The identification of PSS2 as the predominantly expressed somatostatin gene family member in avian autonomic neurons provides a molecular basis for further functional and pharmacological studies.
INDEXING TERMS: sympathetic ganglia, ciliary ganglion, dorsal root ganglia
The members of the somatostatin family of neuropeptides are structurally similar, yet have distinctly different physiological functions. Somatostatin, which is encoded by the PSS1 gene, was the first to be isolated from hypothalamic extracts by its ability to inhibit growth hormone secretion from the anterior pituitary (Brazeau et al., 1973). Two decades later, the first variant of somatostatin was isolated and characterized from the brain of the frog (Ranaridbunda; Vaudry et al., 1992) and its gene, PSS2, which encodes cortistatin, was cloned shortly thereafter (Trabucci et al., 2003). Since then, PSS2 transcripts have been reported in a variety of species, including sturgeon, zebrafish, mouse, rat, chicken, and human (de Lecea, 2008; Tostivint et al., 2008). PSS2 encodes a preproprotein that differs significantly from PSS1 in primary sequence, except for the mature C-terminal 14-amino acid peptide, which differs only by one amino acid residue from somatostatin 14 (SOM) in avians (Fig. 1), and by three amino acid residues in mammals.
Figure 1.
Amino acid sequences of proteins encoded by PSS1 and PSS2 vary in the prepro region but differ by only one residue in the mature peptide. Protein sequences were obtained from the Entrez Protein database and correspond to accession no. CAA42747 for Gallus somatostatin and NP_989786 for Gallus somatostatin 2 (cortistatin). Gaps introduced to optimize alignment of residues are indicated by the horizontal dashes. Residues that are identical between the two proteins are joined by vertical bars; the mature 14-amino acid peptide is underlined. The synthetic 14-amino acids SOM and CST used in the present study were synthesized by using these sequences.
This new neuropeptide is called cortistatin (CST) because of its relatively high expression in the cerebral cortex. Both CST and SOM bind to pituitary cells; however, differing (and in some cases even opposing) effects are elicited by CST and SOM on the cerebral cortex. This may be explained by the fact that CST binds not only to somatostatin receptors 1–5 but also to receptors not activated by SOM, such as the growth hormone secretagogue receptor and the G-protein-coupled receptor MrgX2 (Robas et al., 2003). Furthermore, PSS1 and PSS2 are differentially expressed in the brain and in peripheral organs (Trabucci et al., 2003; Dalm et al., 2004).
Neurons with somatostatin-like immunoreactivity (SOM-LIR) are also found in enteric, sympathetic, and parasympathetic ganglia of the mature autonomic nervous system. SOM-LIR is expressed in cholinergic enteric neurons and a subset of neurons in parasympathetic ganglia; in the superior cervical ganglion, SOM-LIR is expressed in a small subpopulation of neurons (Lundberg et al., 1982; Wright and Luebke, 1989), whereas more SOM-LIR neurons are seen in trunk prevertebral ganglia (Hökfelt et al., 1977; Anderson et al., 2001). It has been assumed that SOM-LIR is due to the presence of SOM-14 or its N-terminal extended SOM-28; however, with the discovery of two genes that encode highly similar mature peptides, it is not clear whether the SOM-LIR observed is actually due to transcription of the PSS1 or PSS2 gene. If CST-14 differs by only one to two amino acids from SOM-14, then many antibodies made against SOM-14 are likely to cross-react with CST-14.
In the avian ciliary ganglion, SOM-LIR identifies the choroid subpopulation of the avian ciliary ganglion (Epstein et al., 1988; DeStafano et al., 1993) and our previous studies showed that a balance of activin and follistatin expression in peripheral targets retrogradely controls the expression of this neuropeptide during development (Darland et al., 1995; Darland and Nishi, 1998). However, in these early studies, we were unable to amplify cDNA encoding prepro-somatostatin from ciliary ganglia that fully expressed SOM-LIR and, recently, we discovered that PSS1 RNA could not be detected by in situ hybridization. We discovered that SOM-LIR in the ciliary ganglion is due to PSS2 rather than PSS1, and this observation led to the broader characterization of PSS1 and PSS2 in the nervous system of chicken embryos that we report here.
MATERIALS AND METHODS
Solid-phase enzyme-linked immunosorbant assay (ELISA)
NUNC MaxiSorb High Protein Binding Capacity 96-well ELISA plates (Fisher Scientific, Pittsburgh, PA) were coated overnight with 2 µg/well of SOM-14 (Preprotech, Rocky Hill, NJ), CST-14 (oxidized), or CST-14 (reduced) (synthesized and purified by the Peptide Synthesis Core, Department of Biochemistry, University of Vermont College of Medicine) diluted in phosphate-buffered saline (PBS; 120 mM NaCl, 20 mM sodium phosphate buffer, pH 7.4). After blocking with PBS containing 10% (v/v) heat-inactivated horse serum (Life Technologies, Bethesda, MD) and 0.2 (w/v) sodium azide (Sigma-Aldrich, St. Louis, MO), the wells were incubated in serial dilutions of rat anti-SOM 14 (mAb clone YC7 culture supernatant; Accurate Chemical and Scientific, Westbury, NY) for at least 1 hour, washed 3 times with PBS containing 0.05% (v/v) Tween-20 (Sigma-Aldrich), and incubated in rabbit-anti-rat antibody covalently coupled to alkaline phosphatase (Promega, Madison, WI). After washing, wells were incubated in alkaline phosphatase buffer (0.2 M Tris, pH 9.5) containing 1 mg/ml p-nitrophenyl phosphate (Sigma-Aldrich), and the resulting chromogenic product was monitored on a UVMax 96-well plate kinetic microplate reader (Molecular Devices, Sunnyvale, CA) at 405 nm on the kinetic setting over a 2-minute reaction period. After confirmation that absorbance increased linearly over the period of measurement, all reactions were expressed as mOD/min.
