Abstract
Phenolics including many polyphenols and flavonoids have the potentials to become chemoprevention and chemotherapy agents. However, poor bioavailability limits their biological effects in vivo. This paper reviews the factors that affect phenolics absorption and their bioavailabilities from the points of view of their physicochemical properties and disposition in the gastrointestinal tract. The up-to-date research data suggested that solubility and metabolism are the primary reasons that limit phenolic aglycones’ bioavailability although stability and poor permeation may also contribute to the poor bioavailabilities of the glycosides. Future investigations should further optimize phenolics’ bioavailabilities and realize their chemopreventive and chemotherapeutic effects in vivo.
Keywords: Chemoprevention, Chemotherapy; Phenolic; Bioavailability Challenge; Polyphenol; Flavonoids
1. Introductions
Both epidemiologic and experimental evidences revealed that modifications in lifestyle including diet, can have a major impact on the risk for various types of cancers [1]. Based on this evidence, there has been an increasing interest in cancer chemoprevention via the use of dietary phytochemicals over the past 20 years. Among these compounds, phenolic compounds such as flavonoids (1), stilbenes (2), coumarins (3), quinones (4), and phenolic acids (5) [2, 3], have perhaps attracted the most attention. Phenolics are characterized by having at least one aromatic ring with hydroxyl group(s), which are widely distributed in fruits, vegetables, cereals, dry legumes, chocolate, wine, and beverages (e.g. tea, coffee) [4]. Phenolic compounds are ubiquitous in the plant kingdom. More than 8,000 phenolic compounds have been isolated in a wide variety of forms [5], including compounds such as EGCG (6) isolated from green tea, resveratrol (7) derived from grape seed, and genistein (8) from soybean. The chemistry, bioavailability, and benefit or toxicity on health of phenolics have been reviewed in several publications [4, 6].
Numerous experiments have successfully linked natural occurring dietary phenolics to anti-proliferative properties and thereby these compounds are regularly referred to as anticancer. For example, juices of prickly pears prevented growth of prostate and colon cells [7], phenolic-rich berry juice possessed antiproliferative activity against Caco-2 cells [8], and ginger-derived phenolics were shown to have chemopreventive and chemotherapeutic effects [9]. Modern pharmacological research indicated that the mechanisms of action of phenolics include induction of apoptosis [10], inhibition of tumor angiogenesis [11], reduction of the expression of the proinflammatory gene cyclooxygenase-2 [12], down-regulation of the expression of pRb, cyclins, and CDKs [13], inhibition of AKR1C3, an target of hormeno-dependent cancer treatment [14], and up regulation of MIC-1 gene expression [15]. Perhaps due to these exciting findings, more health conscious people are advocating the consumption of phenolic-rich food or dietary supplements.
In contrast, some scientists questioned the ability of phenolics to exert clinical anticancer activities. Despite of the fact that experimental studies on cultured cell lines or animals models have established positive relationship between dietary phenolics and cancer, it is very difficult to extrapolate the results of these studies (often conduced in vitro or in rodents) to cancer prevention or therapy in humans. One of the reasons is that these studies have often been conducted at doses or concentrations far beyond those that can be achieved in humans for disease-prevention or therapy. For example, EGCG was shown to prevent melanoma tumor cell lines from growth with IC50 value from 11 to 89 μM [16]. But in humans, the Cmax of EGCG were 0.237-0.328 μg/mL (51.7 - 71.6 nM) after taking 1000 mg green tea extract or 250 g fresh grape plus EGCG riched-nutrient mixture [17]. Similarly for trans-resveratrol (7), a compound active against all 3 major stages of carcinogenesis (initiation, promotion, and progression) [18], the activities were shown at a concentration greater than 1000 ng/mL [19]. In contrast, the Cmax of trans-resveratrol in 12 volunteers (6 male, 6 felmae) ranged from 21.6 ± 9.7 to 28.0 ± 22.0 ng/mL in a single dose study (200 mg trans-resveratrol) or from 27.1 ± 14.4 to 34.5 ± 32.1 ng/mL in a multiple dose study (200 mg trans-resveratrol thrice daily). This result suggested that the in vivo plasma concentration of trans-resveratrol was far below the in vitro biological effect concentration.
In addition, high doses of phenolics consumption may even be harmful for our health. Anti-oxidation is one of the reasons that phenolics can prevent growth of cancer cells. However, Decker argued that phenolics have both anti-oxidation and pro-oxidation effects [20]. Karakaya pointed out “at high concentrations, phenolics or their oxidation products may interact with proteins, carbohydrates, and minerals”, which will harmful to our body[21]. Regulation of cell signaling pathway is one of the predominant mechanisms of action. However, “regulation of cell signaling pathways by dietary polyphenols can also lead to cell proliferation/survival or inflammatory responses due to increased expression of several genes” [22]. Therefore, one could argue that amounts of dietary phenolics consumed should be well titrated in order to achieve the optimal effects.
The consumption and bioavailability of dietary phenolics has become a major concern in phenolic chemopreventive and cancer therapy research. Several reviews concluded that the oral bioavailability of dietary phenolics was poor but the reason is multifaceted [21, 23, 24]. This review focuses on the mechanisms responsible for oral bioavailability challenges associated with development of anti-cancer phenolics.
2. Solubility and Absorption
2.1 Solubility
Solubility is the maximum amount of a solid, liquid, or gaseous chemical substance dissolved in a certain volume of liquid solvent to form a homogeneous mass. It is a property of substance (i.e., a phenolic), which is affected by physicochemical characteristics of the substance (e.g. chemical structure, crystalline state), compositions of the solvent (e.g. solvent type, pH), temperature, and even the dissolution procedure [25]. Solubility is important for oral bioavailability because only dissolved substance can be absorbed across the gastrointestinal epithelium. Another equally important and related parameter is the dissolution rate, or the speed at which a substance is dissolved in the liquid. The latter is because gastrointestinal tract has established rhythm and motility, and an orally administered dose of phenolics normally has 4-6 hr to get absorbed or the remaining dose will be considered lost or non-absorbable. Therefore, it is often desirable to get orally administered phenolics to dissolve rapidly.
2.2. Relationship between solubility and absorption
Müller has reviewed the molecular properties favoring oral absorption of drugs [26]. Ideally, well-absorbed compounds should have molecular weight less than 550, suitable lipophilicity and hydrophilicity, uncharged or partially uncharged at pH = 7.4, and certain numbers of rotatable bonds and of hydrogen bond donors or receivers. It is actually difficult to study the relationship between water solubility and absorption, because the absorption processes are very complex. Although most of the in vitro and in vivo studies were conducted using aqueous media, there is no evidence to show that the common approach of including up to 5% of organic phase in the in vitro cell culture studies will bias the results to such an extent that they will not be reproducible in vivo since most organic solvents used are not allowed in humans. For example, a common practice employed in vitro studies is to use 1% DMSO in the cultured media. In another words, a standard control experiment is a media with 1% DMSO, even though there is no evidence to show that DMSO will not enhance the uptake of agents by the cells because of DMSO treatment. In any rate, improving the solubility of many of the phenolics is an important task for both in vitro and in vivo studies.
Several studies had focused on improving water solubility of anticancer agents. For example, the addition of a salt moiety onto the structure of resveratrol was shown to improve its water solubility [27]. But there was no direct evidence to show when water solubility was improved, the absorption and the bioavailability was improved as a result of the improvement. Further efforts in this area are needed.
2.3 Solubility of selected phenolics
Solubility of phenolic acids
Ellagic acid (EA, 9) is a representative phenolic acid considered to be an anticancer and antiproliferation agent [28]. It was reported to prevent growth of multiple cancer cell lines (e.g. Caco-2, MCF-7, Hs 578T, and DU 145) in vitro. The absorption of EA was proved to be poor in rat after oral administration of pomegranate leaf extract [29]. The poor absorption could at least partially due to its sparing solubility in water (9.7 μg/ml) [30, 31] at pH=7.4 phosphate buffer [31].
Solubility of stilbenes
Natural stilbenes (e.g. resveratrol) and semi-synthetic simple and derivates have potential anticancer and chemopreventive activities [32]. Resveratrol was treated as a lipophilic phenolic and was easy to dissolve in ethanol, DMSO, and other organic solvents. No research has focused on the behavior of resveratrol in aqueous solutions at concentrations >1 mg/mL [33]. There is no direct evidence to show that the poor bioavailability of resveratrol was because of its low water solubility. But researchers are trying to synthesize resveratrol analogues to maintain its biological activities but with improved water solubility, because the latter could impact absorption [34].
Solubility of lignans and flavonoids
Lignans is one of the major sources of phenolics. Natural occurring and semi-synthetic lignans have the potential to be used for treatment or prevention of tumors [35]. These kinds of compounds are more lipophilic, meaning water solubility could be poor. No solubility-absorption relationship for lignans has been reported. Flavonoids possess usually one or more hydroxyl groups, their molecular weights are usually less than 500, and water solubility is usually not a major impediment for absorption.
3. Stability and Bioavailability
Phenolic compounds are known for their anti-oxidation activity. Since they are good antioxidation agents, they themselves may be easily oxidized, especially when multiple hydroxyl groups are adjacent on the same aromatic ring (i.e., ortho isomer or catechol). Light exposure often exasperates the stability problem of phenolics. In addition, harsh environment of the GI tract could accelerate some phenolics decomposition. Moreover, some of phenolics are easily decomposed by intestinal flora. Therefore, chemically degradation or bacteria decomposition will occur for these phenolic compounds before being absorbed into circulation system, which could affect their bioavailabilities.
3.1 Chemical stability
Anthocyanins are very unstable under alkaline conditions
Dietary components containing anthocyanins (10) were reported to be cytotoxic. Dai reported that anthocyanins extracted (ACE) from blackberry were chemically unstable in biologically relevant buffers such as pH= 7.4 PBS, pH =7.4 PBS with 10% FBS, RPMI 1640 medium supplemented with 10% FBS at 25°C or 37 °C [36]. The half-life of total anthocyanins was only 5.0 and 6.2 hours at 37°C in these buffers. Another study also showed that anthocyanins degradation in vitro [37]. In 37°C water or 37°C pH=7.4 phosphate buffer, the half-lives of delphinidin (11), pelargonidin (12), and cyaniding (13) were less than 2 hours (first observation time point). When 3-OH group was conjugated with sugar, the compounds were relatively stable in water at 37°C, but remained unstable in pH=7.4 phosphate buffers.
Acid environment accelerates anthocyanins degradation
Procyanidin oligomers were readily cleaved in mild acid solutions to form flavan-3-ol and quinone methide. Studies showed that 60-80% of procyanidin oligomers (trimer to hexamer) were decomposed into dimier or monomer procyanidins within 90 min under conditions of acidic environment of the gastric juice [38].
Chemical stability is also an issue for flavan-3-ols
Many studies have investigated stabilities of tea catechins. Zhu reported that (+)-catechin (14), (-)-epicatechin(15), and epicatechin-(4β- 6)-epicatechin (16) degraded nearly completely within 8 hours in both simulated intestinal juice or at alkaline pH (pH 7.4, 9.0) [39]. When pH is less than 4, catechins aqueous solution was very stable, whereas they were unstable at pH value higher than 6. Furthermore, the decomposion of catechins was temperature-dependent: at temperature below 44°C, degradation was more profound than epimerization whereas at temperature above 44°C, epimerization was faster than degradation [40].
