Abstract
A macrocyclic dinuclear copper complex, [Cu2II(1)Br3(H2O)]Br has been synthesized and characterized by X-ray crystallography, in which the macrocycle is folded to form a bowl-shaped cavity. The sensing ability of the receptor has been studied for halides by UV-VIS spectroscopy in water-acetonitrile (1:3 v/v) and water. The results indicate that the new receptor exhibits strong affinity and selectivity for iodide.
The field of anion coordination chemistry continues to expand with new synthetic molecules capable of recognizing anions with environmental and biomedical relevance.1 Various types of synthetic receptors have been developed which employ hydrogen bonds offered by specific binding sites as in azamacrocyles,2 amides,3 thioamide,4 urea5 and pyrroles6 to bind anions with size and shape selectivity in various media. Without the involvement of hydrogen bonds, quaternized ammonium hosts by electrostatic forces,7 and azamacrocyles8a and phosphonium hosts8b by ion paring (in aqueous medium where hydrogen bonding is weak)8 are known to form anion complexes in aqueous medium. An alternative approach is to use dinuclear metal complexes9 as shown in seminal papers by Nelson10 and Fabbrizzi11 with two copper ions in cryptand-based receptors, providing vacant axial sites that are available to coordinate an anion through Lewis-acid base interactions. The presence of two metal ions further increases the rigidity of a cryptand, and the metal-metal distance within the cavity determines the binding strength, displaying selectivity for an anion with correct bite length. For example, the dicopper(II) complex of m-xylyl-based cryptand (Cu(II)–Cu(II) = 6.10 Å) showed strong affinity for an anion of comparable size as N3− or NCS− but failed to bind small halides.10 However, the dicopper(II) complex of furan-based cryptand in which the two metal centers are separated by 3.87 Å, formed complexes with halides showing strong selectivity for chloride.11 This approach was further employed recently in the dinuclear Cu(II) complex of an expanded cryptand binding nucleotides with high selectivity for GMP,12 and in the Co(II) complex of m-xylyl cryptand (Co(II)–Co(II) = 4.866(3) Å) binding chloride.13
Although transition metal complexes with the monocyclic analogues are well documented and a high level of understanding has been achieved on physicochemical and structural aspects,14 their application as anion receptors is comparatively little known.15 Monocycle-based dinuclear complexes are expected to be more rigid than their precursors but would be less rigid than their bicyclic analogues, thereby expecting not to be restricted only for an anion with specific bite length. In our continuing effort in designing anion binding hosts,16 we synthesized a macrocycle-based compound 1, whose central nitrogens are attached with methyl groups to provide an additional rigidity, and obtained the square pyramidal dinuclear copper(II) complex, [Cu2II(1)Br3(H2O)]+ that are suitable to recognize an anion. Herein, we report the synthesis and crystal structure of the new dinuclear complex, and its halide binding properties in acetonitrile-water mixture (3:1) and water, showing strong affinity for iodide.
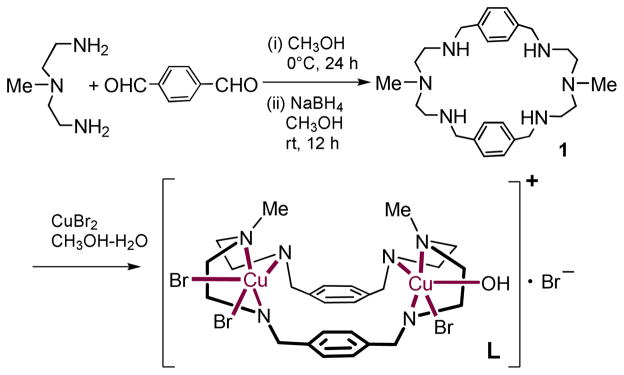
The free macrocycle 1 was prepared as reported previously.17 The synthesis of macrocyclic dinuclear copper complex was accomplished from the reaction of 1 with two equivalents of anhydrous CuBr2 in H2O/CH2OH mixture. Crystals suitable for X-ray analysis were obtained by slow evaporation of a water solution of the complex.
