Abstract
Bone formation and resorption are sensitive to both external loads arising from gravitational loading as well to internal loads generated by muscular activity. The question as to which of the two sources provides the dominant stimulus for bone homeostasis and new bone accretion is arguably tied to the specific type of activity and anatomical site but it is often assumed that, because of their purportedly greater magnitude, muscle loads modulate changes in bone morphology. High-frequency mechanical signals may provide benefits at low- (<1g) and high- (>1g) acceleration magnitudes. While the mechanisms by which cells perceive high-frequency signals are largely unknown, higher magnitude vibrations can cause large muscle loads and may therefore be sensed by pathways similar to those associated with exercise. Here, we review experimental data to examine whether vibrations applied at very low magnitudes may be sensed directly by transmittance of the signal through the skeleton or whether muscle activity modulates, and perhaps amplifies, the externally applied mechanical stimulus. Current data indicate that the anabolic and anti-catabolic effects of whole body vibrations on the skeleton are unlikely to require muscular activity to become effective. Even high-frequency signals that induce bone matrix deformations of far less than five microstrain can promote bone formation in the absence of muscular activity. This independence of cells on large strains suggests that mechanical interventions can be designed that are both safe and effective.
Keywords: Muscle, Bone, Mechanical Signals, Vibrations, High Frequency, Cortical, Trabecular
Introduction
Bone's high sensitivity to mechanical signals may someday provide the basis for non-pharmaceutical interventions capable of increasing bone mass during growth, minimizing skeletal erosion during adulthood, and restoring tissue integrity following losses due to injury, disease or occupation (e.g., space-flight). During the last century, most investigations into the relation between anabolic mechanical signals and the skeleton have focused on forces that are relatively large in nature, such as imposed by high-impact exercise. More recently, these investigations were expanded to include loading challenges applied at higher loading frequencies (>10Hz), commonly referred to as vibrations. Technically, sinusoidal vibrations can be defined by specifying two of the following three variables; frequency (f, number of oscillations per second, Hz), magnitude of the induced peak acceleration (a, expressed typically with the acceleration of the Earth as a referent where 1g= 9.81m/s2), and/or the total displacement produced by the vibrating actuator (D, total peak-to-peak displacement of the oscillating plate, typically in μm, mm or cm). For instance, the acceleration expressed in multiples of g is: a≈0.20·10−3·D·π2·f2, where D is expressed in mm and f in Hz. Acceleration and frequency can be readily measured with an accelerometer attached to the plate and should be verified, rather than relying on a manufacturer's data sheet1.
Vibrations as a means of introducing mechanical loading to the skeleton are receiving increased attention in both exercise and medical areas. Vibrating plates have become particularly ubiquitous in fitness studios with many plates capable of producing a stimulus with peak accelerations of 20g. While interventions employing relatively large accelerations may indeed stop bone loss and build new tissue2,3, they may also expose many systems of the human body to significant health risks, including musculoskeletal and neural damage4. The guidelines delineated in ISO 2631 for the potential danger of exposing the human body to whole body vibrations allow the application of supra-1g accelerations only for very short periods of time (or not at all)5. For this review, low-level vibrations were defined at less than 1g-peak accelerations producing vertical plate displacements of less than 1mm. Interestingly, even at extremely small accelerations and amplitudes, these high-frequency (10–100Hz) stimuli have been shown to provide skeletal benefits by increasing its mass and strength6,7.
The molecular and cellular mechanisms by which mechanical signals become anabolic or anti-catabolic to bone are largely unidentified8. In spite of much debate, it is not even clear whether the mechanical input received by bone cells originates from reactionary forces produced by the skeleton with a substrate (e.g., ground reaction forces) or whether muscle activity is the primary source of mechanical information9–11. In other words, is a skeletal outcome caused by the mechanical force (acceleration) traveling from the interface of the vibrating plate with the feet to a given anatomical site or does the externally applied mechanical signal cause muscles to resist and therefore load the bone? Or does the mechanical signal produce muscle hypertrophy which subsequently serves as an anabolic stimulus to bone because of the greater loads that it may impose upon the skeleton (Figure 1)? High correlations between muscle mass and a skeletal phenotype, particularly in children, may argue for the involvement of muscle in bone's adaptive response11. However, the lack of spatial and temporal specificity of these correlations or the severe consequences of removing gravitational loads from the skeleton may provide evidence that bone's response is directly modulated by the transmission of the ground reaction forces10. Ground reaction forces are inherently linked to muscle activity and it could be argued that it doesn't really matter whether a bone receives the mechanical signal transmitted through the skeleton or through muscle contractions. Nevertheless, such information may prove to be critical towards devising physical interventions that enjoy high-efficacy, convenience, and a high degree of safety.