Some of flavonoids are unstable in physiological environment
Boulton reported that quercetin (17) degraded in HBSS buffer (pH, 7.4). When radio-labeled quercetin was incubated in HBSS buffer at 37°C for 6 hours, C-ring was broken, and protocatechuic acid together with two unidentified degradation products was detected as the degradation products. However, the degradative pathway could be inhibited by including nontoxic concentrations of EDTA in the media [41].
3.2 Plasma stability
Plasma stability is a very important issue in oral drug development. It has a direct impact on drug performance in vivo and serves as a criterion for drug candidate screening in the early stage of preclinical study. Unstable compounds in plasma tend to have rapid clearance and short half-life, resulting in poor bioavailability and poor biological performances [42]. Moreover, plasma instability leads to biased pharmacokinetic results because degradation may still occur even if blood samples were taken. Caffeic acid phenethyl ester (CAPE, 18), a polyphenolic plant product, has been found to exhibit medically useful properties including cancerpreventive and anti-tumor properties [43]. The CAPE is an aryl ester, which can be hydrolyzed in plasma enzymes. Wang reported that CAPE was degraded in rat plasma following first-order kinetics. The half-life of CAPE (5 μg/ml) at 4, 25, and 37°C were 10.21, 0.73, 0.35 hour(s) respectively. It was further reported that addition of 0.4% sodium fluoride together with pH adjustment to pH6, which is a common way to prevent compounds from enzymatic hydrolysis in plasma [44], could maintain the integrity of CAPE in rat plasma.
3.3 Gut microflora transformation
Microflora transformation of phenolics is also an important factor for the stability of phenolics in the gut, even though glucuronidation and sulfation are the major metabolic pathways of phenolics. For instance, Gonthier reported that when rats was given red wine, polyphenol microflora transformation metabolites (3-hydroxphenolpropionic acid, 3-hydroxybenzoic acid, 3-hydroxyhippuric acid, hippuric acid, p-coumaric acid) were detected in urine and the total amount of these metabolites was about 10% of total administered polyphenols [45]. Assuming this is the result of microflora biotransformation, it was proposed that phenolic metabolites (formed as the result of conjugation in the small intestine and liver) and lower molecular weight products (formed by the metabolism of the colonic microflora) are responsible for the observed bioactive effect in vivo [46]. However, vast majority of the in vitro and in vivo experiments have demonstrated that phenolics themselves possess cancer chemoprevention and chemotherapy effects [3]. Therefore, determination of microflora metabolism of phenolics should be significantly strengthened if our goal is to get a better understanding of the metabolism of phenolics in vivo.
Humans and other mammals are colonized by vast and complex microorganisms. The main bacteria in human GI track are anaerobic bacteria. Bacteria found in the upper intestinal tract (stomach and small intestine) are different from those in the lower intestinal tract (colon). Colonic microflora transformation is often thought to be the major flora metabolism for phenolics. About 500 different bacterial species are recovered in the lower intestine, where the most common anaerobic microorganisms are bifidobacteria, lactobacilli and bacteroides [47]. The microflora that could participate in phenolic metabolism are Bacteroides, Clostridium, Eubacterium, Ruminococcus, Eggertheilla [48]. But individuals may have different and/or altered flora profiles, which may affect their metabolism [49]. The following reactions are often reported for phenolics microflora metabolism.
Ring fission
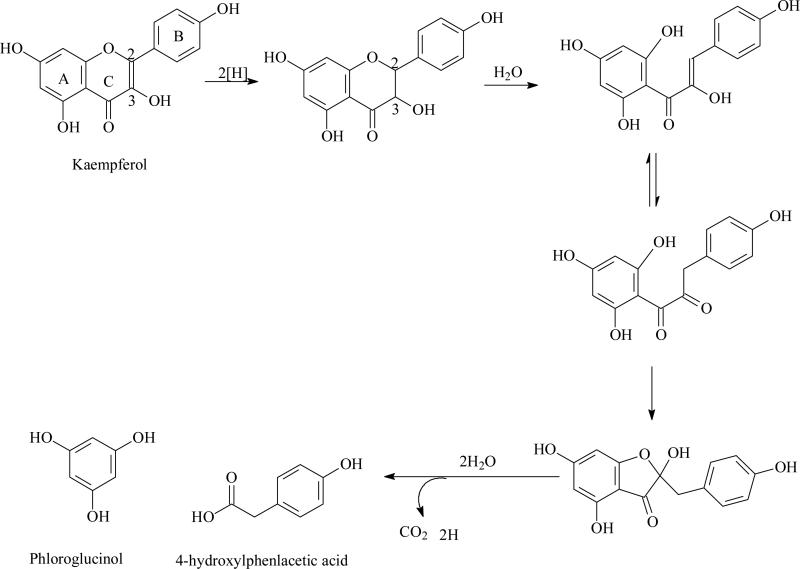
Flavonoid ring fission in mammals was first studied more than 50 years ago [50]. The products of ring fission degradation are usually hydroxyphenylacetic/phenylpropionic acid and phloroglucinol. The pathway of flavonoids degradation may include three main steps (Fig.1): 1. the double bonds between position 2 and 3 was hydrogenized to form dihydroflavonoids; 2. C-ring was broken to form chalcone; 3. chalcone was hydrolyzed together with or without lose carbonyl from C-ring to afford hydroxyphenylacetic/phenylpropionic acid from B ring or phloroglucinol from A ring [51]. For examples, quercetin (17) was degraded by human gut anaerobe into 3,4-dihydroxyphenylacetic acid derived from B ring and phloroglucinol derived from A ring [51, 52] whereas kaempferol (19) was metabolized into 4-hydroxylphenlacetic acid from B ring and phloroglucinol from A ring [53]. Luteolin (20) was transformed into 3,4-dihydroxyphenylpropionic acid and phloroglucinol [51]. For naringenin, (21) a dihydroflavonoid, is C-ring fissioned directly into chalcone to afford phenylacetic acid and phloroglucinol [53]. Anthocyanins was firstly hydrolyzed into chalcones, which were further being degraded into hydroxyphenylacetic/ phenylpropionic acids and phloroglucinols [54] or α-aldehyde and monomeric phenolics[55].
Fig. (1).
Proposed pathway of degradation of kaempferol by human intestinal microflora
Reduction
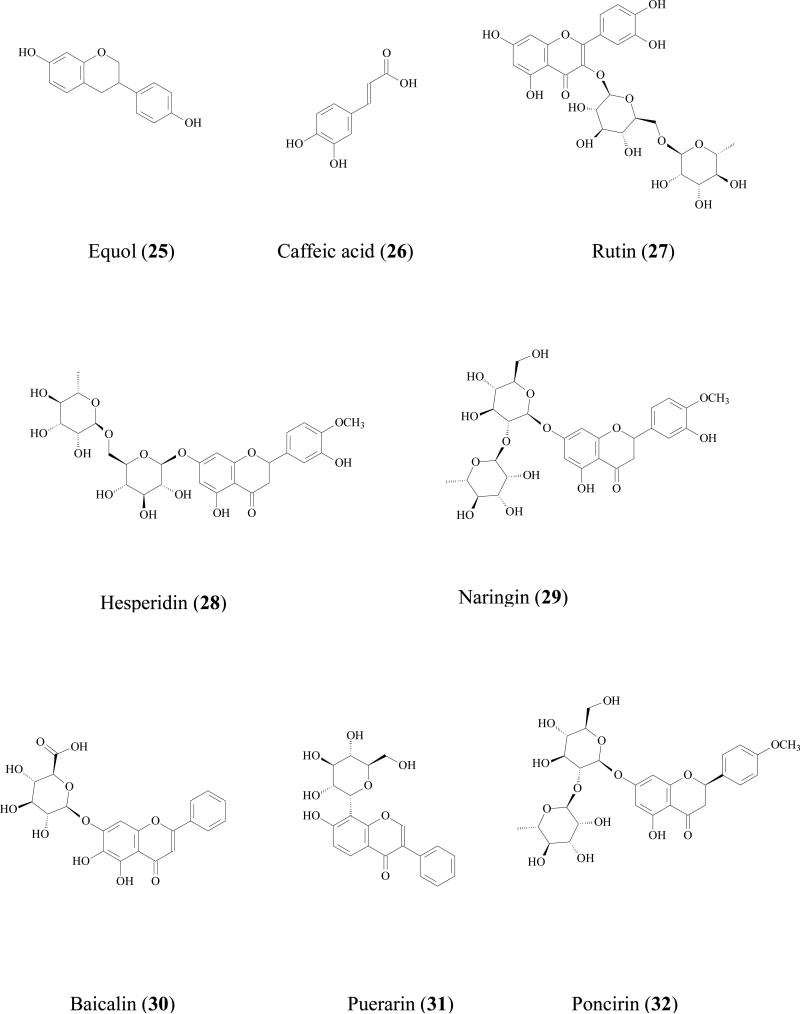
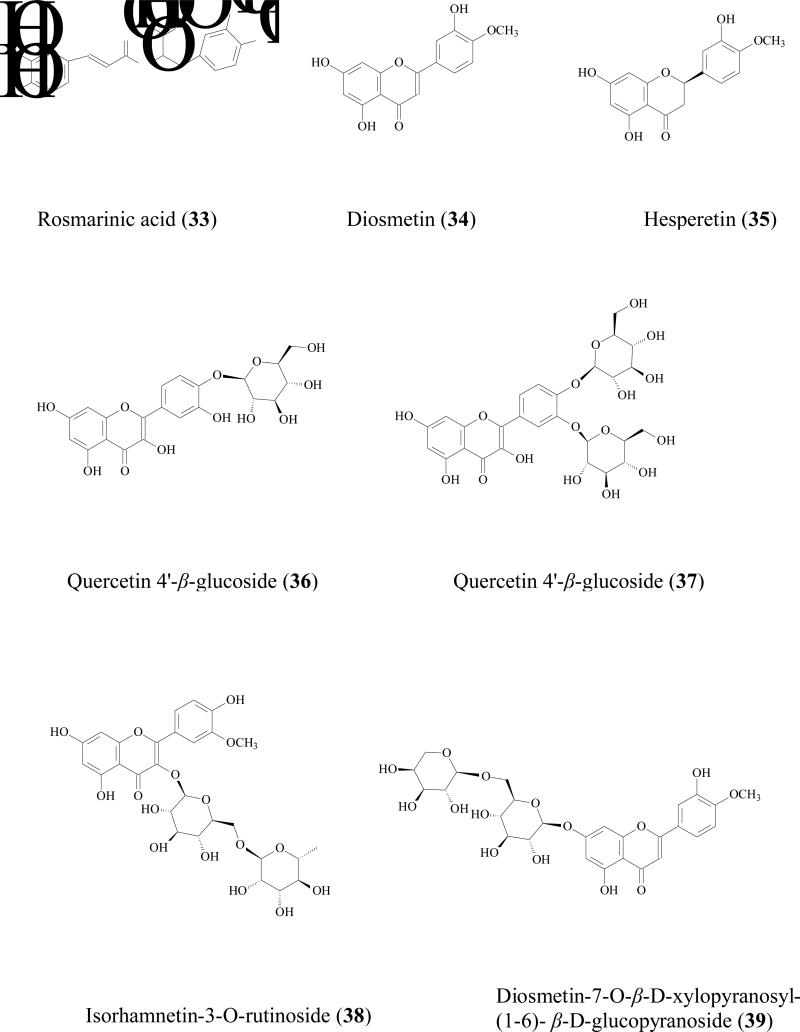
Yuan reviewed the metabolic pathway of soy bean isoflavone daidzin (22) by microflora in GI tract. When un-fermented soybean was ingested, the daidzin was hydrolyzed into daidzein (23) which was further hydrogenised into 2,3-dihydrodaidzein by microflora. The carbonyl of dihydro product was further reducted to afford equol or C-ring was fissioned to afford O- desmethylangolensin (24) [56]. Moreover, in a double-blind study of 14 American women volunteers, it was found that equol (25) appeared after 4 h and remained elevated after 48 h. The AUC for equol was significantly higher after ingestion of the daidzin tablets, although glucuronidation and sulfation are always the major metabolites of soy isoflavonoids. Simple phenolic acid can also further be reduced by reduction reactions. For instance, caffeic acid (26) was degraded into m-coumaric acid, m-hydroxyphenylpropionic acid, and 3,4-dihydroxyphenylpropionic acid by human gut flora [57].