The X-ray analysis18 reveals that the complex is crystallized in the space group, Pn to yield a molecular formula, C26H44Br3Cu2N6O·Br (R) in which two Cu(II) ions reside at both N3 sites in the macrocycle. Each Cu(II) is coordinated with three macrocyclic nitrogens and one bromide at equatorial plane, but one has an axial bromide, while the other has an axial water, thus forming a square pyramidal geometry. Such effect leads to the macrocycle adopting a bowl-shaped cavity (Figure 1) with the Cu–Cu distance of 7.101 (4) Å. The Cu–N distances are 2.025(4) to 2.056(4) Å that are comparable to the corresponding Cu–N distances (1.973(5) to 2.056(5) Å) observed in m-xylyl-based macrocycle.10 The Cu–Braxial distance, 2.959(4) Å is significantly longer than those observed in Cu–Brequatorial distances (Cu2–Br2 = 2.3890(7), and Cu1–Br1 = 2.4171(7) Å) – a phenomenon that is known as a Jahn-Teller distortion.15a One bromide remains outside the cavity interacting with the secondary nitrogen (N2) through NH (N2···Br4 = 3.261 (3) Å) to satisfy the charge requirement of the complex”.
Figure 1.
Crystal structure of [Cu2II(1)Br3(H2O)]Br.
The receptor was found to be insoluble in aprotic solvents like CH3CN or CHCl3, but soluble in CH3CN-H2O mixture. Therefore, a solution of CH3CN containing 25% of H2O was used for the titration experiments of R (1 × 10−4 M) preformed by UV-VIS spectroscopic method using tetrabutylammonium halides (1 × 10−2 M) at room temperature. The receptor showed an absorption band at 296 nm in the absence of an anion. The addition of F− to the receptor solution resulted in a gradual decrease in the intensity while shortening the wavelength (blue shift) showing an isobectic point at 277 nm (Figure 2). The similar trend was observed when Cl− was added to R, whereas the addition of Br− did not result any appreciable change in λmax or intensity. This observation is consistent with our crystallographic evidence showing one outer-sphere Br− outside the cavity (Br4 in Figure 1).
Figure 2.
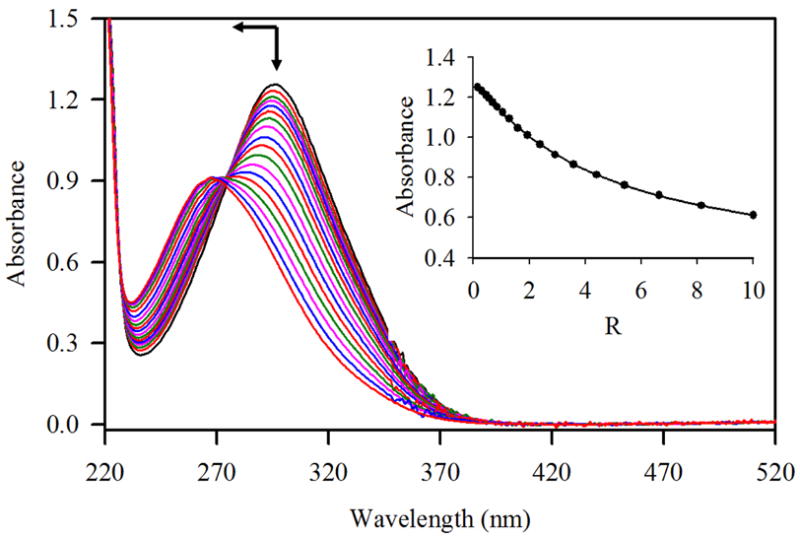
Changes in absorption spectra of R (1 × 10−4 M) with an increasing amount of I− (R = [I−]0/[R]0= 0 - 10) in CH3CN-H2O (3:1 v/v). The titration curve is shown in the inset.