Immunohistochemistry
Tissues were isolated from chicken embryos at the stated ages and fixed by immersion in Zamboni’s fixative (4% paraformaldehyde, 15% saturated picric acid in 0.2 M sodium phosphate buffer, pH 7.4) at 4°C for 2 days. Tissues were washed extensively in PBS and equilibrated in 30% sucrose. Frozen sections of 20-µmthickness were cut on a Microm HM560 cryostat (Richard Allen Scientific, Kalamazoo, MI), mounted on Permafrost 2 microscope slides (Fisher Scientific, Fair Lawn, NJ), washed, and incubated in blocking solution (PBS containing 10% horse serum [Invitrogen/Life Technologies, Carlsbad, CA], 0.5% Triton X-100, 0.2% sodium azide). Antibodies used for the immunohistochemical studies are described in Table 1. Tissue sections were incubated overnight in anti-Islet1/2 (39.4D5, Developmental Studies Hybridoma Bank, Iowa City, IA). Coverslips were washed, and then incubated in goat anti-mouse Alexa 488 (Invitrogen) diluted 1:750 in blocking solution for 1–2 hours, followed by an overnight incubation in 1:100 dilution of anti-SOM 14 as described above, followed by incubation in goat-anti-rat cy3 diluted 1:750 (Invitrogen).
TABLE 1.
Antibodies Used in This Study
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| Somatostatin and cortistatin | Human SOM 1–14, conjugated to thyroglobulin | Accurate Chemical (Westbury, NY), Cat. no. YMC 1020, rat monoclonal YC7 | 1:100 |
| Islet-1 | Recombinant rat Islet 1 | Developmental Studies Hybridoma Bank (Iowa City, IA), mouse monoclonal 39.4D5 | 1:20 |
| Digoxigenin | Digoxigenin | Roche Diagnostic (Mannheim, Germany), cat. no. 11 093 274 001, sheep polyclonal | 1:3,000 |
Images of immunofluorescence were acquired on a Nikon C1 Confocal mounted on a Nikon Eclipse E800 microscope at laser intensities and settings determined by the positively stained image so as to minimize saturation of pixels. Tif images were transferred to Adobe Photoshop CS3 Extended Version 10.0.1 or Adobe Photoshop Elements 6.0 (Adobe Systems, San Jose, CA) and adjusted for brightness and contrast to reflect the captured image when the specimen was scanned. To create the montages, image size reductions and cropping were necessary.
Antibody characterization
Antibodies used are listed in Table 1. We used mouse monoclonal anti-Islet1/2 as a pan- neuronal marker to facilitate identification of ganglia in sections of chicken embryos. This monoclonal antibody (39.4D5), also known as 4D5 (Tsuchida et al., 1994), was produced against the carboxy-terminal residues 178–349 of rat Islet-1; it recognizes both chicken Islet-1 and Islet-2 proteins (Varela-Echavarria et al., 1996) and stains nuclei of a variety of neuronal and endocrine cells in zebrafish, African clawed frog, chicken, rat, and mouse, as previously reported for a rabbit polyclonal (Ericson et al., 1992). The same clone also stains the nuclei of all developing chicken ciliary ganglion neurons throughout E5.5–14 (Lee et al., 2001), as well as nuclei of developing chicken dorsal root ganglion neurons (Cui and Goldstein, 2000) and embryonic chicken sympathetic neurons (Straub et al., 2007).
Rat monoclonal anti-somatostatin was produced by Dr. A.C. Cuello (McGill University, Montreal, Canada) by immunizing rats with human SOM-14 conjugated to thyroglobulin; the epitope recognized by this monoclonal antibody is within the 14 amino acids that form the circular portion of the oxidized SOM peptide. This antibody does not cross-react with enkephalins, other endorphins, substance P, or calitonin gene-related peptide (CGRP; manufacturer’s information sheet). Immunohistochemical staining for somatostatin in the ciliary ganglion is lost when the antibody is preabsorbed with 2 µM synthetic SOM-14 or CST-14 (see Suppl. Fig. 1). Our studies in this paper also show that anti-somatostatin cross-reacts with chicken CST-14 in a solid-phase enzyme-linked immunoassay (see methods above).
Sheep anti-digoxigenin (Roche Diagnostics, Mannheim, Germany) used for detecting hybridization of digoxigenin labeled probes has 100% reactivity with digoxigenin and digoxin, but no cross-reactivity with other steroids, such as estrogens or androgens. Little to no background is observed in tissues that have not been hybridized with a probe or with a sense probe (see Suppl. Fig. 2).
Quantitative real-time PCR
Fertile white leghorn chicken eggs (Oliver Merrill, Londonderry, NH) were incubated at 38°C. Embryos were harvested at the indicated days of incubation and staged according to Hamburger and Hamilton (1992). Tissues were rapidly removed and frozen on dry ice. Total RNA was extracted by using TriReagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer’s directions.
One microgram total cellular RNA was used in the First-Strand cDNA synthesis using SuperScript II Reverse Transcriptase (RT), and oligo dT primers (Invitrogen) to selectively transcribe mRNA transcripts. The total RT reaction volume per sample was 20 µl. Five percent of the cDNA (1 µl) was used for each real-time quantitative polymerase chain reaction (qPCR) reaction. Each 25 µl qPCR reaction was prepared in triplicate on a 96-well plate; each cDNA sample was added to 24 µl of the reaction cocktail, comprised of 12.5 µl TaqMan Universal PCR Mastermix (Applied Biosystems/Life Technologies, Carlsbad, CA), 10.25 µl double deionized (dd) H2O, and 1.25 µl primer/probe mix. Reactions were run on an ABI 7700 in the Molecular Cellular Core operated by the Center of Biomedical Research Excellence (COBRE) in Neuroscience at the University of Vermont. Primers and probes were designed as previously described (Hruska et al., 2007) and the efficiencies of the primer-probe combinations were validated on 10-fold serial dilutions of template (plasmid encoding the target gene or cDNA).
Calculations of actual efficiencies for all primers and probes used in the study were performed by determining the fit of the earliest portion of the reaction after the threshold was exceeded to the theoretical exponential curve expected. Efficiencies of all reactions were consistently high and ranged from 70 to 90%. Target genes for expression profiling were PSS1 (somatostatin) and PSS2 (cortistatin). Expression levels were normalized to CHRPS (chick ribosomal binding protein s17). Whenever possible, all samples for a single experiment were reacted on the same plate so data could be collected for all conditions in a single run.
Primer/probe mixes
PSS1: 10 µM forward and reverse primers (forward: 5′-CCGAGCAGGATGAAGTGA; reverse: 5′-GGACAGGTGGGTTTCAAAG); 2 µM probe (5′-[6FAM]CTCGGCAACTCAA ACCCCG[ BHQ]).