Hydrolysis
Gut microflora hydrolysis is often thought to benefit flavonoid glycoside bioavailability. When sugar unit is removed, the resulting aglycone can be absorbed more readily. For example, rutin (27), hesperidin (28), naringin (29), baicalin (30), puerarin (31), daidzin (22) and poncirin (32) were hydrolyzed to their respective aglycones by human intestinal microflora, and the resulting aglycones were absorbed better [58]. But in some cases parent compounds (e.g., glycosides) are the biological active sources. Then, when hydrolysis occurs, the bioavailability will be decreased. For example, rosmarinic acid (33) is a natural occurring phenolic acid ester of caffeic acid and 3,4-dihydroxyphenylacetic acid with efficient of antioxidation activity. The ester bond of rosmarinic acid was hydrolyzed by human microflora to form two simple less active phenolic acids, [59]. Proanthocyanidins are the most abundant polyphenols in many kinds of food (various fruits, legume seeds, chocolate) and beverages (fruit juices, wine, beer, cider, tea). Pure proanthocyanidins have been suggested to prevent cancers. When proanthocyanidin polymer was incubated with human colonic microflora in vitro, it was rapidly hydrolyzed into catechin (14) which was further degraded into less active phenylacetic, phenylpropionic, and phenylvaleric acids [60]. This type of hydrolysis therefore may decrease the parent compounds’ in vivo bioactivities.
4. Permeation and Transport Mechanisms
4.1 Barriers to oral bioavailability
The intestinal epithelium is the first biological barrier to be crossed by phenolics, therefore a better understanding of the specific intestinal cell mechanisms involved in the permeation processes is of interest when assessing their absorption. The primary function of small intestine is to digest and absorb nutrients from gut lumen. Structure and function of intestinal epithelium membrane has been well described [61]. The intestinal epithelium consists of a single layer of epithelial cells that undergo rapid and continuous renewal, an essential process for digestion and absorption of nutrients. In small intestine, the epithelium displays finger-shaped projection or villi, which is 0.5-1.5 mm in length. The absorption occurs on 90-95% of villus surface. Uptake (e.g. PEPTs, OATPs, OATs, GLUTs) and efflux transporters (e.g. BCRPS, MRPs, Pgps) are distributed in the surface of intestinal epithelial cell. These cells are tightly linked together by tight-junctional proteins.
Permeability is the ability of a molecule to go across a membrane barrier, which also determines the phenolic absorption across intestinal epithelium. Absorption of a molecule across the intestinal epithelium is a complex process involving multiple pathways (Fig.2). The absorption of the drug is determined by two major factors, uptake and efflux. The intestinal uptake/efflux takes place via transcellular/paracellular passive diffusion and facilitated/active transport mechanisms separately or in some types of combinations.
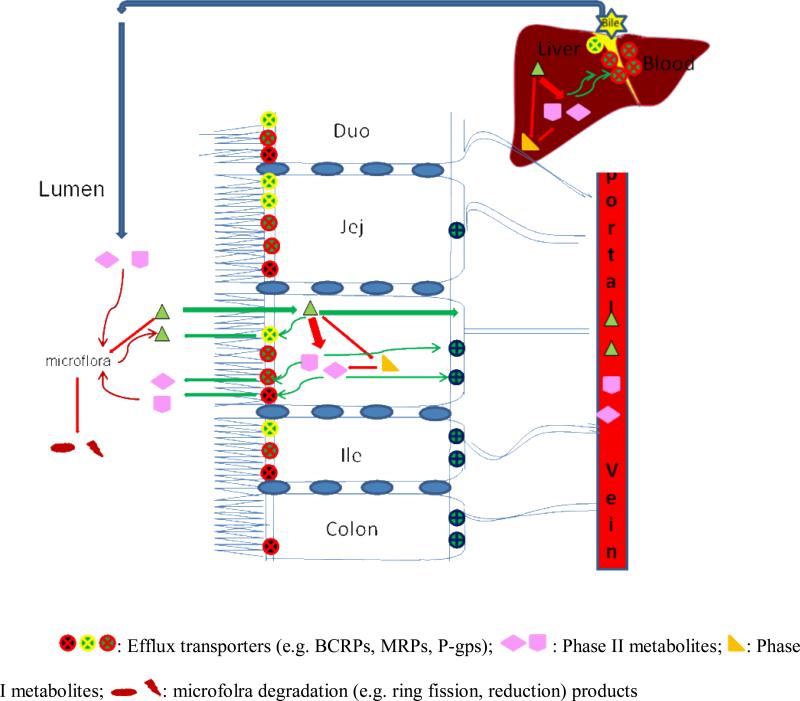
Fig. (2).
Phenolics absorption and metabolism in intestine and liver. 1. Phenolics can be absorbed through passive diffusion or mediated uptake into intestine epithelial wall. 2. In epithelial cell phenolics can pass through cell basolateral barrier arriving portal vein bloodsteam or can be metabolized by phase I and II enzymes (e.g. CYPs, UGTs, SULTs) into different metabolites. Phase I metabolites can be further conjugated into phase II metabolites. Metabolites can be efflux back into lumen or flux into bloodsteam. 3. Phase I and II metabolism occurs in liver also. Similarly, phase I metabolites can be further conjugated into phase II metabolites. Phase I metabolites can be excreted back into intestinal lumen through bile duct. 4. Phase II conjugates can be hydrolyzed by microflora and phenolic aglycones are recovered and can be absorbed again (recycling). Phenolics can also be decomposed by microflora into reduction or ring fission products.
4.2 Passive diffusion
For the passive diffusion mechanism, permeability is much higher for lipophilic compounds than for hydrophilic ones, because compounds must pass through lipid bilayer of the membrane. Moreover, neutral molecular are more permeable comparing with charged molecular. The molecular size is also critical. The parallel artificial membrane permeability assay (PAMPA) is an alternative to biological models to predict drug passive diffusion permeability [62]. This model can outperform cell culture model in speed, versatility, and cost, representing a compelling biologically relevant model used to predict passive diffusion.
Lipophilicity, degree of ionization, and molecular size were used to predict permeability of flavonoid aglycones and glycosides via passive diffusion. The rutinoside glycosides of diosmetin (34), hesperitin (35), and naringenin (21) along with their aglycones were subjected to Caco-2 and soy lecithin lipidic membrane PAMPA models. It was showed that permeabilities of three glycoside compounds were extremely low because of their higher molecular weight and less lipophilicity when comparing with their aglycones. Permeabilities of aglycones from apical to basolateral side were much better and in the range of an in 1.0-8.0× 10-5 cm/sec [63].
Flavonoid glycosides were mainly absorbed by passive diffusion [64]. They are too polar to be absorbed rapidly from the small intestine. Many if not most of the dietary phenolics are presence in food as glycoside. For instance, almost all soy isoflavones exist as glycosides, in soy and unfermented soy foods. Isoflavone glycosides are not absorbed intact across the enterocytes of healthy adults because of their higher hydrophilicity and bigger molecular weights [65]. The glycoside absorption is improved upon the cleavage of the glycoside linkage by glycosidase in the gut wall or present in the microflora.
4.2 Efflux
Human intestinal epithelial wall expresses several families of efflux transporters including ATP-binding cassette transporters such as p-glycoprotein (P-gps), multidrug resistance-associated proteins (MRPs), and breast cancer resistance protein (BCRP). These efflux transporters will affect drug bioavailabilities of orally administrated substrates. The most pharmacologically relevant ABC transporters are expressed in the apical membrane of cells and include P-gp (ABCB1), MRP2 (ABCC2) and BCRP (ABCG2). The transporters may impact bioavailability of their substrates by mediating hepatobiliary and direct intestinal excretion of these compounds [66]. Human intestinal efflux transporters distribution and their functions have been recently reviewed [67]. Phenolics that cross the apical membrane may be substrates for different transporters, which extrude compounds back into the intestinal lumen and limit their bioavailabilities.