Figure 1.

Three different pathways by which mechanical signals produced during whole body vibrations may be sensed by cells within a bone of the appendicular skeleton. Cells that may convert the mechanical signal into biochemical language include osteoblasts/osteoclasts/lining-cells on bone surfaces, osteocytes within the calcified matrix, and mesenchymal precursors within the bone marrow.
Standing on a vertically vibrating plate (whole body vibration) at very high acceleration magnitudes (>10g) activates neuromuscular units and may require high-force production during muscle contractions to maintain balance on plates that engender vertical displacements in excess of 1mm. Because of the large amount of work performed by muscle units subjected to large-magnitude vibrations, the contention whether increased bone mass in response to such a regime is accounted for by elevated ground-reaction or muscle forces will ostensibly extend along the lines of reasoning for exercise induced mechanical stimuli. In contrast, the degree by which muscular activity plays a role in modulating bone's plasticity to extremely low-level vibrations at displacement amplitudes of less than 1g and 100μm – a barely discernible stimulus that requires no training adaptation and could be passively tolerated and be safe for hours – is far less apparent. Muscle could be involved in the pathway by which low-level vibrations become anabolic to bone either directly by providing a stimulus during the treatment regime or indirectly by increasing its mass in response to the treatment and thereby exerting larger forces on the bone during habitual activities upon treatment. Here, we review the influence of very small and safe high-frequency mechanical signals on the musculoskeletal system and discuss whether the skeletal benefits of low-magnitude vibrations can be accounted for by direct effects on the skeleton or whether there is evidence that increased muscle activity is necessary for vibrations to become efficacious.
Transmissibility of the signal in the skeleton
High transmissibility of the oscillatory signal from the vibrating plate into the weight-supported skeleton would be a critical prerequisite for a physical signal if it was to directly target specific skeletal sites. In other words, if increased bone mass was observed in the lumbar spine in spite of failure of the signal to travel to this anatomical site, it was likely accounted for by secondary effects such as increased muscular activity or the release of cytokines/hormones. While the transfer of tissue strains arising from the dynamic alterations in g-force into the weightbearing skeleton is conceptually simple, it has to be demonstrated experimentally as dampening may occur. Only few studies have investigated transmissibility of ground based vibration at frequencies above 12Hz12. Towards establishing a relation between the acceleration magnitude of the vibrating plate and its transmission through the appendicular and axial skeleton, accelerometers mounted on Steinman pins imbedded in the spinous process of L3 and the greater trochanter measured accelerations from six volunteers standing on a vertically oscillating plate13. To determine damping as a function of posture, data were also collected from subjects while standing with bent knees. Interestingly, negligible loss of acceleration was observed in the femur and spine up to frequencies of 30Hz, indicating excellent transmissibility. As expected, higher frequencies and bending of the knees reduced transmissibility.
It is also interesting to note that transmissibility becomes complex when whole body vibrations are above 1g in magnitude. As those accelerations will exceed the gravitational acceleration of the Earth, the feet of the subject will lose contact with the vibrating plate unless significant dampening occurs. For both conditions, loss-of-contact or dampening, it is not straight forward to estimate the precise nature of the physical signal to which a bone (and its cellular sensors) is exposed to during high-magnitude accelerations. Indeed, large-magnitude but not small-magnitude accelerations may be amplified, rather than dampened, by the musculo-skeletal system at specific joints14. These non-linerarities of the musculo-skeleton at higher acceleration magnitudes may not only pose an additional health risks but may also hamper investigations into the precise identity of the signal promoting osteogenesis. Regardless, that a substantial fraction of ground-based low-level accelerations is transmitted to regions of the weight-bearing skeleton does not favor a mechanism based on ground reaction forces over a muscle-based mechanism. It demonstrates, however, that it is entirely possible that bone cells, even at regions distant from the site of induction of the mechanical signal, typically the foot, can sense the stimulus directly.