PSS2: 10 µM forward and reverse primers (forward: 5′-GTGTCTGTTCTGCTGCTGGT; reverse: 5′-CACGTCTGAGCTCTCCAAGA); 2 µM probe (5′-[6FAM]CCACTGC GCTGCCTGGGAA[BHQ]).
CHRPS: 10 µM forward and reverse primers (forward: 5′-CTTCATCAGGTGGGTGACAT; reverse: 5′-GGACAGGTGGGTTTCAAAAG); 2 µM probe (5′-[6FAM]CGCCATCATCCCCAGCAAGA[BHQ]).
Relative quantification methods from real-time data
Cycle threshold (Ct) values for the target gene (PSS1 or PSS2) were normalized to the control gene (CHRPS) by using the equation 10,000/2Ct(target)-CT(control) in accordance with ABI instructions. Efficiencies of target and control reactions were very similar and did not differ significantly from one another (Student’s two-tailed t-test). Mean values and standard deviations were calculated for each experiment and analyzed by using a one-way analysis of variance (ANOVA) for CNS versus PNS tissues for either PSS1 or PSS2 separately or two-way ANOVA for developing tissues versus PSS1 or PSS2) and for activin-treated cultures at different time points (activin versus PSS1 or PSS2). Because primers and probes differ for PSS1 versus PSS2, we avoided direct comparisons of PSS1 to PSS2 from the same samples.
In situ hybridization
Fertile white leghorn chicken eggs (Gallus gallus domesticus; Stefan Körner, Frankfurt, Germany) were stored at 18°C. On day 0 of incubation, they were placed into an incubator set at 38.5°C and 60% humidity. Embryos were harvested at the indicated days and staged according to Hamburger and Hamilton (1992). Head, body, and isolated ciliary ganglia were fixed in 4% paraformaldehyde/PBS overnight at 4°C. Then tissues were equilibrated in 30% sucrose at 4°C overnight. The tissues were embedded and frozen in embedding medium (Tissue Tek, Sakura Finetek, Torrance, CA) and sectioned by using a Leica CM3050 S cryostat (Leica, Wetzlar, Germany). Then 14-µm horizonal sections containing the ciliary ganglion or the sympathetic and the dorsal root ganglia of the wing limb area were collected on slides (Menzel Gläser Superfrost Plus, Braun-schweig, Germany) for in situ hybridization.
Total RNA was isolated from E13 neural tube, dorsal root ganglia, and sympathetic ganglia for the PSS1 probe and from E15 ciliary ganglia for the PSS2 probe by using RNeasy Mini Kit (Qiagen, Chatsworth, CA) according to the manufacturer’s instruction. cDNA synthesis was performed according to the manufacturer’s instructions (SuperScript III First-Strand Synthesis System for RT-PCR; Invitrogen). For the reverse transcription, oligo(dT) primers were used. The PSS-specific primers used for PCR amplification were designed according to the known Gallus gallus PSS1 sequence (Genbank accession no. X60191) and Gallus PSS2 sequence (Genbank accession no. NM_204455).
A template for PSS1 (28–638; 602 bp) was generated by RT-PCR (forward primer: 5′-CGGCGAGATGCTGTCGTG; reverse primer: 5′-AATCGCGGAGTGCATGTCAC), subcloned into pCRII-TOPO (Invitrogen), and sequenced. A template for PSS2 (1– 447; 447 bp) was generated by RT-PCR (forward primer: 5′-CAGGAACAGCTCCCTCTC; reverse primer: 5′-TCTCAGGTGGGAGGACAG), subcloned into pGEM-Teasy (Promega), and sequenced. The template for SCG10 encoded the full-length cDNA for SCG10 (890 bp; GenBank accession no. L14938) and was kindly provided by P.L. Jeffrey (Children’s Medical Research Institute, Wentworthville, Australia). Digoxigenin-labeled SCG10 cRNA, PSS1 cRNA, and PSS2 cRNA were synthesized by using the DIG-Nucleic Acid Detection Kit (Roche Diagnostics).
Sections were incubated overnight at 68°C with digoxigenin-labeled antisense and sense PSS1 or PSS2 cRNA probe (diluted 1:100) in hybridization solution. The hybridization solution was composed of 50% formamide, 10% dextran sulfate, 1 mg/ml yeast RNA, 12.6mMTris, pH 7.5, 185 mM NaCl, 10 mM NaH2PO4, 5 mM EDTA, pH 7.5, 0.5X Denhardt’s solution (1X Denhardt’s solution: 0.02% [w/v] bovine serum albumin [BSA], 0.02% [w/v] Ficoll, 0.02% [w/v] polyvinylpyrilidon). Sections were incubated in 1X; SSC (1X SSC: 150 mM NaCl, 15 mM Na-citrate, pH 7.0), 50% formamide, 0.1% Tween 20 at 68°C for 1 hour, and then equilibrated in MABT solution (100 mM maleic acid, 150 mM NaCl, pH 7.5, 0.1% Tween 20) at room temperature for 1 hour followed by blocking the unspecific binding with 20% chick serum in MABT solution at room temperature for 3 hours. Then sections were incubated with sheep anti-digoxigenin antibody (Roche Diagnostics) diluted 1:3,000 in MABT solution with 20% chick serum overnight at room temperature. After washing in MABT for 1 hour at room temperature, the sections were equilibrated in AP solution (100 mM Tris, pH 9.5, 50 mM MgCl2, 0.1% Tween 20, 0.024% levamisol) twice for 10 minutes at room temperature.
Staining was carried out in the dark at room temperature with 4.5 µg/µl NBT (4-nitro blue tetrazolium chloride; Roche Diagnostics) and 1.75 µg/µl BCIP (5-bromo-4-chloro-3-indolyl-phosphate; Roche Diagnostics) in AP solution until staining became visible. Staining was stopped by washing with PBS. After air-drying, sections were embedded in Kaiser’s Glycerin Gelatin (Merck, Darmstadt, Germany) and coverslipped. Stained sections were imaged on a Zeiss Axioplan with top frame Axiophot2. Images were transferred as .tif files to Adobe Photoshop CS Version 8.0, and manipulations were limited to linear level adjustments, rotations, and image size reductions.