MRPs are involved in phenolics glycosides efflux
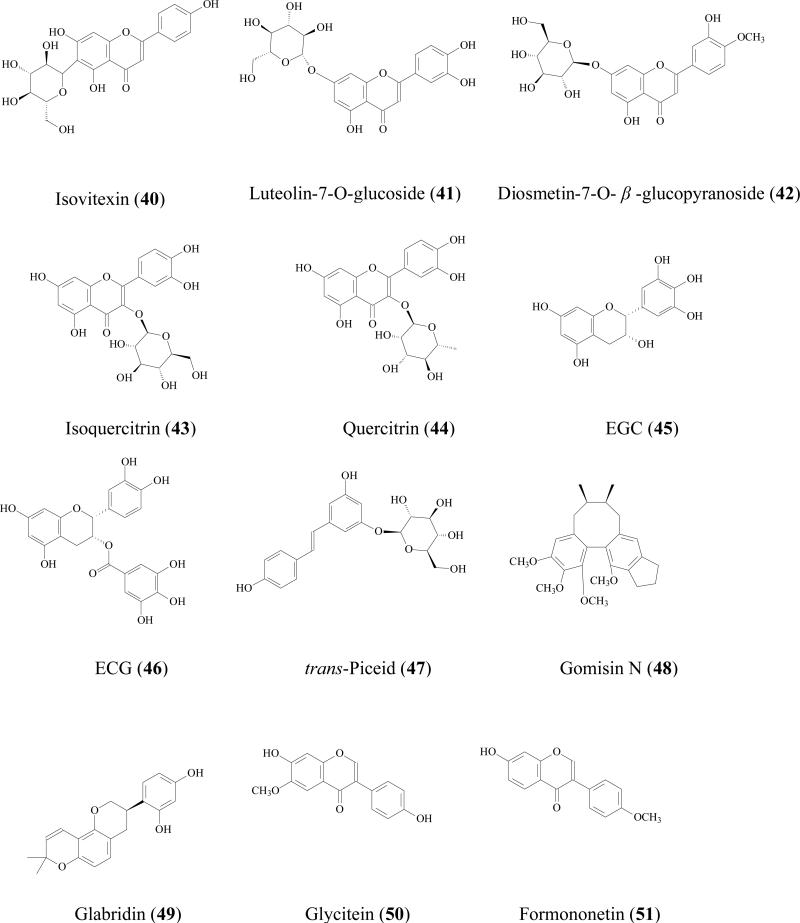
Flavonoid glycosides are reported to be substrates of MRPs transporters in Caco-2 cell culture model by using inhibitors. For example, Quercetin 4'-β-glucoside (36), a major dietary component capable of reducing certain cancer risks including stomach carcinoma and lung cancer[68], is not well absorbed [69]. The apical to basolateral permeability was less than 0.02×10-6 cm/sec, while the basolateral to apical side permeability was 1.6 ±0.2 ×10-6 cm/sec (more than 80 fold higher) indicating the involvement of efflux transporter(s) [70]. Further study showed the efflux ratio reduced in the presence of MK-571 suggesting MRP2 was the efflux transporter[69]. By using the similar method, quercetin 3, 4'-β-diglucoside absorption (37) [69], isorhamnetin-3-O-rutinoside (38), diosmetin-7-O-β-D-xylopyranosyl-(1-6)- β-D-glucopyranoside (39), isovitexin (40), baicalin (30), luteolin-7-O-glucoside (41), diosmetin-7-O- β -glucopyranoside (42), isoquercitrin (43), quercitrin (44), and daidzin (22) were also proved to be substrates of MRP2 [64].
Flavan-3-ols are also substrates of MRPs
Tea catechins, such as (EC (15), EGC (45), ECG (46), EGCG (6), have been suggested to have anticancer effects both in animals and in humans [71, 72]. But the oral bioavailability of tea flavonoids has been suggested to be low in rats [73] and humans [74]. For example, oral bioavailability of ECG in Sprague Dawley rats was only 6.0% [75]. Low absorption was one of the major problems for these catechins [76]. Bharathi reported that the ECG (50μM) uptake by Caco-2 cell at 60 min was 810±141 pmol/mg protein while in the presence of 50μM MK-571, uptake was significantly increased more than 2 folds to 1698±308 pmol/mg protein [77]. The ECG uptake by MRP2-overexpressing MRP2-MDCKII cell was only half of that by normal MDCKII cell. Transepithelial transport of ECG across Caco-2 cells showed that its apical to basolateral permeability was concentration-dependent. When MK-571 was used, permeability of EGCG was doubled at a higher concentration (200 μM) but did not change at a lower concentration (50 μM). These evidences revealed that MRPs may impacts the absorption of catechins.
Stilbenes are also MRPs substrates
Trans-resveratrol (7) and its glycoside trans-piceid (47) are the major stilbenes in wine. Trans-resveratrol seems to have a greater biological benefit that trans-piceid [78]. Hydrolysis often occurs in human small intestine, which will increase the quantity of aglycone so that stilbene in wine is available for rapid absorption [79]. Although trans-resveratrol was highly absorbed in rats [80], its efflux still should not be neglected. Henry et al reported that both trans-resveratrol and trans-piceid were efflux by MRP2 [81]. In the Caco-2 cell efflux experiment, after 90 s, there was only 31% of trans-resveratrol and 19% of trans-piceid remained in the cells, suggesting that these two molecules were rapidly excreted by Caco-2 cells. When MK-571 was used, the cellular accumulation of these two compounds increased 45 % for trans-resveratrol and 32 % for trans-piceid. Although there were very few data in humans, the bioavailability of resveratrol and its aglycone is likely to be limited by efflux.
Lignans are substrates of MRP2
Efflux of gomisin N (48) was inhibited by MK-571 and verapamil, indicating both MRP2 and P-gp were involved in its efflux [82]
Some of phenolics are also substrates of P-gp/MDR1 transporters
Glabridin is a major flavonoids aglycone in root of Glycyrrhiza glabra. Cao et al reported that the systemic bioavailability of glabridin (49) was approximately 7.5% in Sprague-Dawley rats at 5 and 20 mg/kg [83]. Caco-2 transport experiments showed that efflux ratio of glabridin was 3.3- to 8.4 in the experimental concentration range (0.1 - 100 μM). In the presence of Pgp/MDR1 inhibitor, 100 μM verapamil, efflux was significantly inhibited. Furthermore, the efflux rate of glabridin (0.1 - 50 μM) in MDR1 over-expressed MDCKII cells was significantly (approximately 5- to 7-fold) higher than that in control MDCKII cells. In vivo study showed that AUC0–24h and Cmax of glabridin were 3.77- and 2.83-fold higher in MDR1a(-/-) mice than those in wild-type mice given the same dose. These findings indicated that glabridin was effluxed by PgP/MDR1 transporter, which will limit its absorptions.
5. Metabolism
The intestinal epithelial membrane is a defensive barrier against absorption of xenobiotics including many of the phenolics. Xenobiotics that possess suitable permeability can be absorbed through the intestinal wall. Once these compounds pass through this biological barrier, metabolism in intestinal epithelial cells and/or in hepatocytes will become the major barriers to their bioavailability. These barriers are classically termed “first-pass” metabolism, or metabolism that occurs before xenobiotics reached the circulation system for the first time.
Many phenolics are good substrates for certain intestinal and hepatic uridine-5'-diphosphate (UDP)-glucuronosyltransferases (UGTs) and sulfotransferases (SULFs), as well as for certain cytochrome P450 enzymes. Metabolism occurs in intestinal epithelial cells before phenolics are presented to hepatocytes via the portal vein (Fig.2). Moreover, phenolic conjugated metabolites are often good substrates of apically located efflux transporters such as MRPs, Pgp, and BCRP. Hence, significant amounts of conjugates will be excreted back into intestinal lumen after absorbed phenolics are metabolized in the intestine. For those phenolics that escaped intestinal metabolism, liver will be the next organ that metabolize a large (if not all of) portion of the escaped phenolics via a variety of mechanisms (hydrolysis, methylation, oxidation, sulfation and glucuronidation), and phenolics themselves and/or their metabolites may also be excreted via bile[24]. The excreted phenolics can be reabsorbed by the intestine epithelial wall. Their metabolites can be hydrolyzed by microflora enzymes into aglycones, which can then come back into the plasma, completing the recycling loop. A portion of the unabsorbed phenolics and their metabolites could also be further metabolized by the microflora into smaller phenolic acids as we describe in stability section. It is believed that extensive conjugation of the free hydroxyl groups is the main reason for the low oral bioavailability of the dietary flavonoids and other polyphenols [84]. An unanticipated benefit of this extensive metabolism via conjugation and extensive excretion of the metabolites into the intestinal lumen by enterocytes and via bile and subsequent return to the aglycone is that this class of compounds typically has reasonable apparent half-lives, even though they have very poor bioavailabilities [85, 86].
5.1 Phase II metabolism of phenolics
Phase II metabolism, such as glucuronidation and sulfation, is the main route of metabolism for phenolics. Conjugation of free phenolic group(s) via glucuronidation and /or sulfation will increase their polarity and water solubility, enabling their ultimate elimination from the body [87]. Although conjugation is often thought as a detoxification process, it is in fact also a process that limits the benefit of phenolics to humans. We are currently performing research to determine if this process can be manipulated to allow entry of biologically beneficial compounds without increasing exposures to carcinogens. In any rate, these conjugates are too hydrophilic to diffuse across cell membrane by the passive diffusion and they are known to be substrates of efflux transporters such as MRPs and BCRPs [86].
Glucuronidation
UGTs are membrane-bound enzymes that situate in the endoplasmic reticulum. Their distribution has been well described in different organs of humans [88]. Although UGTs are found in multiple organs, such as lung, kidney, breast, bladder, ovary, uterus, testis, and stomach, they are primarily in the liver, small intestine, and colon. In the recent decades, extensive glucuronidation of phenolics in the intestine and the liver have been demonstrated in various studies. Although phenolic aglycones are rapidly absorbed by GI tract, their plasma concentrations were low. Glucuronidation mediated by various UGTs is recognized as one of the most important metabolic pathways of phenolics in both liver and intestine.
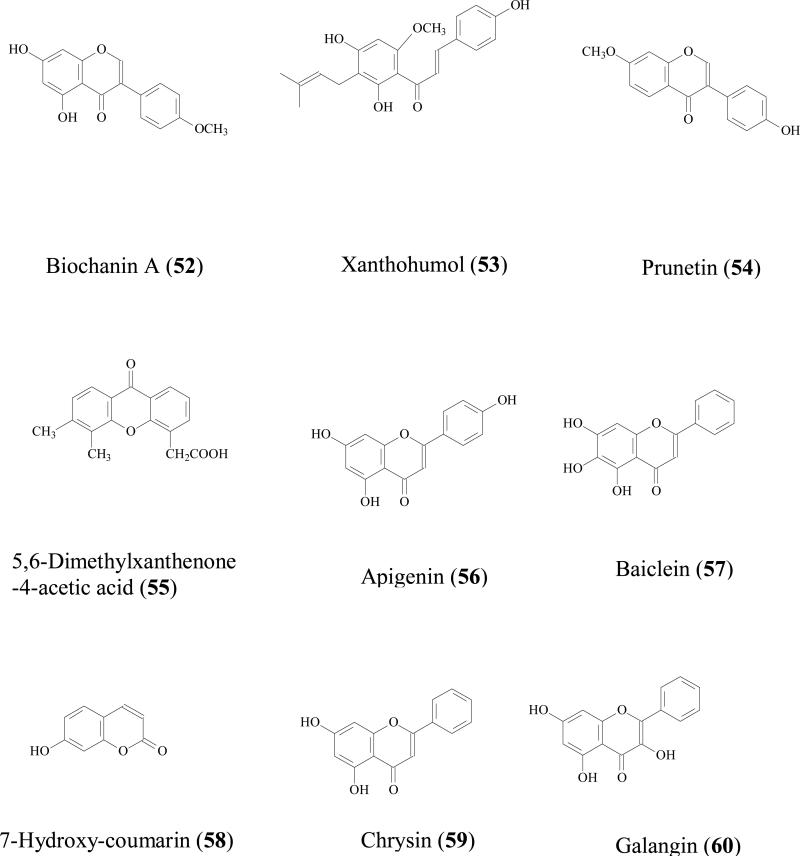
It is generally accepted that UGTs expression in intestine is much lower than that in liver, but due to the location, small intestine is the first organ encountering the absorbed phenolics that are present at relatively high concentration, often in 10s of μM. Therefore, the contribution of intestine in limiting the oral phenolic bioavailability should not be underestimated. For example, Cermak et al found that the swine portal blood contained only quercetin metabolites and no parent compound, suggesting quercetin (17) was totally biotransformed in the GI tract before reaching the liver [89]. Both in vitro and in vivo study showed that glucuronide is often the main intestinal metabolite. Since phenolic usually possess multiple hydroxyl groups, different glucuronides with respect to the position of glucuronidation was possible. For example, when quercetin (17) was incubated with rat intestine S9 fraction, quercetin-3-glucuronide, quercetin-7-glucuronide, quercetin-3'-glucuronide, quercetin-3'-methyl-7- glucuronide, quercetin-4'-methyl-3'- glucuronide were all identified as the metabolites [90]. Soybean isoflavones (genistein (8), daidzein (23), glycitein (50), formononetin (51), and biochanin A (52) were glucuronidated in Caco-2 models and 7-hydroxyl group was the main site for glucuronidation [91]. Mono-glucuronidation occurred for trans/cis-resveratrol at 3/4' position when being incubated with human small intestine microsomes [92].