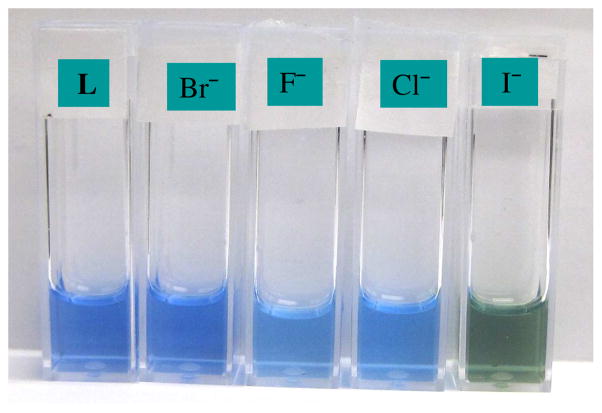
The response of R to I− was quite different than the rest of the halides, showing a remarkable enhancement of the absorption (Figure 3) while the λmax shifted slightly (blue shift of 3 nm) as compared with chloride (13 nm) and fluoride (29 nm). The absorption was significantly affected upon the interaction with iodide, displaying a new band at 360 nm. The addition of iodide to R (1 × 10−3 M) in CH3CN-H2O also resulted into the visual color change (blue to green) as demonstrated in Figure 4. The blue color of the receptor solution was changed into light blue for fluoride or chloride, however, no color change was seen for bromide. The relative changes in wavelength or intensity as a function of an anion concentration, satisfied 1:1 equilibrium isotherms as calculated from the non-linear regression analysis,19 showing strong affinity for I− (Table 1). The observed selectivity pattern of R for halides (I− > Cl− >F−> Br−) is different than dicopper(II) complex of m-xyly cryptand (Cl− > F− >Br− >I−) reported earlier.[11] In our study, the selectivity roughly correlates with the size of halides. The spectral changes are perhaps due to the replacement of attached groups linked the copper ions by an added anion. We also performed UV-VIS binding studies in water under neutral condition (pH = 7.0), however, the receptor displayed negligible changes in the absorption spectra in the presence of halide, showing a weak affinity for I− (125 compared to 23,900 M−1 in CH3CN-H2O) and almost no affinity for F−, Br− or Cl−.
Figure 3.
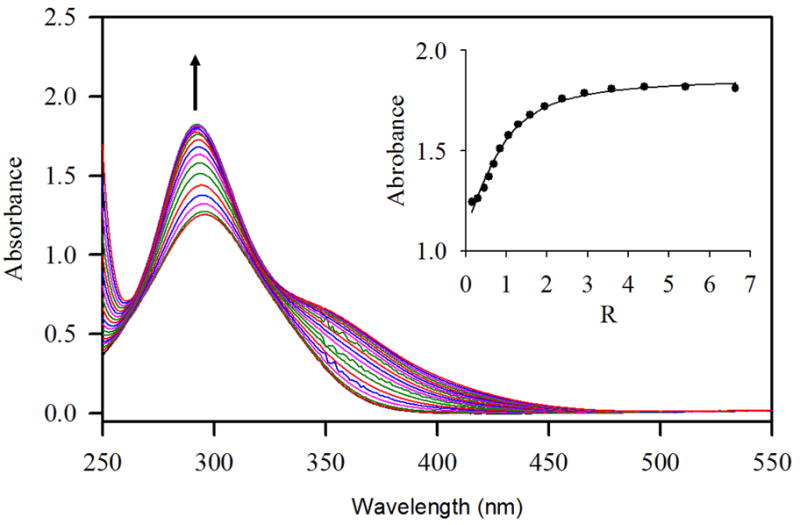
Changes in absorption spectra of R (1 × 10−4 M) with an increasing amount of F− (R = [F−]0/[R]0= 0 - 10) in CH3CN-H2O (3:1 v/v). The titration curve is shown in the inset.
Figure 4.
Color changes observed upon the addition of different halides (10 equiv.) to R (1 × 10−3 M) in CH3CN-H2O (3:1 v/v).
Table 1.
Equilibrium constants (K, M−1) of R for anions as determined from UV-VIS titrations in CH3CN-H2O (3:1, v/v).