Cell culture
Ciliary ganglia were removed from Hamburger and Hamilton (HH) stage 34 (E8) chicken embryos, dissociated, and cultured as previously described (Nishi, 1996), with the following modifications: the medium was Dulbecco’s modified Eagle’s medium and was supplemented with 10% (v/v) heat-inactivated horse serum (GIBCO/Life Technologies, Carlsbad, CA), 20 U/ml penicillin, 2 mg/ml streptomycin (GIBCO/Life Technologies), 2 mM glutamine (GIBCO/Life Technologies), and 10 ng/ml [his]6 chCNT-Fyyy (bacterially expressed recombinant chicken ciliary neurotrophic factor [CNTF; also known as growth-promoting activity {GPA}]), which was purified by the Nishi lab (Heller et al., 1995). Some cultures were also supplemented with 20 ng/ml recombinant human activin obtained from the National Hormone and Pituitary Program (National Institute of Diabetes, Digestive Disorders and Kidney, Rockville, MD).
RESULTS
Immunohistochemical staining revealed many SOM-LIR neurons in sympathetic ganglia at E8 (Fig. 2A,B), and at E14 (Fig. 2D), which is in agreement with previous studies (Hayashi et al., 1983; Maxwell et al., 1984). SOM-LIR is maintained in a small subset of sympathetic neurons through E18, the oldest stage analyzed (data not shown). We also confirmed our previous studies (Coulombe and Nishi, 1991) that SOM-LIR is in a small percentage of neurons in ciliary ganglia at E8 (Fig. 2C), increasing to approximately half of the neurons by E14 (Fig. 2E; for quantification of SOM-LIR neuron number, see Bunker and Nishi, 2002; Hruska et al., 2007). In contrast, little or no SOM-IR is observed in developing dorsal root ganglia at these stages of development. Because the anti-somatostatin antibody we used was generated against SOM-14, we tested whether it could distinguish between CST-14 and SOM-14 in a solid-phase ELISA. As shown in Figure 3, the rat monoclonal antibody we used for immunohistochemistry (Fig. 2; Bunker and Nishi, 2002; Hruska et al., 2007) is unable to distinguish between somatostatin and cortistatin.
Figure 2.
Somatostatin-like immunoreactivity is detectable during development of sympathetic and ciliary ganglia, but not sensory ganglia. Embryos were harvested at the indicated days of incubation, fixed, cryostat sectioned, and stained with Islet-1 (green), a transcription factor that is localized to the nuclei of all neurons, and rat anti-somatostatin (magenta). A: Transverse section across an E8 chicken embryo at low power showing the positions of the lumbar motor neurons (LMN), sympathetic ganglion (SG), and dorsal root ganglion (DRG). B: Higher power image of the sympathetic ganglion boxed in A. Arrowheads identify neurons with SOM-IR. C: Low-power image of an E8 ciliary ganglion (cil) with SOM-IR expressing cells (arrowheads). D: Transverse section across the lumbar region of the spinal column of an E14 embryo showing SOM-IR in a subset of neurons of the sympathetic ganglion (SG), shown at higher power in the inset. E: E14 ciliary ganglion with a number of SOM-IR neurons. Digital images were only adjusted for brightness to match the level of staining observed by epifluorescence through the microscope eyepieces. Scale bar = 50 µm in B (applies to B and inset in D); 100 µm in E (applies to A,C–E).
Figure 3.
An antibody prepared against SOM-14 cross-reacts with CST-14. Serial two-fold dilutions of the rat monoclonal antibody used to identify SOM-IR immunohistofluorescence in Figure 2 was tested in a solid-phase ELISA against synthetic circularized SOM-14 (squares), circularized CST-14 (circles), linearized CST-14 (triangles), or no peptide (diamonds). There is strong cross-reactivity to both circularized and linearized CST-14. Unit for the x-axis is the fraction of the starting concentration of the antibody as provided by the supplier (a culture supernatant). Absolute values of anti-SOM-specific immunoglobulin protein are unknown. Values given are the mean of 4 wells per point, and the error bars represent standard error of the mean. ELISA was performed twice with similar results.
Because our anti-SOM could not distinguish between the PSS1 (somatostatin) versus PSS2 (cortistatin) gene products, we used real-time qPCR to quantify the levels of transcripts encoding PSS1 and PSS2 in neural tissues isolated from developing chicken embryos. For these studies, relative quantification was used and expression of the target genes was normalized to the constitutively expressed gene chicken ribosomal binding protein s17 (Darland et al., 1995; Hruska et al., 2007). Thus, the relative levels of transcripts can be compared for each gene across samples, but the values of PSS1 versus PSS2 in a single tissue cannot rigorously be compared with one another.
Table 2 shows that normalized levels of PSS1 are 100–1,000 times more abundant in the forebrain, cerebellum, tectum, and brainstem, compared with sympathetic, ciliary, or dorsal root ganglia, in which the signals were barely detectable. In contrast, PSS2 is readily detected in peripheral ganglia, with exceptionally high levels in ciliary ganglia. Thus, there is a greater abundance of PSS1 in the CNS versus peripheral autonomic ganglia, whereas PSS2 transcripts are more abundant in autonomic ganglia.
TABLE 2.
PSS1 and PSS2 Expression in the CNS Versus PNS1
| Tissue | PSS1 | PSS2 |
|---|---|---|
| Forebrain | 551 ± 120 | 49 ± 18 |
| Cerebellum | 341 ± 135 | 80 ± 14 |
| Tectum | 952 ± 243 | 78 ± 20 |
| Brainstem | 724 ± 136 | 355*± 127 |
| SG | 2.6*± 2.2 | 446*± 241 |
| DRG | 2.5*± 1.0 | 117 ± 146 |
| CG | 0.34*± 0.1 | 1211*± 127 |
Tissue was isolated from E14 chicken embryos, and transcripts encoding PSS1 and PSS2 were quantified by real-time qPCR using Taqman probes. Values for each target gene were normalized to chicken ribosomal binding protein s17 (CHRPS), yielding arbitrary mRNA units (AU) as described in Materials and Methods. Values represent the AU of three independent tissue and RNA isolations ± standard deviation.
These values differ from values of the same gene for forebrain, tectum, and cerebellum by P < 0.001, one-way ANOVA, Tukey’s post hoc test. CNS, central nervous system; PNS, peripheral nervous system; SG, sympathetic lumbar ganglia; DRG, dorsal root ganglia; CG, ciliary ganglia.
The detailed analysis of the developmental expression of PSS1 shows high expression in E8 lumbar sympathetic ganglia, followed by a steady decline to levels that are 1/20th of the starting levels by E14 (Fig. 4A). In lumbar dorsal root ganglia, PSS1 levels are low but detectable at E8, peak at threefold higher levels at E12, and then decline to levels comparable to sympathetic ganglia by E14. Finally, in ciliary ganglia, PSS1 expression is comparable to that of dorsal root ganglia at E8 and declines to virtually undetectable levels by E14.