Flavonoids glucuronidation has been reviewed and UGT isoform-specific patterns were summarized [86]. For instance, xanthohumol (53) was efficiently glucuronidated by UGT1A8, 1A 9, and 1A10, followed by UGT1A 1, 1A7, and 2B7; whereas UGT1A3, 1A4, and 1A6 had minor contribution [93]. Our recent experiments also revealed UGT isoform metabolic patterns of several soy isoflavones: genistein (8), daidzein (23), glycitein (50), prunetin (54), biochanin A (52), and formononetin (51). It was shown that these isoflavones were metabolized most rapidly by one of the following four UGT isoforms:UGT1A1, UGT1A8, UGT1A9 and UGT1A10 [94]. Another review revealed that flavonoids glucuronidation in small intestine is structure dependent [95]. When flavonoids with a substituted hydroxyl group on the B-ring (i.e., hesperetin, 35) were less predisposed to glucuronidation, whereas the flavonoids containing 3', 4'-ortho-dihydroxy (or catechol) B-ring were transformed predominantly as glucuronides. In addition, monophenolic B-ring flavonoids were also extensively glucuronidated, in particular naringenin (21), which was only detected in serosal fluid as naringenin-7-glucuronide.
Although liver glucuronidation may be limited comparing with that of intestine as shown above, it is of great importance to emphasize that hepatic glucuronidation does occur due to high contents of UGTs in liver [88]. Hepatic glucuronidation occurred for flavonoids/isoflavonoids [96], flavones [97], anthocyanins [98], stilbenes [99], lignans [100] in both in vitro or in vivo models. Furthermore, It was reported that the capacity for flavonoid glucuronidation by rat liver microsomes is age-dependent [101]. In male F334 hepatic microsomes metabolism study, the formation rates of quercetin-7- glucuronide were 3.4, 3.1 and 3.8 nmol/min/mg at age of 4, 18, and 28 months respectively. While the peak formation rates of quercetin-3'- glucuronide and quercetin-4'- glucuronide was observed at 18 month. Liver glucuronidation reaction is also gender related. For 5,6-dimethylxanthenone-4-acetic acid (55), an synthesized anticancer agent belonging to xanthenone family, its metabolism rate in male rats (0.75±0.03 nmol/min per mg) was significantly higher than that in female rats (0.45±0 .01 nmol/min per mg) [102].
Colon also has various levels of UGTs expression
In vitro microsome reaction or perfusion model showed that phenolics glucuronidation occurring in colon. But, as we discussed in stability section, deconjugation or ring fission by microflora is often thought to be the major reaction in colon because of the abundant amount of microflora.
Stomach glucuronidation
There is a few data mentioned the stomach glucuronidation. For example, trans and cis-resveratrol were conjugated with glucuronic acid when being incubated with human stomach microsome [92]. Another example is quercetin glucuronidation in stomach microsomes derived from Wistar rats [103].
Species-dependent glucuronidation
Caco-2 cell culture, microsome reaction, and animal perfusion models are often used to study and predict phenolic glucuronidation, but glucuronidation is species-dependent. For instance, in quercetin (17) metabolism study, employing human small intestine S9 fractions, quercetin-4'- glucuronide was abundant besides quercetin-3-glucuronide, quercetin-7-glucuronide, and quercetin-3'-glucuronide. But when using rat intestine S9 fraction, quercetin-4'- glucuronide was not found in the incubation media [90]. In the same study, on the other hand, quercetin-3'-methyl-7- glucuronide and quercetin-4'-methyl-3'- glucuronide were identified from rat S9 fraction reaction, while in human S9 fraction reaction, none of these two metabolites were observed [90]. Another experiment showed that in rat live and intestinal microsome reaction, two epicatechin-monoglucuronides were obtained as major metabolites, but it was shown that epicatechin was not glucuronided by human liver, intestine and colon microsomes[72].
Organ-specific glucuronidation
Glucuronidation reaction is also organ-specific. For instance, in a glucuronidation study in vitro, incubation of quercetin (17) with rat liver S9 fraction afforded 14% and 9% of quercetin-7-glucuronide and quercetin-3'-glucuronide, respectively, whereas small intestine S9 fraction generated 44% and 21% of these two metabolites [90]. The involvement of UGT isoforms in phase II metabolism is organ-specific. It was reported that in the human liver UGT1A9 was the major isoform involved whereas in the intestine UGT1A1 and UGT1A8 were the major isoforms involved in the metabolism of quercetin and luteolin [104]
Mono- and di-glucuronides of phenolics
Although mono-glucuronide is the main phenolic glucuronidation product both in vitro and in vivo, di-glucuronide have been observed occasionally. For example in liver perfusion of apigenin (56), diglucuronide of apigenin was qualitatively identified [105]. In vivo study also revealed the existence of diglucuronide metabolites of phenolics. Baicalein (57) diglucuronidates was identified as the major metabolites from bile when it was administrated orally to male Westar rats [106]. When diosmetin (34), a flavonoid with anticancer bioactivity isolated from Caucasian vetch, was orally administrated to male Westar rats, diosmetin-3',7-diglucuronide was positively identified [107]. Quercetin diglucuronides was also observed in male weanling F-344 rat. After quercetin was given orally for 6 weeks, quercetin-diglucuronides were identified from intestine at 0.05 nmol/g fresh tissue (8.6% in total identified metabolites) [108]. Quercetin-di-glucuronide was also detected in urine and from different part of small intestine of male Sprague-Dawley rats after it was administrated orally [109].
Efflux of glucuronides
Glucuronidated metabolites are usually substrate of efflux transporters (e.g. MRPs, P-gps, BCRPs). When glucuronidation occurs, the conjugates are often excreted out of epithelial cells, which is also called phase III efflux or phase III metabolism. For example, previously published studies from a few laboratories including our own revealed that MRPs and BCRP effluxed flavonoid glucuronides, and these efflux transporters compensated effectively among themselves to enable rapid intestinal excretion of flavonoid glucuronides (i.e., naringenin, genistein, daidzein, glycitein, formononetin, biochanin A, hesperetin) [66, 110].
Sulfation
Sulfation, an important phase II metabolic pathway for phenolics, is catalyzed by sulfotransferases (SULTs). First reported in 1876, sulfation has been shown to be important metabolism pathway of xenobiotics [111]. In humans SULT superfamily, 3 subfamilies SULT1, SULT2, and SULT4 have been identified, and these subfamilies contains at least thirteen distinct members. The main SULT isoform in the human liver is SULT1A1 [72, 112], which is also located in other organs such as brain, kidney, breast, intestine. SULT1A3 is highly expressed in small intestine but barely detectable in adult liver. Human sulfotransferases and their role in chemical metabolism has been reviewed recently [111]. Since intestine and liver are both the effective “first-pass” organs, both SULT1A1 and SULT1A3 may play a significant role in sulfation of phenolics.
Mono sulfate is the most abundant type of sulfate of phenolics in intestine and liver
Like glucuronidation, most of the sulfation products are mono-sulfates at different position of hydroxyl group(s) [93, 95]. For example, formononetin (51), a soybean isoflavonoid, was conjugated to form mono sulfates in Caco-2 cell model, rat perfusion model, and intestine S9 reaction [113]. Mono sulfates of prunetin (54) and apigenin (56) were the major metabolites these two flavonoids in Caco-2 model as well [91, 114]. Resveratrol mono-sulfate was also reported as the main sulfate metabolite in Caco-2 model [115]. 7-hydroxy-coumarin-sulfated was detected in human plasma, urine, and faces after 7-hydroxy-coumarin (58) was administrated in 70% aqueous ethanol solution [116]. Lignans (e.g. sesaminol)[117], simple phenolics, (nitrophenol, cresol, 1-naphthol), were also reported to be substrates of SULTs[111].
Sulfation is also species-dependent and regioselective
Quercetin-3'-sulfate and quercetin-7-sulfate were major sulfate metabolites (13% and 52% of total metabolites respectively) when using Wistar rat liver S9 fraction, whereas sulfation studies using human liver and intestine S9 fractions did not generate detected amounts of either sulfate conjugate. In Wistar rat intestine S9 fraction, these two sulfates were not found, but in human intestinal S9 fraction reaction, the metabolism was quite rapid and relative percentages of these two sulfates generation were 9% for quercetin-7-sulfate and 71% for quercetin-3'-sulfate, respectively [90].
Sulfates are good substrates of efflux transporters [118]
In our previously Caco-2 model study, apigenin (56) was mainly conjugated into sulfate and glucuronide and MRP and OAT were identified as the involved intestinal efflux transporters.[114]. By using the same model, flavonoids, isoflavonoid, flavones, and stilbenes [119] conjugates were shown to be effluxed by MRPs, P-gp, or BCRPs transporters [66, 110]. Extensive conjugation of phenolics and rapidly excretion of conjugated metabolites is responsible for poor bioavailability of phenolic.
5.2 Relationship between sulfation and glucuronidation
When both metabolic pathways are active for a particular compound, the resulting metabolite is most likely to be either glucuronides or sulfates. Occasionally, however, a phenolic may form a mixed conjugate with both sulfate and glucuronide. For example, baicalein (57) was metabolized into baicalein-6-glucuronide-7-sulfate, which was then excreted from the rat bile [106]. On the other hand, epicatechin (15) was shown to be sulfated but not glucuronidated in the human liver cytosol, suggesting some element of compensation between the two pathways[72].
Occasionally, glucuronides could be converted to sulfates. For example, quercetin-3'-glucuronides and quercetin-7-glucuronides can be hydrolyzed by endogenous beta-glucuronidase to aglycone, followed by sulfation to quercetin-3'-sulfate. Interestingly, quercetin-4'-glucuronides did not undergo this metabolism, suggesting not all glucuronides are equally sensitive to the glucuronidase [118].
Lastly, sulfation and glucuronidation activity is gender-dependent. In mature femal Wistar rats, when radio labeled apigenin (56) was administrated orally, the mono-glucuronide and mono-sulfate conjugates in urine were 10.0–31.6% and 2.0-3.6 %, respectively, whereas in mature male rats, the same two metabolites were 4.9% and 13.9%, respectively [120]. Very little studies have probed the reason for this gender-dependent effects, which may be of concerns, since most efficacy studies of phenolics are conducted in male rodents.