Bone deformations induced by low-level vibrations
Considering that most proposed physical mechanisms by which bone senses and responds to a mechanical signal are in some manner based on mechanical strain and its derivatives, knowledge of the magnitude of deformations induced in the calcified matrix during low-level vibrations may provide a hint regarding the involved mechanism. If they are relatively large, bone's adaptive response could be explained with a simple model such as the mechanostat15. If they are very small, an alternative mechanism may have to be considered. Cortical surface bone strains generated in the proximal tibia during a 0.3g, 45Hz vibratory regime were measured in two adult BALB/cByJ mice16. Under isoflurane anesthesia, a miniature single-element strain gage (1mm gage length) was implanted on the antero-medial surface of the proximal tibia. Upon recovery from surgery and with the animal standing on the vibrating plate, strain data were collected at a resolution of approximately 0.5 microstrain (με). The vibratory oscillations induced peak bone strain oscillations at the antero-medial surface of the tibia on the order of approximately 10με. In the rat, decreasing the acceleration of the signal to 0.15g and increasing the frequency to 90Hz reduces the strain magnitude at the cortical surface to about 2με17. Even when considering that the strains were recorded from a single site and that the magnitude of bone strains at the cortical periosteal surface may differ significantly from intracortical or trabecular matrix strains, it is readily apparent that the strains produced by the device are exceedingly small, several orders of magnitude smaller than the peak strains generated during loco-motory activities18–20. In the absence of systemic effects of low-level vibratory signals on the skeleton21,22, these data suggest the involvement of a physical mechanism largely independent of mechanical strain, regardless of whether deformations arose through muscle- or gravitational forces.
Vibration effects on the musculo-skeleton
To date, three human trials evaluating the potential of high-frequency vibrations at very low-levels (<0.5g) to positively influence bone mass and morphology have been completed. In the first, sixty-two post-menopausal women were randomized into in a double-blind, placebo controlled pilot study23. Thirty-two women stood on vertically vibrating devices at an acceleration magnitude of 0.2g and signal frequency of 30Hz for two ten-minute periods per day. Evaluating those in the highest quartile of compliance (86% compliant), placebo controls lost 2.1% in the femoral neck over the year, while vibration treatment was osteoprotective. In this quartile, the spine of lighter women (<65kg) exhibited a relative treatment benefit of 3.4% greater BMD. In the second study, twenty children with cerebral palsy were randomized into low-level vibration treatment (0.3g, 90Hz, 10min/d) or placebo controls24. Over the 6mo trial, tibial volumetric bone mineral density (BMD) of children who stood on placebo devices decreased by 11.9%, while children who stood on active devices increased by 6.3%. This benefit was achieved with an overall compliance of 44% of the 10 min/d period, implying that the anabolic response was triggered, rather than accumulated, by even brief exposures.
In the third study, a 12mo trial was performed in 48 young women, with half of the subjects subject to 10min/d low-level whole body vibrations (30Hz, 0.3g)25. A per protocol (PP) analysis demonstrated that women had to stand on the vibrating plate for at least 2 min/d to achieve a gain in bone mass, including a 3.9% net benefit in cancellous bone of the spine or a 3.0% net benefit in cortical bone of the femur (Table 1). In this study and in contrast to the previous two, muscle was included as an outcome measure. The low-level mechanical signal elevated muscle mass, with a 7.2% net benefit in the total paraspinous musculature, a 5.2% net benefit in the psoas muscle and a 7.9% net benefit in the erector spinae (Table 1). Together, these investigations demonstrated the ability of the human musculoskeletal system to derive structural benefits from the application of very low-level mechanical signals. As the target populations in these three studies were unlikely to be very active, exposure of their skeletons to a very large number of very small mechanical events could be considered a surrogate for specific aspects of the habitual loading environment. Unfortunately, the limited number of assays employed in these small clinical studies makes it difficult to extract information regarding relations between muscular- and skeletal adaptation and animal models may be more suitable to address these questions.
Table 1.