Figure 4.
Developmental expression of PSS1 and PSS2 in peripheral ganglia. Expression of PSS1 (A) and PSS2 (B) in sympathetic (SG), dorsal root (DRG), and ciliary ganglia (CG) collected from chicken embryos at the indicated days of egg incubation was quantified on the same plate by using quantitative real-time PCR with Taqman probes. Arbitrary units (AU) of target mRNA were calculated by normalizing to the constitutively expressed chicken ribosomal binding protein s17 (CHRPS) by 10,000/2(Ct(target)-Ct(control)). Reaction efficiencies of the PCR reactions in individual wells were highly consistent and ranged from 70 to 90%. A: PSS1 values are expressed as means of qPCR reactions obtained by using material from three independent isolations of tissue (n = 3); SG values at E8, 10, and 12 and the DRG value at E12 differ significantly from CG values at E8 – 14 and DRG values at E8 – 10 (P < 0.01), two-way ANOVA with Bonferroni post test. B: PSS2 values are expressed as means of qPCR reactions obtained by using material from three independent isolations of tissue (n = 3). SG and CG values at E8–14 differ significantly (P < 0.001) from DRG values at any stage, two-way ANOVA with Bonferroni post test.
The developmental pattern of PSS2 in peripheral ganglia differs in several aspects from that of PSS1 (Fig. 4B). In sympathetic ganglia, PSS2 is low at E8, then increases by five- to sixfold by E12, and remains high at E14. In dorsal root ganglia, PSS2 is undetectable between E8 and 10, becomes detectable by E12, and is considerably higher by E14, but the levels at E14 are four- to fivefold lower than sympathetic ganglia and over 100-fold lower than ciliary ganglia. In ciliary ganglia, PSS2 RNA levels at the earliest stage examined (E8) are already 100–200-fold higher than that of sympathetic or dorsal root ganglia, and these levels remain high. Despite the high levels of PSS2 at E8 that persist through E14, expression of SOM-LIR is detectable in a smaller percentage of neurons at E8 versus E14 (Coulombe and Nishi, 1991).
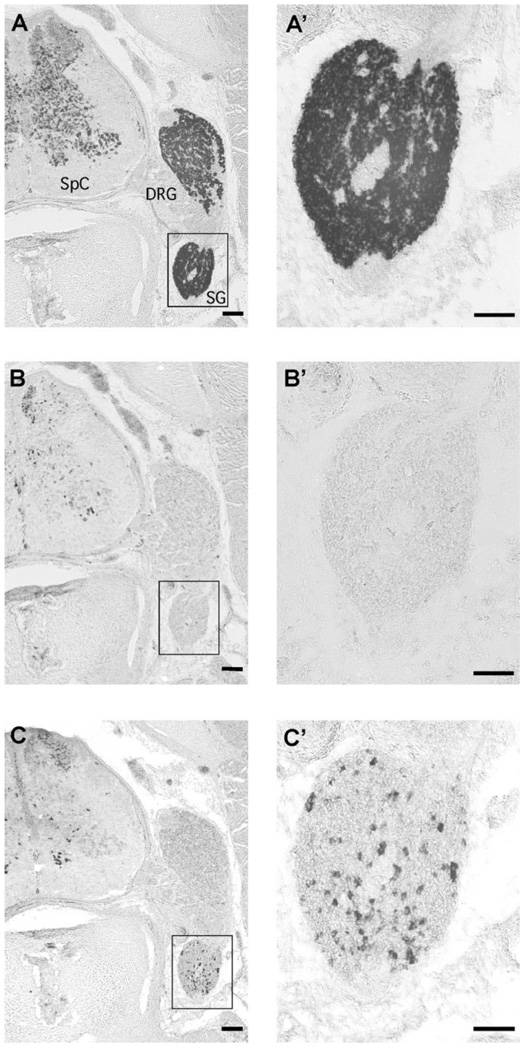
The histological pattern of PSS1 versus PSS2 expression in peripheral ganglia was examined by in situ hybridization with full-length riboprobes (Figs. 5–7). Because the sequences of the cDNAs encoding these transcripts differ significantly, cross-hybridization can be excluded. Furthermore, little or no signal was detected when sense probes were hybridized to the tissue sections (Suppl. Fig. 2). In tissue sections, ganglia were identified by hybridization of probes for SCG10, a pan-neuronal marker (Hannan et al., 1996) (Figs. 5A,A′, 6A,A′, 7A,D). The in situ hybridization corroborated the PSS1 patterns we observed with qPCR, with little or no detectable signal in dorsal root ganglia, whereas a subpopulation of neurons in the ventral region of sympathetic ganglia at E8 labeled with the PSS1 probe (Fig. 5B,B′) with very little detectable hybridization for PSS1 at E14 (Fig. 6B,B′). PSS2 was undetectable in E8 dorsal root ganglia, and only a faint signal was found in a small number of E8 sympathetic neurons (Fig. 5C,C′).
Figure 5.
PSS1 is expressed in sympathetic, but not dorsal root ganglia at E8. In situ hybridization with a digoxigenin-labeled probe was used to localize expression of SCG10 (A,A′), PSS1 (B,B′), and PSS2 (C,C′) in transverse sections through the trunk region of E8 embryos. The boxed areas in A, B, and C are shown at higher magnification in A’, B’, C’. SpC, spinal cord; DRG, dorsal root ganglion; SG, sympathetic ganglion. Arrows in C′ indicate a small number of PSS2-positive cells. In B and C, PSS1 and PSS2 expression is also seen in chromaffin cells of the adrenals (Adr), which lie ventral to the sympathetic ganglia. Scale bar = 200 µm in A–C; 100 µm in A′–C′.
Figure 7.
PSS2 but not PSS1 is expressed in ciliary ganglia at E8 and E14. In situ hybridization with a digoxigenin-labeled probe was used to localize expression of SCG10 (A,D), PSS1 (B,E), and PSS2 (C,F) in sections of E8 (A–C) and E14 (D–F) ciliary ganglia. Scale bar = 100 µm.
Figure 6.