5.3 Phase I metabolism
Phase I reaction, which is mediated by cytochrome P450 (CYP) or flavin mixed function oxidases, includes oxidation, reduction, hydrolysis, hydration, methylation as well as other rare and miscellaneous reaction types [121]. The CYPs are a superfamily of enzymes with over 2000 individual members [122]. In human and other mammalian species, CYP1, CYP2 and CYP3 families are primarily associated with phase I metabolism of most of the xenobiotics [123]. Several databases are available to search human CYP substrates, reactions types, inducers, and inhibitors (e.g., www.gentest.com) [124]. Although most of tissues and organs are well populated with CYPs, liver is usually thought to be the most important phase I metabolism organ. Oxidation, methylation, de-methylation [125], reduction, ring fission reactions [125] are the popular phase I reactions in phenolic metabolism, although the latter is thought to be caused by the intestinal microflora.
Structural specificities
In phase I metabolism of phenolics, the reaction is quite isoform-specific, which is different from UGT- and SULT-mediate conjugation reaction, where several enzyme isoforms tend to produce the same metabolite. The reason for this discrepancy is not entirely clear, but it is often attributed to the critical requirement of substrate-enzyme binding site. In addition, the same substrate may be metabolized by different CYP isoforms to form different metabolites. For example, coumarin was oxidized by human CYP2A6 into 7-hydroxy-coumarin, by human CYP3A4 into 3-hydroxy-coumarin, and by human CYP1A and CYP2E1 into 3,4-exposide-coumarin which was unstable and further convert into O-hydroxyphenylacetaldehyde [126].
Phase I vs. phase II metabolism
For phenolics, phase I metabolic pathway is usually a minor contributor when comparing to phase II pathway. Since phenolics already possess hydroxyl group(s) in their structures, most of the terminal metabolites are conjugates. For example, chrysin (59), a flavonoid with chemopreventive properties, was not metabolized via the phase I pathway in human intestinal Caco-2 cells and hepatic HepG2 cells, even though a large amount of chrysin-glucuronide and chrysin-sulfate were found. It was concluded that both sulfation and glucuronidation were critical determinants of the oral bioavailability of chrysin in humans [127]. Another example is galangin (60) metabolism in human liver microsomes. Although phase I oxidation occurred together with phase II glucuronidation and sulfation, the intrinsic clearance (Vmax/Km) values of glucuronidation (at 3 and 7 hydroxyl group) was 11 and 31 times faster than that of oxidation reaction. Sulfation of galangin was also very efficient[128].
Phase 1 plus phase 2 metabolites of phenolics
Since CYPs co-exist with UGTs/SULTs in human tissue and organs, many of the hydroxylated phase I metabolites formed by CYP will undergo secondary phase II reaction. Indeed, formation of dihydro-resveratrol sulfate in rats after administering resveratrol is an instance where phase II metabolite formation may follow phase I reaction [115].
6. OVERCOME BIOAVAILABILITY CHALLENGES
Permeation and metabolism are recognized to be the major bioavailability challenge for phenolics. Thus, efflux transporter inhibitors and metabolism enzyme modulators could affect phenolic absorption and metabolism which consequently can improve their bioavailabilities. For example, at the present of MK-571, a well known MRP2 inhibitor, the ECG uptake by Caco-2 cell increased 2 folds [77]. Another in vitro example is that the glucuronidation of EGCG was reduced 40 % and 60% at the presence of 100 and 500 μM piperine respectively in mice small intestinal microsome reaction [129]. In vivo studies showed that bioavailabilities of cucurmin were increased 154% and 2000% in wistar rat and human volunteers respectively when cucurimin was administrated together with piperine [130]. The bioavailability of EGCG was also increased (1.3 folds) at the present of piperine in male CF-1 mice [129]. The bioavailability increase was attributed to glucuronidation inhibition by piperine.
One concern is that the safety of traditional inhibitors such as MK-571, verapamil. None toxic natural product modulators, especially dietary components, should be paid more attention. Dietary UGTs, SLUTs, and CYPs modulators have been reviewed by Cermark[131].
Intestinal microflora population can also be modulated by antibiotics or other natural products (e.g. catechins) [132]. Therefore, phenolic microflora degradation may be affected at the presence of these modulators; however, only a few microflora has been identified to response for phenolic degradation. Moreover, this transformation occurs in a quite complex environment in GI track. Future study needs to further demonstrate that how intestinal microflora population change affects phenolic transformation so that degradation can be modulated to improve phenolics bioavailability.
7. Conclusion
Bioavailability of phenolics is of a major concern for the development this class of compounds into chemopreventive or anticancer agents. For phenolics that are aglycones, solubility and stability could limit their bioavailability. The largest contributor to their bioavailability challenge is phase II conjugation into glucuronides and sulfates. For phenolics that are glycosides, enzyme-catalyzed hydrolysis reaction that produces aglycones, have a major impact on their bioavailability. Lastly, recycling of phenolics through phase II metabolic pathway could prolong their half-lives and enhance their bioactivities. Future studies should focus on how to increase the bioavailabilities of phenolics so one day they can become effective chemopreventive and even chemotherapeutic agents.
List of abbreviations
- GI
gastrointestinal
- HBSS
Hanks Balanced Salt Solution
- PEPT
oligopeptide transporter
- OATP
Organic Anion Transporting Polypeptide transporter
- OAT
organic anion transporter
- GLUT
Glucose transporter
- BCRP
Breast Cancer Resistance Protein transporter
- MRP
Multidrug Resistance-Associated Protein transporter
- Pgp
permeability glycoprotein transporter
- UGT
UDP-glucuronosyltransferase
- SULT
cytochrome P450
References
- 1.Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50(1):1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 2.Prasain JK, Barnes S. Metabolism and bioavailability of flavonoids in chemoprevention: current analytical strategies and future prospectus. Mol Pharm. 2007;4(6):846–64. doi: 10.1021/mp700116u. [DOI] [PubMed] [Google Scholar]
- 3.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52(5):507–26. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 4.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–43. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 5.Harnly JM, Bhagwat S, Lin LZ. Profiling methods for the determination of phenolic compounds in foods and dietary supplements. Anal Bioanal Chem. 2007;389(1):47–61. doi: 10.1007/s00216-007-1424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Chavez-Santoscoy RA, Gutierrez-Uribe JA, Serna-Saldivar SO. Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Hum Nutr. 2009;64(2):146–52. doi: 10.1007/s11130-009-0117-0. [DOI] [PubMed] [Google Scholar]
- 8.Bermudez-Soto MJ, Larrosa M, Garcia-Cantalejo J, Espin JC, Tomas-Barberan FA, Garcia-Conesa MT. Transcriptional changes in human Caco-2 colon cancer cells following exposure to a recurrent non-toxic dose of polyphenol-rich chokeberry juice. Genes Nutr. 2007;2(1):111–3. doi: 10.1007/s12263-007-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundu JK, Na HK, Surh YJ. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr. 2009;61:182–92. doi: 10.1159/000212750. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Hao MW, Dong K, Lin F, Ren JH, Zhang HZ. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharm Res. 2009;32(9):1263–9. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohga N, Hida K, Hida Y, Muraki C, Tsuchiya K, Matsuda K, Ohiro Y, Totsuka Y, Shindoh M. Inhibitory effects of epigallocatechin-3 gallate, a polyphenol in green tea, on tumor-associated endothelial cells and endothelial progenitor cells. Cancer Sci. 2009;100(10):1963–70. doi: 10.1111/j.1349-7006.2009.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheckel KA, Degner SC, Romagnolo DF. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J Nutr. 2008;138(11):2098–105. doi: 10.3945/jn.108.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn MY, Jung JH, Na YJ, Kim HS. A natural histone deacetylase inhibitor, Psammaplin A, induces cell cycle arrest and apoptosis in human endometrial cancer cells. Gynecol Oncol. 2008;108(1):27–33. doi: 10.1016/j.ygyno.2007.08.098. [DOI] [PubMed] [Google Scholar]
- 14.Skarydova L, Zivna L, Xiong G, Maser E, Wsol V. AKR1C3 as a potential target for the inhibitory effect of dietary flavonoids. Chem Biol Interact. 2009;178(1-3):138–44. doi: 10.1016/j.cbi.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Golkar L, Ding XZ, Ujiki MB, Salabat MR, Kelly DL, Scholtens D, Fought AJ, Bentrem DJ, Talamonti MS, Bell RH, Adrian TE. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J Surg Res. 2007;138(2):163–9. doi: 10.1016/j.jss.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Ravindranath MH, Ramasamy V, Moon S, Ruiz C, Muthugounder S. Differential Growth Suppression of Human Melanoma Cells by Tea (Camellia sinensis) Epicatechins (ECG, EGC and EGCG). Evid Based Complement Alternat Med. 2009;6(4):523–30. doi: 10.1093/ecam/nem140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gawande S, Kale A, Kotwal S. Effect of nutrient mixture and black grapes on the pharmacokinetics of orally administered (-)epigallocatechin-3-gallate from green tea extract: a human study. Phytother Res. 2008;22(6):802–8. doi: 10.1002/ptr.2372. [DOI] [PubMed] [Google Scholar]
- 18.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49(5):405–30. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 19.Nunes T, Almeida L, Rocha JF, Falcao A, Fernandes-Lopes C, Loureiro AI, Wright L, Vaz-da-Silva M, Soares-da-Silva P. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol. 2009;49(12):1477–82. doi: 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]
- 20.Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55(11 Pt 1):396–8. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 21.Karakaya S. Bioavailability of phenolic compounds. Crit Rev Food Sci Nutr. 2004;44(6):453–64. doi: 10.1080/10408690490886683. [DOI] [PubMed] [Google Scholar]
- 22.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26(6):747–66. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 23.Spencer JP, Schroeter H, Rechner AR, Rice-Evans C. Bioavailability of flavan-3-ols and procyanidins: gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid Redox Signal. 2001;3(6):1023–39. doi: 10.1089/152308601317203558. [DOI] [PubMed] [Google Scholar]