Changes of musculoskeletal variables in control and treated women over the length of the 12mo trial. Variables with significant differences between the two groups are bolded.
| Control | >2min/d of Treatment | |
|---|---|---|
| Total Paraspinous Musculature (%) | 0.8±5.1 | 8.0±9.1 |
| Psoas (%) | 1.6±8.2 | 6.8±6.0 |
| Quadratus Lumborum (%) | 5.4±13.7 | 13.4±15.0 |
| Erector Spinae (%) | −0.2±4.7 | 8.1±14.5 |
| Spine Cancellous Bone Density (%) | −0.1±4.5 | 3.8±4.9 |
| Quadriceps Femoris Area (%) | 3.0±6.8 | 3.9±4.2 |
| Femur Cross-sectional Area (%) | 1.0±2.2 | 2.4±3.7 |
| Femur Cortical Bone Area (%) | 1.3±3.9 | 4.3±3.6 |
Similar to clinical data, animal studies have consistently demonstrated that vibrations, applied at very low levels for short daily durations can increase bone formation26–28, decrease bone resorption16, and result in a skeleton with higher mass and strength6,29,30. As expected from a stimulus that alters cellular metabolism, skeletal changes are accompanied by the differential expression of key molecules in vivo including iNOS, RANKL, or MMP-228. In vitro experiments directly highlight the sensitivity of bone cells to vibratory signals of different magnitudes as shown by the altered transcriptional levels of c-fos and c-myc31, osteocalcin31–33, MMP-932, osteopontin33 or COX-234. Whether signaling pathways are dependent on the magnitude of the vibration, and whether vibrations of any magnitude are regulated differently from exercise induced mechanical signals, is currently unknown.
Similar to clinical studies, data from animal models indicate that in addition to low-magnitude accelerations, higher-magnitude accelerations can also raise bone formation and mass35–38. Only few investigations were designed to directly contrast the effects of low-magnitude versus high-magnitude accelerations. Considering the non-linearity by which vibrations are transmitted into the musculo-skeleton, it may not be surprising that the attempt to associate bone formation with acceleration magnitude has produced equivocal results. For instance, a whole body vibration intervention in mice was equally effective in increasing trabecular bone volume in the tibial metaphysis when the signal was applied at 0.1g and 1.0g29. In the ovariectomized rat, a 3g vibration regime was more efficacious than a 0.5g or 1.5g signal in preventing the detrimental changes induced by the loss of estrogen. However, the loading frequency and number of loading cycles also differed substantially between the three interventions, making it difficult to isolate the effect of acceleration magnitude. Taken together, there is currently no experimental data suggesting that efficacy of vibratory regimens increases with acceleration magnitude.
Substantiated with evidence that bone's anabolic and catabolic activity can be altered by low-level vibratory mechanical signal, its impact on the musculoskeletal system was investigated recently in the mouse39. Eight-week old BALB/cByJ mice subjected to a 45Hz, 0.3g signal had a 14% greater trabecular bone volume in the tibial metaphysis while periosteal bone area, bone marrow area, cortical bone area, and the moments of inertia of this region were all significantly greater (up to 29%). The soleus muscle also realized gains, with an up to 29% greater total cross-sectional area as well as type I and type II fiber area (Figure 2). Thus, similarly to clinical data, both muscle and bone can readily respond to the low-level mechanical signal in a murine model.
Figure 2.
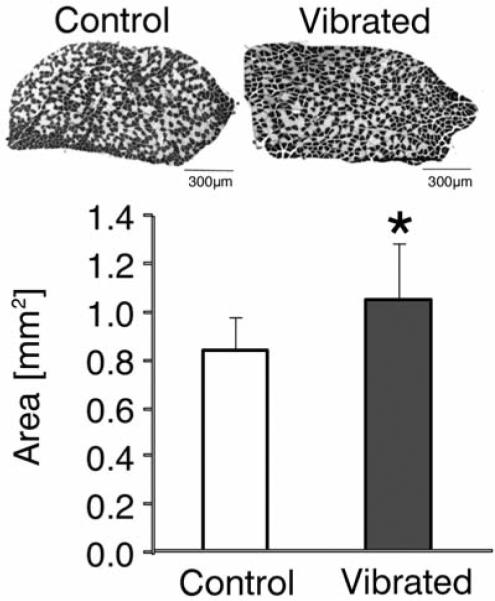
Upon application of low-level (0.3g) whole-body vibrations for 15min/d, differences in cross-sectional muscle area of the soleus were readily available after 6wk. The ratio between type I (slow, stained black) and type II (fast, stained white) muscle fibers remained unchanged by the mechanical intervention.
The specific type of cell that is sensing and responding to high-frequency, low-level mechanical signals has not been elucidated. Studies using larger force magnitudes at much lower frequencies have suggested that the cellular sensors for mechanical signals are embedded within the bone (i.e., osteocytes), rather than on bone surfaces40, given that osteocyte signaling regulates both mechanically induced bone formation41 and resorption42. Nevertheless, both osteoblasts and osteoclasts are also sensitive to mechanical information. Recent evidence suggests that the mechanism by which low-level, high-frequency mechanical stimuli are converted into a biologic response within a bone involves the selective proliferation and differentiation of specific progenitor cells in the bone marrow43,44. Conceivable, any cell located either on a bone surface, residing within the extracellular matrix, or located within the marrow can directly receive a signal from a foot-based vibration device that is transmitted through the appendicular skeleton.