| Anion | Ka (M−1) | |
|---|---|---|
| CH3CN-H2O (3:1 v/v) | H2O | |
| Bromide | No binding | No binding |
| Fluoride | 2270 | 20 |
| Chloride | 3200 | 25 |
| Iodide | 23900 | 125 |
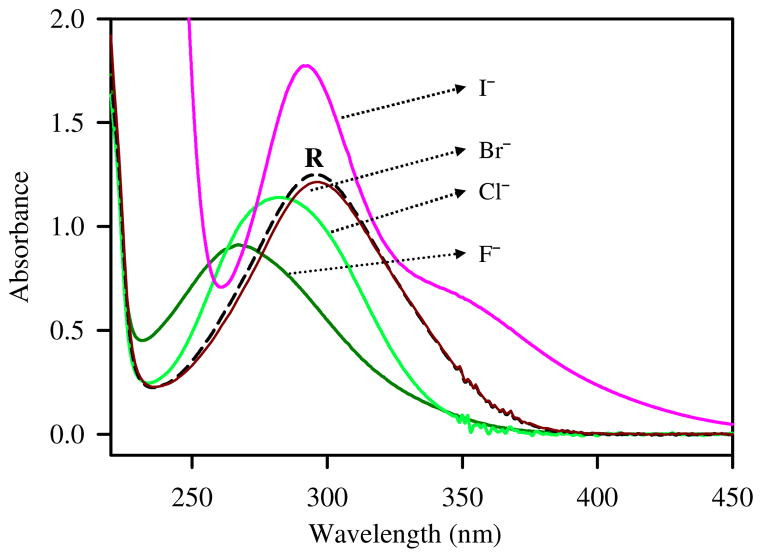
The receptor exhibited different responses to different halides in terms of absorption intensity and band (λmax) (Figure 5). The opposite trend in the absorbance with fluoride and iodide could be related to the charge transfer complexes between R and X−. At the end of the titrations, the absorption band (λmax) of R at 296 nm was shifted to 267, 283 and 293 nm for F−, Cl− and I−, respectively, suggesting that the magnitude of Δλmax is primarily dependent on their relative basicity (F− > Cl− > I−).
Figure 5.
Changes in absorbance of R (1 × 10−4 M) in the presence of 10 equivalents of different anions in CH3CN-H2O (3:1 v/v) at room temperature. λmax: R = 296, I− = 293, Cl− = 283, F− = 267 nm.
In conclusion, we have synthesized and structurally characterized a new macrocycle-based dinuclear complex, as an ideal receptor for iodide. The receptor binds iodide strongly, showing selectivity for it over other halides. The new receptor is capable of discriminating anions that can be monitored from their λmax, absorption intensity or even visual color change. An appreciable variation of the magnitude of λmax with different anions, allows the receptor useful for the direct identification of halides in solution. We are currently pursuing further work in this direction.
Supplementary Material
Scheme 1.
Synthesis of [Cu2II(1)Br3(H2O)]Br
Acknowledgments
The project described was supported by Grant Number G12RR013459 from the National Center for Research Resources. This material is based upon work supported by the National Science Foundation under CHE-0821357. Purchase of the diffractometer was made possible by grant No. LEQSF (1999–2000)-ENH-TR-13, administered by the Louisiana Board of Regents. The authors thank the National Science Foundation (CHE-0130835) and the University of Oklahoma for funds to acquire the diffractometer used in this work.
Footnotes
Supporting Information available: Crystallographic file in CIF format, synthetic procedures of 1 and R, packing diagram of H, UV-VIS titration spectra titration spectra of R with various anions in acetonitrile-water mixture (3:1) and water in pdf format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Bowman-James K. Acc Chem Res. 2005;38:671. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]; (d) Gale PA. Acc Chem Res. 2006;39:465. doi: 10.1021/ar040237q. [DOI] [PubMed] [Google Scholar]; (b) Hossain MA. Curr Org Chem. 2008;12:1231. [Google Scholar]; (c) Caltagirone C, Gale PA. Chem Soc Rev. 2009;38:520. doi: 10.1039/b806422a. [DOI] [PubMed] [Google Scholar]
- 2.Llinares JM, Powell D, Bowman-James K. Coord Chem Rev. 2003;240:57. [Google Scholar]
- 3.(a) Bondy CR, Loeb SJ. Coord Chem Rev. 2003;240:77. [Google Scholar]; (b) Kang SO, Hossain MA, Bowman-James K. Coord Chem Rev. 2006;250:3038. [Google Scholar]
- 4.(a) Hossain MA, Kang SK, Llinares LM, Powell D, Bowman-James K. Inorg Chem. 2003;42:5043. doi: 10.1021/ic034735r. [DOI] [PubMed] [Google Scholar]; (b) Inoue Y, Kanbara T, Yamamoto T. Tetrahedron Lett. 2003;44:5167. [Google Scholar]