- 24.McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51(6):702–13. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 25.Edward H, Kerns LD. Drug-like Properties: Concepts, Structure Design and Methods for ADME to Toxicity Optimization. Elsevier Inc; 2008. p. 56. [Google Scholar]
- 26.Muller CE. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem Biodivers. 2009;6(11):2071–83. doi: 10.1002/cbdv.200900114. [DOI] [PubMed] [Google Scholar]
- 27.Anderson A, Belelli D, Bennett DJ, Buchanan KI, Casula A, Cooke A, Feilden H, Gemmell DK, Hamilton NM, Hutchinson EJ, Lambert JJ, Maidment MS, McGuire R, McPhail P, Miller S, Muntoni A, Peters JA, Sansbury FH, Stevenson D, Sundaram H. Alpha-amino acid phenolic ester derivatives: novel water-soluble general anesthetic agents which allosterically modulate GABA(A) receptors. J Med Chem. 2001;44(22):3582–91. doi: 10.1021/jm010903i. [DOI] [PubMed] [Google Scholar]
- 28.Rossi M, Erlebacher J, Zacharias DE, Carrell HL, Iannucci B. The crystal and molecular structure of ellagic acid dihydrate: a dietary anti-cancer agent. Carcinogenesis. 1991;12(12):2227–32. doi: 10.1093/carcin/12.12.2227. [DOI] [PubMed] [Google Scholar]
- 29.Lei F, Xing DM, Xiang L, Zhao YN, Wang W, Zhang LJ, Du LJ. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796(1):189–94. doi: 10.1016/s1570-0232(03)00610-x. [DOI] [PubMed] [Google Scholar]
- 30.Bala I, Bhardwaj V, Hariharan S, Kumar MN. Analytical methods for assay of ellagic acid and its solubility studies. J Pharm Biomed Anal. 2006;40(1):206–10. doi: 10.1016/j.jpba.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Murugan V, Mukherjee K, Maiti K, Mukherjee PK. Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J Agric Food Chem. 2009;57(11):4559–65. doi: 10.1021/jf8037105. [DOI] [PubMed] [Google Scholar]
- 32.Kimura Y, Sumiyoshi M, Baba K. Antitumor activities of synthetic and natural stilbenes through antiangiogenic action. Cancer Sci. 2008;99(10):2083–96. doi: 10.1111/j.1349-7006.2008.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Nicolas JM, Garcia-Carmona F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J Agric Food Chem. 2008;56(17):7600–5. doi: 10.1021/jf800843e. [DOI] [PubMed] [Google Scholar]
- 34.Pettit GR, Anderson CR, Gapud EJ, Jung MK, Knight JC, Hamel E, Pettit RK. Antineoplastic agents. 515. Synthesis of human cancer cell growth inhibitors derived from 3,4-methylenedioxy-5,4'-dimethoxy-3'-amino-Z-stilbene. J Nat Prod. 2005;68(8):1191–7. doi: 10.1021/np058033l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamblin F, Hano C, Fliniaux O, Mesnard F, Fliniaux MA, Laine E. [Interest of lignans in prevention and treatment of cancers]. Med Sci (Paris) 2008;24(5):511–9. doi: 10.1051/medsci/2008245511. [DOI] [PubMed] [Google Scholar]
- 36.Dai J, Gupte A, Gates L, Mumper RJ. A Comprehensive Study of Anthocyanin-Containing Extracts from Selected Blackberry Cultivars: Extraction Methods, Stability, Anticancer Properties and Mechanisms. Food Chem Toxicol. 2009;47:837–47. doi: 10.1016/j.fct.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Woodward G, Kroon P, Cassidy A, Kay C. Anthocyanin stability and recovery: implications for the analysis of clinical and experimental samples. J Agric Food Chem. 2009;57(12):5271–8. doi: 10.1021/jf900602b. [DOI] [PubMed] [Google Scholar]
- 38.Spencer JP, Chaudry F, Pannala AS, Srai SK, Debnam E, Rice-Evans C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem Biophys Res Commun. 2000;272(1):236–41. doi: 10.1006/bbrc.2000.2749. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Zhang YZ, Zheng H, Xu BB, Gao W. Suppression of the obliteration process by ventilation in a mouse orthotopic tracheal transplantation model. Transplantation. 2009;87(12):1762–8. doi: 10.1097/TP.0b013e3181a6618a. [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Zhou W, Jiang X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J Agric Food Chem. 2008;56(8):2694–701. doi: 10.1021/jf0730338. [DOI] [PubMed] [Google Scholar]
- 41.Boulton DW, Walle UK, Walle T. Fate of the flavonoid quercetin in human cell lines: chemical instability and metabolism. J Pharm Pharmacol. 1999;51(3):353–9. doi: 10.1211/0022357991772367. [DOI] [PubMed] [Google Scholar]
- 42.Kerns EH. Drug-like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization. Elsevier; Burlington, MA, USA: 2008. p. 169. [Google Scholar]
- 43.Weyant MJ, Carothers AM, Bertagnolli ME, Bertagnolli MM. Colon cancer chemopreventive drugs modulate integrin-mediated signaling pathways. Clin Cancer Res. 2000;6(3):949–56. [PubMed] [Google Scholar]
- 44.Scheidweiler KB, Shojaie J, Plessinger MA, Wood RW, Kwong TC. Stability of methylecgonidine and ecgonidine in sheep plasma in vitro. Clin Chem. 2000;46(11):1787–95. [PubMed] [Google Scholar]
- 45.Gonthier MP, Cheynier V, Donovan JL, Manach C, Morand C, Mila I, Lapierre C, Remesy C, Scalbert A. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr. 2003;133(2):461–7. doi: 10.1093/jn/133.2.461. [DOI] [PubMed] [Google Scholar]
- 46.Del Rio D, Costa LG, Lean ME, Crozier A. Polyphenols and health: What compounds are involved? Nutr Metab Cardiovasc Dis. 2009;20:1–6. doi: 10.1016/j.numecd.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Evaldson G, Heimdahl A, Kager L, Nord CE. The normal human anaerobic microflora. Scand J Infect Dis Suppl. 1982;35:9–15. [PubMed] [Google Scholar]
- 48.Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr. 2007;137(3 Suppl 2):751S–5S. doi: 10.1093/jn/137.3.751S. [DOI] [PubMed] [Google Scholar]
- 49.Van Nuenen MH, Venema K, van der Woude JC, Kuipers EJ. The metabolic activity of fecal microbiota from healthy individuals and patients with inflammatory bowel disease. Dig Dis Sci. 2004;49(3):485–91. doi: 10.1023/b:ddas.0000020508.64440.73. [DOI] [PubMed] [Google Scholar]
- 50.Booth AN, Deeds F, Jones FT, Murray CW. The metabolic fate of rutin and quercetin in the animal body. J Biol Chem. 1956;223(1):251–7. [PubMed] [Google Scholar]
- 51.Braune A, Gutschow M, Engst W, Blaut M. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl Environ Microbiol. 2001;67(12):5558–67. doi: 10.1128/AEM.67.12.5558-5567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishnamurty HG, Cheng KJ, Jones GA, Simpson FJ, Watkin JE. Identification of products produced by the anaerobic degradation of rutin and related flavonoids by Butyrivibrio sp. C3. Can J Microbiol. 1970;16(8):759–67. doi: 10.1139/m70-129. [DOI] [PubMed] [Google Scholar]
- 53.Winter J, Moore LH, Dowell VR, Jr., Bokkenheuser VD. C-ring cleavage of flavonoids by human intestinal bacteria. Appl Environ Microbiol. 1989;55(5):1203–8. doi: 10.1128/aem.55.5.1203-1208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aura AM, Martin-Lopez P, O'Leary KA, Williamson G, Oksman-Caldentey KM, Poutanen K, Santos-Buelga C. In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr. 2005;44(3):133–42. doi: 10.1007/s00394-004-0502-2. [DOI] [PubMed] [Google Scholar]
- 55.Keppler K, Humpf HU. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg Med Chem. 2005;13(17):5195–205. doi: 10.1016/j.bmc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol Nutr Food Res. 2007;51(7):765–81. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 57.Gonthier MP, Verny MA, Besson C, Remesy C, Scalbert A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J Nutr. 2003;133(6):1853–9. doi: 10.1093/jn/133.6.1853. [DOI] [PubMed] [Google Scholar]
- 58.Kim DH, Jung EA, Sohng IS, Han JA, Kim TH, Han MJ. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res. 1998;21(1):17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 59.Konishi Y, Kobayashi S. Transepithelial transport of rosmarinic acid in intestinal Caco-2 cell monolayers. Biosci Biotechnol Biochem. 2005;69(3):583–91. doi: 10.1271/bbb.69.583. [DOI] [PubMed] [Google Scholar]
- 60.Deprez S, Brezillon C, Rabot S, Philippe C, Mila I, Lapierre C, Scalbert A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr. 2000;130(11):2733–8. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- 61.Morteau O. Oral tolerance: the response of the intestinal mucosa to dietary antigens. Kluwer academic/plenum publishers; New York: 2004. pp. 1–12. [Google Scholar]
- 62.Yan Z. optimization in Drug Discovery in vitro methods. Humana Press; Totowa, NJ, USA: 2004. pp. 19–35. [Google Scholar]
- 63.Serra H, Mendes T, Bronze MR, Simplicio AL. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg Med Chem. 2008;16(7):4009–18. doi: 10.1016/j.bmc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 64.Tian X, Yang X, Wang K. The efflux of flavonoids morin, isorhamnetin-3-O-rutinoside and diosmetin-7-O-beta-D-xylopyranosyl-(1-6) -beta-D-glucopyranoside in the human intestinal cell line caco-2. Pharm Res. 2006;23(8):1721–8. doi: 10.1007/s11095-006-9030-5. [DOI] [PubMed] [Google Scholar]
- 65.Hur HG, Lay JO, Jr., Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol. 2000;174(6):422–8. doi: 10.1007/s002030000222. [DOI] [PubMed] [Google Scholar]
- 66.Brand W, van der Wel PA, Rein MJ, Barron D, Williamson G, van Bladeren PJ, Rietjens IM. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36(9):1794–802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 99(2):598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 68.Dorant E, van den Brandt PA, Goldbohm RA, Sturmans F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology. 1996;110(1):12–20. doi: 10.1053/gast.1996.v110.pm8536847. [DOI] [PubMed] [Google Scholar]
- 69.Walgren RA, Walle UK, Walle T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem Pharmacol. 1998;55(10):1721–7. doi: 10.1016/s0006-2952(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 70.Walgren RA, Karnaky KJ, Jr., Lindenmayer GE, Walle T. Efflux of dietary flavonoid quercetin 4'-beta-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. J Pharmacol Exp Ther. 2000;294(3):830–6. [PubMed] [Google Scholar]
- 71.Katiyar SK, Agarwal R, Mukhtar H. Green tea in chemoprevention of cancer. Compr Ther. 1992;18(10):3–8. [PubMed] [Google Scholar]
- 72.Vaidyanathan JB, Walle T. Glucuronidation and sulfation of the tea flavonoid (-)-epicatechin by the human and rat enzymes. Drug Metab Dispos. 2002;30(8):897–903. doi: 10.1124/dmd.30.8.897. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25(9):1045–50. [PubMed] [Google Scholar]
- 74.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10(1):53–8. [PubMed] [Google Scholar]
- 75.Zhu M, Chen Y, Li RC. Oral absorption and bioavailability of tea catechins. Planta Med. 2000;66(5):444–7. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]
- 76.Vaidyanathan JB, Walle T. Transport and metabolism of the tea flavonoid (-)-epicatechin by the human intestinal cell line Caco-2. Pharm Res. 2001;18(10):1420–5. doi: 10.1023/a:1012200805593. [DOI] [PubMed] [Google Scholar]
- 77.Vaidyanathan JB, Walle T. Cellular uptake and efflux of the tea flavonoid (-)epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther. 2003;307(2):745–52. doi: 10.1124/jpet.103.054296. [DOI] [PubMed] [Google Scholar]