Future studies that will relate the mechanical environment induced by vibrations to changes in cellular activity may provide clues toward identifying the origin of the biochemical signal leading to bone formation. For instance, if vibrations generate non-uniform distributions of strain and its by-products across a bone and spatially correlate with biologically relevant signals, it could be argued that cells within the matrix act as sensors. Lack of correlations, in particular if the cellular response was relatively uniform in distribution, could be interpreted as the signal originating from the marrow or the involvement of a biologic mechanism that integrates and processes the mechanical information from osteocytic sensors. Considering that the distribution of mechanical parameters engendered by high-magnitude vibrations is likely to be distinct from those induced by low-magnitude vibrations, such studies may also be used to test whether the identity of the sensory system is dependent upon the amplitude of the vibration.
Can bone differentiate between two high-frequency signals?
Vibration frequencies used in the clinical studies ranged from 30–90Hz. As reviewed above, the study which demonstrated anabolism in both muscle and bone of young women employed a frequency of 30Hz. Excitation frequencies of at least 400Hz are required for maximal power output when the muscle itself is stimulated45. In contrast, when a muscle dynamically oscillates without any electrical stimulation, its natural frequency is between 10–50Hz46. If the excitation frequency of an external vibratory mechanical stimulus is close to the natural frequency of the muscle, an increase in muscle activity may be initiated to dampen the vibrations within the tissue. Thus, examining the degree of sensitivity of a bone to a given frequency in vivo may provide information towards the question whether the increase in bone formation may be directly associated with the ground based signal transmitted into the skeleton.
To determine whether one high-frequency signal may be more effective than another, ovariectomized (OVX) Sprague-Dawley rats were subjected to low-level vibrations applied at either 45Hz or 90Hz and compared to OVX age-matched controls17. Five additional rats were used, in vivo, to establish the induced bone surface strains. Following a 28d protocol, bone formation rates in the metaphysis of the proximal tibia were 159% greater in 90Hz rats when compared to age-matched controls, but 45Hz rats were not significantly different from controls. Bone morphology of 90Hz rats indicated significantly greater trabecular bone volume (22% and 25%) and thicker trabeculae (11% and 12%) over either controls or 45Hz rats in the epiphysis of the distal femur, respectively. Despite the enhanced sensitivity of the skeleton towards the 90Hz signal, strain magnitudes and strain rates induced by this frequency were 65% and 38% lower than during 45Hz vibration. While these data may be specific to the rat skeleton deprived of estrogen, it is interesting to note that the vibration frequency that was closer to both the natural frequency of muscle as well as its own peak dynamic frequency was the one ineffective in raising osteoblast activity. These data are therefore inconsistent with the hypothesis that muscle and bone are tuned to similar frequencies. Further, they demonstrate that, unlike muscle in which the degree of plasticity has been associated with the magnitude of force production, stimulus amplitude is unlikely to drive bone's response to low-level vibrations29.
Can bone sense low-level vibrations in the absence of muscular activity?
A critical test to demonstrate that neither increased muscle mass nor increased muscle activity are necessary to raise bone mass upon exposure to low-level vibrations is to inhibit muscle activity during the oscillatory treatment. While such an experiment may appear difficult to realize because postural muscle activity is required for an individual to stand on the vibrating plate, it becomes possible when it is assumed that matrix strain is not a critical factor in bone's response to low-level, high-frequency vibrations. During whole body vibrations, the weight of the subject effectively acts against the vertically upwards moving plate, thereby inducing deformations in the weightbearing skeleton. If deformation per se is not a prerequisite for mechanotransduction, cells may be equally sensitive to simple oscillatory motions (shaking) applied to skeletal segments, allowing to test the hypothesis that neither muscle contractions nor strain in the bone matrix are required for vibratory signals to become efficacious.