PSS2 but not PSS1 is expressed in sympathetic ganglia at E14. In situ hybridization with a digoxigenin-labeled probe was used to localize expression of SCG10 (A,A′), PSS1 (B,B′), and PSS2 (C,C′) in transverse sections through the trunk region of E14 embryos. The boxed areas in A–C are shown at higher magnification in A′–C′. SpC, spinal cord; DRG, dorsal root ganglion; SG, sympathetic ganglion. Scale bar = 200 µm in A–C; 100 µm in A′–C′
However, a subpopulation of sympathetic neurons with strong PSS2 in situ signal was found at E14 (Fig. 6C,C′) and E18 (not shown). PSS1 is already strongly expressed by adrenal chromaffin cells at E8 (Fig. 5B), whereas only few cells are PSS2 positive (Fig. 5C). In contrast to sympathetic and dorsal root ganglia, a larger number of ciliary ganglion neurons expressed PSS2, but not PSS1 at E8 and E14 (Fig. 7; compare B,E with C,F). Thus, the SOM-LIR that we and others have reported in the choroid neuron subpopulation is likely due to cortistatin, the PSS2 gene product.
We also previously reported that activin induced expression of SOM-LIR in E8 ciliary ganglia (Darland et al., 1995; Darland and Nishi, 1998). If this immunoreactivity is due to cortistatin, the PSS2 encoded gene product, then we would expect activin to affect expression of PSS2 rather than PSS1. Accordingly, we treated E8 ciliary ganglion neurons in cell culture with activin and monitored PSS1 and PSS2 expression by qPCR. PSS1 levels are very low and highly variable (Fig. 8A), whereas PSS2 levels are very high 2 hours after plating (Fig. 8B; note that values for mRNA AU for PSS2 are 1,000 times higher than for PSS1). In the absence of activin, the high levels of PSS2 mRNA at E8 decline rapidly within 24 hours to barely detectable levels. In contrast, activin stabilizes the high levels of PSS2 RNA, and has no effect on PSS1 RNA. Thus, activin posttranscriptionally controls expression of PSS2 but not PSS1 in the cultured ciliary neurons. This lends further support to the concept that the SOM-IR we reported in earlier studies (Coulombe and Nishi, 1991; Coulombe et al., 1993; Darland et al., 1995; Darland and Nishi, 1998) is cortistatin rather than somatostatin and suggests a novel mechanism for regulating neuropeptide expression.
Figure 8.
Activin regulates PSS2 but not PSS1 in ciliary ganglion neurons. Ciliary ganglia were removed from chicken embryos at E8, dissociated, and cultured in wells coated with poly-D-lysine and laminin in the presence (black bars) or absence (white bars) of human recombinant activin (20 ng/ml) with 10 ng/ml of chicken ciliary neurotrophic factor (chCNTF) to support survival. Cultures were harvested at the indicated times, RNA was extracted and reverse-transcribed, and PSS1 and PSS2 together with the internal control gene CHRPS were quantified with qPCR by using Taqman probes. Arbitrary units (AU) of target mRNA were calculated by normalizing to the constitutively expressed chicken ribosomal binding protein s17 (CHRPS) by 10,000/2(Ct(target)-Ct(control). Reaction efficiencies of the PCR reactions in individual wells were highly consistent and ranged from 70 to 90%. A: PSS1 values represent the mean AU obtained from three replicate cultures (n = 3), and error bars represent standard deviation. Note that PSS1 values are very low in comparison with PSS2 values; means for PSS1 do not differ significantly from one another, two-way ANOVA, Bonferroni test. B: PSS2 values values represent the mean AU obtained from three replicate cultures (n = 3), and error bars represent standard deviation. PSS2 expression starts high at 2 hours, but drops to values at 24 hours that are signficantly lower than those at 2 hours (P <0.001, two-way ANOVA, Bonferroni test). PSS2 expression is maintained if cells are exposed to activin. Cultures with activin at 24 hours differ signficantly from control at 24 hours (P <0.001, two-way ANOVA, Bonferroni post test).
DISCUSSION
The principal findings reported here are that PSS1 is transiently expressed during the development of the PNS, whereas PSS2 becomes upregulated in sympathetic, dorsal root, and ciliary ganglion neurons. We conclude that SOM-LIR in parasympathetic and sympathetic neurons is due primarily to expression of the PSS2 gene product, implicating CST as the physiologically relevant neuropeptide in the avian autonomic nervous system.
Because somatostatin and cortistatin have different physiological effects in the mammalian CNS, this knowledge will facilitate future physiological investigations of the function of somatostatin-like peptides in peripheral tissues.
Our conclusions rely on the combination of immunocytochemistry using an antibody that we show cross-reacts with both SOM-14 and CST-14 to demonstrate expression of the peptide together with identification and quantification of the transcripts encoded by PSS1 and PSS2 by qPCR and in situ hybridization. We show that the anti-SOM-14 does not distinguish between SOM-14 and CST-14, implying that SOM-28 and CST-24 will also be recognized by the antibody. Whereas the anti-SOM-14 does not distinguish between somatostatin and cortistatin expression, transcripts encoded by PSS1 and PSS2 can be differentially detected by using full-length probes because the genes differ greatly in the prepro region, which represents more than 80% of the nucleotide sequence. Detection of transcripts does not guarantee that protein is expressed; however, lack of detection of the PSS1 transcript likely ensures that the protein is not expressed at detectable levels.
Thus, our results show that PSS2, but barely detectable PSS1, is expressed at E14 in sympathetic, dorsal root, and ciliary ganglion neurons. The difference in the levels of PSS2 expression detected by in situ hybridization versus immunohistochemistry may be explained either by a lower sensitivity of immunohistological detection or by the expression of protein being posttranscriptionally regulated. Indeed, translational regulation of somatostatin expression has been described previously in cultures of rat sympathetic neurons (Spiegel et al., 1990). Although it would be ideal to have antibodies that differentially detect cortistatin versus somatostatin to confirm the presence of the peptides, this is not possible due to the structural similarities of the mature peptides. Antibodies against the pre-proproteins will distinguish the precursors; however, such antibodies have been raised against mouse and human prepro-cortistatin and would not be expected to stain chicken precursors. Furthermore, neuropeptides are processed in the Golgi; thus only the mature peptides would accumulate to detectable levels in synaptic dense-core vesicles that are the hallmark of neuropeptide transmitters, making differential detection of mature neuropeptides problematic. In fact, repeated attempts to create a cortistatin-selective antibody have resulted in failure, and commercially available antisera stain neurons in PSS2 knockout mice, so are likely to be nonspecific (P. de Lecea, personal communication).