- 5.(a) Brooks SJ, Caltagirone C, Cossins AJ, Gale PA, Light M. Supramol Chem. 2008;20:349. [Google Scholar]; (b) Pérez-Casas C, Yatsimirsky AK. J Org Chem. 2008;73:2275. doi: 10.1021/jo702458f. [DOI] [PubMed] [Google Scholar]; (f) Lorenzo A, Aller E, Molina P. Tetrahedron. 2009;65:1397. [Google Scholar]
- 6.(a) Custelcean R, Delmau LH, Moyer BA, Sessler JL, Cho WS, Gross D, Bates GW, Brooks SJ, Light ME, Gale PA. Angew Chem Int Ed. 2005;44:2537. doi: 10.1002/anie.200462945. [DOI] [PubMed] [Google Scholar]; (b) Sessler JL, Cho DG, Stepien M, Lynch V, Waluk J, Yoon ZS, Kim D. J Am Chem Soc. 2006;128:12640. doi: 10.1021/ja064675z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Schmidtchen FP. Angew Chem, Int Ed Engl. 1977;16:720. [Google Scholar]; (b) Ichikawa K, Hossain MA. Chem Commun. 1996:1721. [Google Scholar]
- 8.(a) García-España E, Díaz P, Llinares JM, Bianchi A. Coord Chem Rev. 2006;250:2952. [Google Scholar]; (b) Pomecko R, Asfari Z, Hubscher-Bruder V, Bochenska M, Arnaud-Neu F. Supramol Chem. 2007;19:459. [Google Scholar]
- 9.O’Neil EJ, Smith BD. Coord Chem Rev. 2006;250:3068. [Google Scholar]
- 10.Harding CJ, Mabbs FE, MacInnes EJL, McKee V, Nelson J. J Chem Soc Dalton Trans. 1996:3227. [Google Scholar]
- 11.Amendola V, Bastianello E, Fabbrizzi L, Mangano C, Pallavicini P, Perotti A, Manotti Lanfredi A, Ugozzoli F. Angew Chem, Int Ed. 2000;39:2917. [PubMed] [Google Scholar]
- 12.Amendola V, Bergamaschi G, Buttafava A, Fabbrizzi L, Monzani E. J Am Chem Soc. 2010;132:147. doi: 10.1021/ja9046262. [DOI] [PubMed] [Google Scholar]
- 13.Chen JM, Zhuang XM, Yang LZ, Jiang L, Feng XL, Lu TB. Inorg Chem. 2008;47:3158. doi: 10.1021/ic702143d. [DOI] [PubMed] [Google Scholar]
- 14.(a) Hosseini MW, Lehn JM. Helv Chim Acta. 1987;70:1312. [Google Scholar]; (b) Nation DA, Martell AE, Carroll RI, Clearfield A. Inorg Chem. 1996;35:7246. doi: 10.1021/ic960661q. [DOI] [PubMed] [Google Scholar]; (c) Wang Z, Reibenspies J, Martell AE. Inorg Chem. 1997;36:629. [Google Scholar]; (d) Tomat E, Cuesta L, Lynch VM, Sessler JL. Inorg Chem. 2007;46:6224. doi: 10.1021/ic700933p. [DOI] [PubMed] [Google Scholar]
- 15.(a) Nation DA, Martell AE, Carroll RI, Clearfield A. Inorg Chem. 1996;35:7246. doi: 10.1021/ic960661q. [DOI] [PubMed] [Google Scholar]; (b) Fabbrizzi L, Marcotte N, Stomeo F, Taglietti A. Angew Chem Int Ed. 2002;41:3811. doi: 10.1002/1521-3773(20021018)41:20<3811::AID-ANIE3811>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.(a) Saeed MA, Fronczek FR, Hossain MA. Chem Commun. 2009:6409. doi: 10.1039/b916099j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saeed MA, Fronczek FR, Huang MJ, Hossain MA. Chem Commun. 2010;46:404. doi: 10.1039/b923013k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson D, Dey KR, Fronczek FR, Hossain MA. Tetrahedron Lett. 2009;50:6537. doi: 10.1016/j.tetlet.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crystal data for R: C26H44Br3Cu2N6O·Br, M = 903.39, monoclinic, a = 7.9327(10) Å, b = 9.1110(11) Å, c = 22.667(2) Å, α = 90°, β = 99.465(6)°, γ = 90°, V = 1616.0(3) Å3, T = 90.0(5) K, space group Pn, Z = 2, μ(MoKa) = 6.292 mm−1, 30150 reflections measured, 9778 independent reflections (Rint = 0.023). The final R1 was 0.038 (I > 2σ(I)). The final R1 was 0.049 (all data). The goodness of fit on F2 was 1.027. Flack parameter = 0.484(8). CCDC 764942.
- 19.Schneider HJ, Kramer R, Simova S, Schneider U. J Am Chem Soc. 1988;110:6442. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.