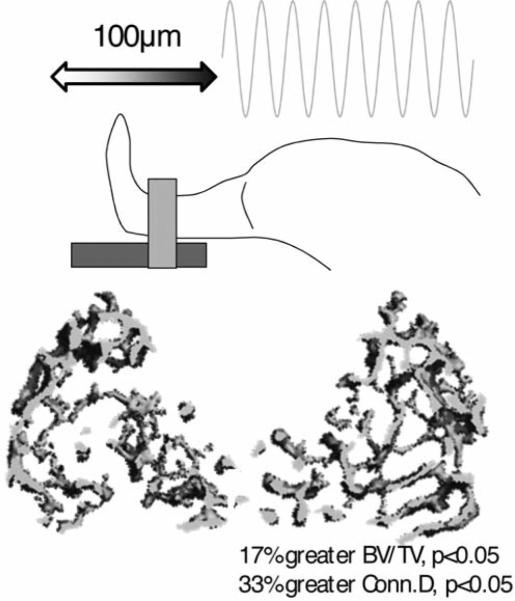
A loading apparatus was developed to accelerate specific segments of the murine skeleton without loading them (Figure 3). In other words, bone was subjected to oscillatory motions without the direct application of deformations to the tissue. The left tibia of eight adult mice was exposed to small (0.3g or 0.6g) 45Hz sinusoidal accelerations for 10min/d, while the right tibia served as internal control. During treatment, mice were anesthesized and therefore, all muscle tone was removed. Mice were allowed to freely ambulate between treatments. After 3wk, trabecular metaphyseal bone formation rates were 88% greater in tibiae accelerated at 0.3g than in their contralateral control, similar to the 66% increase in formation rates of bones accelerated at 0.6g. Stimulated tibiae also displayed significantly greater cortical area (+8%) and thickness (+8%), together suggesting that tiny acceleratory motions – independent of direct loading of the matrix – can influence bone formation and bone morphology.
Figure 3.
With the anesthetized mouse in a supine position, the tibia can be readily subjected to high-frequency horizontal sinusoidal motions produced by a linear actuator (top panel). Even a brief daily exposure to this mechanical signal in the absence of muscle tone can produce skeletal benefits such as greater trabecular bone volume fraction and greater trabecular connected in the metaphysis of the tibia.
In subsequent studies22,47, we confirmed these findings in a model in which loads induced by locomotion were removed from the tibiae via hindlimb unloading. Oscillatory accelerations, applied in the absence of weight bearing, resulted in 70% greater bone formation rates in the trabeculae of the metaphysis, but similar levels of bone resorption when compared to contralateral controls. Quantity and quality of trabecular bone also improved as a result of the acceleration stimulus, as evidenced by significantly enhanced morphological and mechanical properties (Figure 3). As the imposed acceleratory signal was effective both in normally ambulating mice as well as in mice in which large-magnitude muscle contractions were essentially eliminated, the metabolic state of the muscle does not appear to influence the efficacy of the mechanical signal in bone.
Together, these in vivo data not only indicate that mechanosensory elements of resident bone cell populations can perceive and respond to very small magnitude acceleratory signals but also demonstrate that bone can readily respond to low-level vibrations in the absence of muscle activity and even in the presence of sarcopenia. These findings are necessary but not sufficient to accept the hypothesis that anabolism is the result of direct skeletal transmission of the acceleratory signal from the oscillating plate to the bony region of interest because high-frequency muscle stimulation in the absence of any externally applied loads or motions can also increase bone formation48,49. Thus, if the application of whole body vibrations elicits firing of muscle fibers at a similar frequency, it is possible that not only substrate reaction forces, but also muscle activity may contribute to bone's cellular response during whole body vibrations.
Temporal sequence of musculoskeletal plasticity
If it was true that muscle loading is greater than gravitational loading and, therefore, bone morphology is predominantly determined by muscle loads, then it is reasonable to argue that muscular adaptations in response to a mechanical stimulus should temporally precede skeletal adaptations. These types of comparisons are commonly made in exercise studies and data have been used to both support or refute muscle-bone relations. Unfortunately, none of the clinical trials using low-level vibrations gathered sufficient data for such temporal associations. We recently completed a study in which 8wk old mice were subjected to eight distinct 3wk low-level whole body vibration regimes for which acceleration magnitude (0.3g or 0.6g), loading duration (15–60min/d) and the number of daily bouts (1–3 per day) was varied (unpublished data). The extent of altered bone formation and morphology in the tibia was heavily dependent on the parameters of the stimulus with some types of regimes significantly increasing bone mass while others were ineffective. Interestingly, none of the eight vibrations schemes was able to induce changes in soleus areal properties even though soleus cross-sectional area is increased when extending the experimental duration from 3wk to 6wk39. While these results need to be interpreted with care because of normal muscle growth during the experimental period, they are consistent with data from exercise studies in which mechanical loads induced greater bone mineral density (BMD) without significant difference in muscle strength50. If bone adaptation is not be a temporally secondary event to muscle adaptation, then increased muscle mass does not serve as the stimulus for increasing bone mass.