The functions of the transiently expressed SOM-LIR and PSS1 in early sympathetic ganglia are not well understood. SOM-LIR has been observed during early embryonic development of sympathetic and sensory neurons (Hayashi et al., 1983; Garcia-Arraras et al., 1984; Maxwell et al., 1984) and our studies suggest that PSS1, and therefore SOM-14 or SOM-28, are the active peptides expressed at these early stages. The early embryonic expression of these genes may simply reflect a process that has been observed in other systems in which developing neurons express many different transmitters and environmental signals specify the elimination of all but the appropriate subtype-specific transmitters needed (Borodinsky et al., 2004).
Our observations have significant impact on the physiological function of SOM-LIR in the avian ciliary ganglion. SOM-LIR is a feature that distinguishes choroid neurons from ciliary neurons (Epstein et al., 1988; DeStefano et al., 1993). Expression of SOM-LIR is regulated by interactions with the appropriate target tissue (Coulombe and Nishi, 1991) and the factor that controls SOM-LIR was identified as activin (Coulombe et al., 1993; Darland et al., 1995; Darland and Nishi, 1998). Somatostatin released by choroid neurons acts presynaptically on choroid neuron terminals by inhibiting voltage-activated calcium currents, thus inhibiting release of acetylcholine (Pilar et al., 1996). Our finding that PSS2 is preferentially expressed over PSS1 in ciliary ganglia, together with our data that activin acts to stabilize and induce PSS2, but not PSS1, suggests that cortistatin is likely to be the physiologically relevant peptide in choroid neurons. It will be of significant interest to determine whether cortistatin and somatostatin have differing effects physiologically.
It is not clear why our studies find very little expression of PSS1 or PSS2 together with SOM-LIR in dorsal root ganglia. In mammals, a subset of nociceptive neurons express SOM-LIR, and SOM agonists act as anti-inflammatory agents (Malcangio, 2003). Perhaps these properties develop at stages past E18, the latest stage at which we examined sensory ganglia, or avian dorsal root ganglia do not express SOM-LIR. However, our studies suggest that it will be essential to determine whether the SOM-LIR in sensory neurons is due to somatostatin or cortistatin. Our data also raise the issue of whether the subpopulation of prevertebral sympathetic neurons observed in the celiac ganglion of adult guinea pigs that label with antibodies prepared against SOM-14 are actually expressing somatostatin or cortistatin (Anderson et al., 2001).
The present finding that cortistatin is expressed in autonomic neurons may facilitate the analysis of cortistatin signaling mechanisms and physiological functions. CST-14, SOM-14, and SOM-28 are circularized due to a disulfide bridge between two cysteines. Contained within the circularized portion of the peptide is a phe-trp-lys motif necessary for biological activity (reviewed in Broglio et al., 2007). CST-14 binds with high affinity to all five subtypes of somatostatin receptors. However, additional cortistatin-specific receptors are thought to exist because cortistatin induces distinct physiological effects from somatostatin when these two peptides are injected intracerebroventrically: cortistatin produces hypomotility, whereas somatostatin produces hypermotility; cortistatin increases slow-wave sleep and decreases REM sleep, whereas somatostatin increases REM sleep. In addition, only cortistatin binds to the growth hormone secretagogue receptor, also known as the ghrelin receptor, and has been reported to bind to the orphan receptor Mrg×2. It will be interesting to investigate whether targets innervated by parasympathetic or sympathetic neurons express these genes or new G-protein-coupled receptors that selectively respond to cortistatin.
In summary, our studies suggest that cortistatin has previously unappreciated functions in the periphery via release from the autonomic nervous system. In addition, the early expression of somatostatin/PSS1 during embryonic development suggests a potential developmental function during neurogenesis or gliogenesis. It will be of significant interest to determine the specific effects of cortistatin and somatostatin in developing and mature autonomic neuron circuits.
Supplementary Material
ACKNOWLEDGMENTS
We thank the UVM Neuroscience Center of Biomedical Research Excellence Cellular and Molecular Core facility (5P20-RR016435) for use of the ABI 7700.
Grant sponsor: NIH; Grant numbers: NS25767 and DA17784 (to R.N.); Grant sponsor: the Deutsche Forschungs Gemeinschaft; Grant number: RO2551/1-1 (to H.R.); Grant sponsor: the Schram-Foundation; Grant number: T287/16252 (to H.R.).
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Anderson RL, Morris JL, Gibbins IL. Neurochemical differentiation of functionally distinct populations of autonomic neurons. J Comp Neurol. 2001;429:419–435. doi: 10.1002/1096-9861(20010115)429:3<419::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Broglio F, Papotti M, Muccioli G, Ghigo E. Brain-gut communication: cortistatin, somatostatin and ghrelin. Trends Endocrinol Metab. 2007;18:246–251. doi: 10.1016/j.tem.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Bunker GL, Nishi R. Developmental cell death in vivo: rescue of neurons independently of changes at target tissues. J Comp Neurol. 2002;452:80–92. doi: 10.1002/cne.10363. [DOI] [PubMed] [Google Scholar]
- Coulombe JN, Nishi R. Stimulation of somatostatin expression in developing ciliary ganglion neurons by cells of the choroid layer. J Neurosci. 1991;11:553–562. doi: 10.1523/JNEUROSCI.11-02-00553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe JN, Schwall R, Parent AS, Eckenstein FP, Nishi R. Induction of somatostatin immunoreactivity in cultured ciliary ganglion neurons by activin in choroid cell-conditioned medium. Neuron. 1993;10:899–906. doi: 10.1016/0896-6273(93)90205-6. [DOI] [PubMed] [Google Scholar]
- Cui S, Goldstein RS. Early markers of neuronal differentiation in DRG: islet-1 expression precedes that of Hu. Brain Res Dev Brain Res. 2000;121:209–212. doi: 10.1016/s0165-3806(00)00034-1. [DOI] [PubMed] [Google Scholar]