- 78.Merillon JM, Fauconneau B, Teguo PW, Barrier L, Vercauteren J, Huguet F. Antioxidant activity of the stilbene astringin, newly extracted from Vitis vinifera cell cultures. Clin Chem. 1997;43(6 Pt 1):1092–3. [PubMed] [Google Scholar]
- 79.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436(1):71–5. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 80.Soleas GJ, Angelini M, Grass L, Diamandis EP, Goldberg DM. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001;335:145–54. doi: 10.1016/s0076-6879(01)35239-4. [DOI] [PubMed] [Google Scholar]
- 81.Henry C, Vitrac X, Decendit A, Ennamany R, Krisa S, Merillon JM. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J Agric Food Chem. 2005;53(3):798–803. doi: 10.1021/jf048909e. [DOI] [PubMed] [Google Scholar]
- 82.Madgula VL, Avula B, Choi YW, Pullela SV, Khan IA, Walker LA, Khan SI. Transport of Schisandra chinensis extract and its biologically-active constituents across Caco-2 cell monolayers - an in-vitro model of intestinal transport. J Pharm Pharmacol. 2008;60(3):363–70. doi: 10.1211/jpp.60.3.0012. [DOI] [PubMed] [Google Scholar]
- 83.Cao J, Chen X, Liang J, Yu XQ, Xu AL, Chan E, Wei D, Huang M, Wen JY, Yu XY, Li XT, Sheu FS, Zhou SF. Role of P-glycoprotein in the intestinal absorption of glabridin, an active flavonoid from the root of Glycyrrhiza glabra. Drug Metab Dispos. 2007;35(4):539–53. doi: 10.1124/dmd.106.010801. [DOI] [PubMed] [Google Scholar]
- 84.Lv P, Huang XW, Lv QJ. [Advances in studies on absorption, distribution, metabolism of flavonoids]. Zhongguo Zhong Yao Za Zhi. 2007;32(19):1961–4. [PubMed] [Google Scholar]; Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother. 1997;51(8):305–10. doi: 10.1016/s0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- 85.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38(8):771–85. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Zuo Z, Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm. 2007;4(6):833–45. doi: 10.1021/mp700077z. [DOI] [PubMed] [Google Scholar]
- 87.Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106(1):97–132. doi: 10.1016/j.pharmthera.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Ohno S, Nakajin S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2009;37(1):32–40. doi: 10.1124/dmd.108.023598. [DOI] [PubMed] [Google Scholar]
- 89.Cermak R, Landgraf S, Wolffram S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J Nutr. 2003;133(9):2802–7. doi: 10.1093/jn/133.9.2802. [DOI] [PubMed] [Google Scholar]
- 90.Van der Woude H, Boersma MG, Vervoort J, Rietjens IM. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol. 2004;17(11):1520–30. doi: 10.1021/tx049826v. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55(2):159–69. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 92.Sabolovic N, Humbert AC, Radominska-Pandya A, Magdalou J. Resveratrol is efficiently glucuronidated by UDP-glucuronosyltransferases in the human gastrointestinal tract and in Caco-2 cells. Biopharm Drug Dispos. 2006;27(4):181–9. doi: 10.1002/bdd.498. [DOI] [PubMed] [Google Scholar]
- 93.Ruefer CE, Gerhauser C, Frank N, Becker H, Kulling SE. In vitro phase II metabolism of xanthohumol by human UDP-glucuronosyltransferases and sulfotransferases. Mol Nutr Food Res. 2005;49(9):851–6. doi: 10.1002/mnfr.200500057. [DOI] [PubMed] [Google Scholar]
- 94.Tang L, Singh R, Liu Z, Hu M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6(5):1466–82. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spencer JP. Metabolism of tea flavonoids in the gastrointestinal tract. J Nutr. 2003;133(10):3255S–61S. doi: 10.1093/jn/133.10.3255S. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L, Lin G, Zuo Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm Res. 2007;24(1):81–9. doi: 10.1007/s11095-006-9126-y. [DOI] [PubMed] [Google Scholar]
- 97.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5'-AMP-activated protein kinase. J Biol Chem. 2007;282(41):30143–9. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hulten K, Winkvist A, Lenner P, Johansson R, Adlercreutz H, Hallmans G. An incident case-referent study on plasma enterolactone and breast cancer risk. Eur J Nutr. 2002;41(4):168–76. doi: 10.1007/s00394-002-0373-3. [DOI] [PubMed] [Google Scholar]
- 99.Maier-Salamon A, Hagenauer B, Reznicek G, Szekeres T, Thalhammer T, Jager W. Metabolism and disposition of resveratrol in the isolated perfused rat liver: role of Mrp2 in the biliary excretion of glucuronides. J Pharm Sci. 2008;97(4):1615–28. doi: 10.1002/jps.21057. [DOI] [PubMed] [Google Scholar]
- 100.Jan KC, Hwang LS, Ho CT. Tissue distribution and elimination of sesaminol triglucoside and its metabolites in rat. Mol Nutr Food Res. 2009;53(7):815–25. doi: 10.1002/mnfr.200800380. [DOI] [PubMed] [Google Scholar]
- 101.Bolling BW, Court MH, Blumberg JB, Chen CY. The kinetic basis for age-associated changes in quercetin and genistein glucuronidation by rat liver microsomes. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou S, Kestell P, Tingle MD, Paxton JW. Gender differences in the metabolism and pharmacokinetics of the experimental anticancer agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA). Cancer Chemother Pharmacol. 2002;49(2):126–32. doi: 10.1007/s00280-001-0383-5. [DOI] [PubMed] [Google Scholar]
- 103.Murota K, Terao J. Quercetin appears in the lymph of unanesthetized rats as its phase II metabolites after administered into the stomach. FEBS Lett. 2005;579(24):5343–6. doi: 10.1016/j.febslet.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 104.Boersma MG, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben NH, van Iersel ML, van Bladeren PJ, Rietjens IM. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol. 2002;15(5):662–70. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- 105.Gradolatto A, Canivenc-Lavier MC, Basly JP, Siess MH, Teyssier C. Metabolism of apigenin by rat liver phase I and phase ii enzymes and by isolated perfused rat liver. Drug Metab Dispos. 2004;32(1):58–65. doi: 10.1124/dmd.32.1.58. [DOI] [PubMed] [Google Scholar]
- 106.Abe K, Inoue O, Yumioka E. Biliary excretion of metabolites of baicalin and baicalein in rats. Chem Pharm Bull (Tokyo) 1990;38(1):209–11. [PubMed] [Google Scholar]
- 107.Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21(6):1525–8. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- 108.Graf BA, Ameho C, Dolnikowski GG, Milbury PE, Chen CY, Blumberg JB. Rat gastrointestinal tissues metabolize quercetin. J Nutr. 2006;136(1):39–44. doi: 10.1093/jn/136.1.39. [DOI] [PubMed] [Google Scholar]
- 109.Mullen W, Rouanet JM, Auger C, Teissedre PL, Caldwell ST, Hartley RC, Lean ME, Edwards CA, Crozier A. Bioavailability of [2-(14)C]quercetin-4'-glucoside in rats. J Agric Food Chem. 2008;56(24):12127–37. doi: 10.1021/jf802754s. [DOI] [PubMed] [Google Scholar]
- 110.Xu H, Kulkarni KH, Singh R, Yang Z, Wang SW, Tam VH, Hu M. Disposition of naringenin via glucuronidation pathway is affected by compensating efflux transporters of hydrophilic glucuronides. Mol Pharm. 2009;6(6):1703–15. doi: 10.1021/mp900013d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci. 2006;90(1):5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- 112.Ganguly TC, Krasnykh V, Falany CN. Bacterial expression and kinetic characterization of the human monoamine-sulfating form of phenol sulfotransferase. Drug Metab Dispos. 1995;23(9):945–50. [PubMed] [Google Scholar]
- 113.Jeong EJ, Jia X, Hu M. Disposition of formononetin via enteric recycling: metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol Pharm. 2005;2(4):319–28. doi: 10.1021/mp0498852. [DOI] [PubMed] [Google Scholar]
- 114.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307(1):314–21. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 115.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 116.Ford RA, Hawkins DR, Mayo BC, Api AM. The in vivo dermal absorption and metabolism of [4-14C] coumarin by rats and by human volunteers under simulated conditions of use in fragrances. Food Chem Toxicol. 2001;39(2):153–62. doi: 10.1016/s0278-6915(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 117.Liao CD, Hung WL, Lu WC, Jan KC, Shih DY, Yeh AI, Ho CT, Hwang LS. Differential Tissue Distribution of Sesaminol Triglucoside and Its Metabolites in Rats Fed with Lignan Glycosides from Sesame Meal with or without Nano/Submicrosizing. J Agric Food Chem. 2009;58(1):563–9. doi: 10.1021/jf9028046. [DOI] [PubMed] [Google Scholar]
- 118.O'Leary KA, Day AJ, Needs PW, Mellon FA, O'Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–91. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- 119.Kaldas MI, Walle UK, Walle T. Resveratrol transport and metabolism by human intestinal Caco-2 cells. J Pharm Pharmacol. 2003;55(3):307–12. doi: 10.1211/002235702612. [DOI] [PubMed] [Google Scholar]
- 120.Gradolatto A, Basly JP, Berges R, Teyssier C, Chagnon MC, Siess MH, Canivenc-Lavier MC. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Dispos. 2005;33(1):49–54. doi: 10.1124/dmd.104.000893. [DOI] [PubMed] [Google Scholar]
- 121.Gibson GG. Introduction to Drug Metabolism. Nelson Thornes Publishers; Cheltenham, United Kingeom: 2003. pp. 2–14. [Google Scholar]
- 122.Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997;29(1-2):413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- 123.Lewis DF. Human cytochromes P450 associated with the phase 1 metabolism of drugs and other xenobiotics: a compilation of substrates and inhibitors of the CYP1, CYP2 and CYP3 families. Curr Med Chem. 2003;10(19):1955–72. doi: 10.2174/0929867033456855. [DOI] [PubMed] [Google Scholar]
- 124.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34(1-2):83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 125.Breinholt V, Hossaini A, Svendsen GW, Brouwer C, Nielsen E. Estrogenic activity of flavonoids in mice. The importance of estrogen receptor distribution, metabolism and bioavailability. Food Chem Toxicol. 2000;38(7):555–64. doi: 10.1016/s0278-6915(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 126.Lewis DF, Ito Y, Lake BG. Metabolism of coumarin by human P450s: a molecular modelling study. Toxicol In Vitro. 2006;20(2):256–64. doi: 10.1016/j.tiv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Galijatovic A, Otake Y, Walle UK, Walle T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica. 1999;29(12):1241–56. doi: 10.1080/004982599237912. [DOI] [PubMed] [Google Scholar]
- 128.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30(5):576–81. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- 129.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134(8):1948–52. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 130.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 131.Cermak R. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin Drug Metab Toxicol. 2008;4(1):17–35. doi: 10.1517/17425255.4.1.17. [DOI] [PubMed] [Google Scholar]
- 132.Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57(15):6485–501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]