Metabolic evidence
Even without evidence that increased muscle mass serves as a signal for increasing bone mass, one could hypothesize that low-level whole body vibrations lead to greater levels of physical activity and metabolism when the mice are not roaming the vibrating plate. To this end, we tested whether the mechanical intervention elevates activity levels and muscle fuel utilization. At 7wk of age, male chow-fed C57BL/6J mice were assigned to control and experimental mice (n=8, each). For 12wk, 15min/d, experimental mice were subjected to low-level whole-body vibrations (90Hz, 0.2g). At the second and ninth week of the experimental protocol, mice were individually housed in calorimetric cages for 48h, respectively. Prior to returning the mice to their regular cages, they were fasted for 15h and then re-fed. Throughout each 48h period, activity levels of each individual mouse were measured by multiple infrared beams installed in the cages. The respiratory quotient (RQ), the ratio of the amount of carbon dioxide produced to the amount of oxygen consumed was measured in each mouse during the second 48h period. A RQ value of 1.0 indicates exclusive carbohydrate oxidation whereas a RQ value of 0.70 reflects exclusive fatty acid usage. During each of the 12wk, food consumption as well as body mass did not differ between the two groups. Indirect calorimetry showed that during the night, mice primarily oxidized carbohydrates (RQ=0.95±0.03 for control, 0.97±0.02 for vibrated) while during the day, a partial fasting period for mice, a mixture of carbohydrates and fatty acids was oxidized (0.91±0.04 vs 0.92±0.04). During any given period, there were neither significant differences in the RQ nor in the activity levels between control and experimental mice (Figure 4). The absence of significant differences in skeletal muscle fatty acid utilization and activity levels limits the role of metabolic factors in explaining skeletal benefits induced by low-level vibrations outside the treatment window.
Figure 4.

Activity levels and energy expenditure assessed by indirect calorimetry nine weeks into an experimental protocol during which mice were subjected to 15min/d, of low-level whole-body vibrations (90Hz, 0.2g) or allowed to freely roam their cages.
The lack of altered metabolism is also interesting given that the exposure of mice to short exposures of low-level whole body vibrations can limit adipogenesis while stimulating osteoblastogenesis. For instance, a 6wk vibratory intervention increased the overall marrow-based stem cell population by 37% and the number of mesenchymal stem cells by 46%43. After 14 wk, visceral adipose tissue formation was suppressed by 28%, whereas trabecular bone volume fraction in the tibia was increased by 11%. As bone and muscle cells originate from the same pool of progenitor cells, it is entirely conceivable that mechanically altered bone marrow cell populations link the changes between different tissues within the musculoskeletal system.
Conclusions
The structural benefits that bone can gain through exposure to low-level vibrations are apparent. Considering that muscle generates signals in the same frequency range during habitual activities such as standing, the plasticity of skeletal muscle to a signal this small may appear even more surprising. In spite of its sensitivity to an externally applied high-frequency mechanical stimulus, there is currently no evidence that muscle, either directly or indirectly, plays a major role in modulating the anabolic or anti-catabolic response of the skeleton to low-level oscillations. The high degree of transmissibility of the foot-based signal into the axial skeleton, the ability of bone to respond to oscillatory mechanical signals even without matrix deformation or muscle activity, the lack of a temporally sequential muscle-bone response, or the absence of increased muscle fuel utilization may point towards a pathway in which bone cells can directly sense the signal transmitted through the skeleton. Nevertheless, there is no conclusive evidence either for or against a muscle based or ground reaction force based mechanism. Additive and synergistic interactions between muscle- and external mechanical signals, through mechanical or biochemical factors, are entirely possible. Towards unraveling the mechanisms at which high-frequency mechanical signals modulate cellular activity in the musculo-skeleton, two types of studies appear particular critical at this point. The first is an accurate determination of the mechanical environment that vibration regimes generate at different levels and hierarchies of the musculo-skeletal system, information that will be required to identify physical mechanisms. The second will need to focus on identifying molecular and cellular mechanisms in response to a given high-frequency signal. Together, they may facilitate the development of efficacious physical interventions that target specific biologic systems.