- Dalm VA, Van Hagen PM, de Krijger RR, Kros JM, Van Koetsveld PM, Van Der Lely AJ, Lamberts SW, Hofland LJ. Distribution pattern of somatostatin and cortistatin mRNA in human central and peripheral tissues. Clin Endocrinol (Oxf) 2004;60:625–629. doi: 10.1111/j.1365-2265.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- Darland DC, Nishi R. Activin A and follistatin influence expression of somatostatin in the ciliary ganglion in vivo. Dev Biol. 1998;202:293–303. doi: 10.1006/dbio.1998.8998. [DOI] [PubMed] [Google Scholar]
- Darland DC, Link BA, Nishi R. Activin A and follistatin expression in developing targets of ciliary ganglion neurons suggests a role in regulating neurotransmitter phenotype. Neuron. 1995;15:857–866. doi: 10.1016/0896-6273(95)90176-0. [DOI] [PubMed] [Google Scholar]
- de Lecea L. Cortistatin—functions in the central nervous system. Mol Cell Endocrinol. 2008;286:88–95. doi: 10.1016/j.mce.2007.12.014. [DOI] [PubMed] [Google Scholar]
- DeStefano M, Ciofi LA, Mugnaini E. Neuronal ultrastructure and somatostatin immunolocalization in the ciliary ganglion of chicken and quail. J Neurocytol. 1993;22:868–892. doi: 10.1007/BF01186358. [DOI] [PubMed] [Google Scholar]
- Epstein ML, Davis JP, Gellman LE, Lamb JR, Dahl JL. Cholinergic neurons of the chicken ciliary ganglion contain somatostatin. Neuroscience. 1988;25:1053–1060. doi: 10.1016/0306-4522(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- Garcia-Arraras JE, Chanconie M, Fontaine-Perus J. In vivo and in vitro development of somatostatin-like-immunoreactivity in the peripheral nervous system of quail embryos. J Neurosci. 1984;4:1549–1558. doi: 10.1523/JNEUROSCI.04-06-01549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. (Originally published in 1951) Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Henke RC, Weinberger RP, Sentry JW, Jeffrey PL. Differential induction and intracellular localization of SCG10 messenger RNA is associated with neuronal differentiation. Neuroscience. 1996;72:889–900. doi: 10.1016/0306-4522(95)00593-5. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Edgar D, Thoenen H. The development of substance P, somatostatin and vasoactive intestinal polypeptide in sympathetic and spinal sensory ganglia of the chick embryo. Neuroscience. 1983;10:31–39. doi: 10.1016/0306-4522(83)90078-7. [DOI] [PubMed] [Google Scholar]
- Heller S, Finn TP, Huber J, Nishi R, Geissen M, Puschel AW, Rohrer H. Analysis of function and expression of the chick GPA receptor (GPAR-alpha) suggests multiple roles in neuronal development. Development. 1995;121:2681–2693. doi: 10.1242/dev.121.8.2681. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Elfvin LG, Elde R, Schultzberg M, Goldstein M, Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977;74:3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Ibanez-Tallon I, Nishi R. Cell-autonomous inhibition of alpha 7-containing nicotinic acetylcholine receptors prevents death of parasympathetic neurons during development. J Neurosci. 2007;27:11501–11509. doi: 10.1523/JNEUROSCI.3057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Smiley GG, Nishi R. Cell death and neuronal replacement during formation of the avian ciliary ganglion. Dev Biol. 2001;233:437–448. doi: 10.1006/dbio.2001.0236. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hökfelt T, Anggard A, Terenius L, Elde R, Markey K, Goldstein M, Kimmel J. Organizational principles in the peripheral sympathetic nervous system: subdivision by coexisting peptides (somatostatin-, avian pancreatic polypeptide-, and vasoactive intestinal polypeptide-like immunoreactive materials) Proc Natl Acad Sci U S A. 1982;79:1303–1307. doi: 10.1073/pnas.79.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M. GDNF and somatostatin in sensory neurones. Curr Opin Pharmacol. 2003;3:41–45. doi: 10.1016/s1471-4892(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Maxwell GD, Sietz PD, Chenard PH. Development of somatostatin-like immunoreactivity in embryonic sympathetic ganglia. J Neurosci. 1984;4:576–584. doi: 10.1523/JNEUROSCI.04-02-00576.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R. Autonomic and sensory neuron cultures. Methods Cell Biol. 1996;51:249–263. doi: 10.1016/s0091-679x(08)60632-9. [DOI] [PubMed] [Google Scholar]
- Pilar G, Gray DB, Meriney SD. Membrane delimited and intracellular soluble pathways in the somatostatin modulation of ACh release. Life Sci. 1996;58:1979–1986. doi: 10.1016/0024-3205(96)00188-9. [DOI] [PubMed] [Google Scholar]
- Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278:44400–44404. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Wong V, Kessler JA. Translational regulation of somatostatin in cultured sympathetic neurons. Neuron. 1990;4:303–311. doi: 10.1016/0896-6273(90)90104-n. [DOI] [PubMed] [Google Scholar]
- Straub JA, Sholler GL, Nishi R. Embryonic sympathoblasts transiently express TrkB in vivo and proliferate in response to brain-derived neurotrophic factor in vitro. BMC Dev Biol. 2007;7:10. doi: 10.1186/1471-213X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostivint H, Lihrmann I, Vaudry H. New insight into the molecular evolution of the somatostatin family. Mol Cell Endocrinol. 2008;286:5–17. doi: 10.1016/j.mce.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Trabucci M, Tostivint H, Lihrmann I, Blaehser S, Vallarino M, Vaudry H. Characterization of the cDNA encoding a somatostatin variant in the chicken brain: comparison of the distribution of the two somatostatin precursor mRNAs. J Comp Neurol. 2003;461:441–451. doi: 10.1002/cne.10690. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Varela-Echavarria A, Pfaff SL, Guthrie S. Differential expression of LIM homeobox genes among motor neuron subpopulations in the developing chick brain stem. Mol Cell Neurosci. 1996;8:242–257. doi: 10.1006/mcne.1996.0061. [DOI] [PubMed] [Google Scholar]
- Vaudry H, Chartrel N, Conlon JM. Isolation of [Pro2,Met13]somatostatin-14 and somatostatin-14 from the frog brain reveals the existence of a somatostatin gene family in a tetrapod. Biochem Biophys Res Commun. 1992;188:477–482. doi: 10.1016/0006-291x(92)92409-q. [DOI] [PubMed] [Google Scholar]
- Wright LL, Luebke JI. Somatostatin-, vasoactive intestinal polypeptide- and neuropeptide Y-like immunoreactivity in eye- and submandibular gland-projecting sympathetic neurons. Brain Res. 1989;494:267–275. doi: 10.1016/0006-8993(89)90595-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.