Acknowledgements
Financial support by NASA and NIAMS is gratefully acknowledged. We thank Dr. Irwin Kurland and Chuck Trujillo for assistance with the metabolic cages.
References
- 1.Christiansen BA. Whole-body vibration and weight loss: truth or consequence? Int J Obes (Lond) 2009;33:384–3. doi: 10.1038/ijo.2008.261. [DOI] [PubMed] [Google Scholar]
- 2.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–9. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 3.Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. doi: 10.1186/1471-2474-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Vibration exposure and bio-dynamic responses during whole-body vibration training. Med Sci Sports Exerc. 2007;39:1794–800. doi: 10.1249/mss.0b013e3181238a0f. [DOI] [PubMed] [Google Scholar]
- 5.Bernard B, Nelson N, Estill CF, Fine L. The NIOSH review of hand-arm vibration syndrome: vigilance is crucial. National Institute of Occupational Safety and Health. J Occup Environ Med. 1998;40:780–5. doi: 10.1097/00043764-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Judex S, Boyd S, Qin YX, Turner S, Ye K, Muller R, Rubin C. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng. 2003;31:12–20. doi: 10.1114/1.1535414. [DOI] [PubMed] [Google Scholar]
- 7.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism: Low mechanical signals strengthen long bones. Nature. 2001;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 8.Judex S, Zhong N, Squire ME, Ye K, Donahue LR, Hadjiargyrou M, Rubin CT. Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem. 2005;94:982–94. doi: 10.1002/jcb.20363. [DOI] [PubMed] [Google Scholar]
- 9.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–51. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 10.Judex S, Carlson KJ. Is bone's response to mechanical signals dominated by gravitational loading? Med Sci Sports Exerc. 2009;41:2037–43. doi: 10.1249/MSS.0b013e3181a8c6e5. [DOI] [PubMed] [Google Scholar]
- 11.Robling AG. Is bone's response to mechanical signals dominated by muscle forces? Med Sci Sports Exerc. 2009;41:2044–9. doi: 10.1249/MSS.0b013e3181a8c702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann Biomed Eng. 1997;25:831–9. doi: 10.1007/BF02684167. [DOI] [PubMed] [Google Scholar]
- 13.Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci. 1999;54:B352–B357. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- 14.Kiiski J, Heinonen A, Jarvinen TL, Kannus P, Sievanen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23:1318–25. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 15.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 16.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Judex S, Zernicke RF. Does the mechanical milieu associated with high-speed running lead to adaptive changes in diaphyseal growing bone? Bone. 2000;26:153–9. doi: 10.1016/s8756-3282(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 19.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–10. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 20.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107(2):321–7. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 21.Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445–52. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 22.Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–40. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 23.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–51. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 24.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 25.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 26.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–2. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 27.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225–9. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 28.Jankovich JP. The effects of mechanical vibration on bone development in the rat. J Biomech. 1972;5:241–50. doi: 10.1016/0021-9290(72)90038-3. [DOI] [PubMed] [Google Scholar]
- 29.Christiansen BA, Silva MJ. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng. 2006;34:1149–56. doi: 10.1007/s10439-006-9133-5. [DOI] [PubMed] [Google Scholar]
- 30.Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–57. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 31.Tjandrawinata RR, Vincent VL, Hughes-Fulford M. Vibrational force alters mRNA expression in osteoblasts. FASEB J. 1997;11:493–7. doi: 10.1096/fasebj.11.6.9194530. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka SM, Li J, Duncan RL, Yokota H, Burr DB, Turner CH. Effects of broad frequency vibration on cultured osteoblasts. J Biomech. 2003;36:73–80. doi: 10.1016/s0021-9290(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 33.Sato N, Kubo K, Yamada M, Hori N, Suzuki T, Maeda H, Ogawa T. Osteoblast mechanoresponses on Ti with different surface topographies. J Dent Res. 2009;88:812–6. doi: 10.1177/0022034509343101. [DOI] [PubMed] [Google Scholar]
- 34.Bacabac RG, Smit TH, Van Loon JJ, Doulabi BZ, Helder M, Klein-Nulend J. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20:858–64. doi: 10.1096/fj.05-4966.com. [DOI] [PubMed] [Google Scholar]
- 35.Oxlund BS, Ortoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32:69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 36.Sehmisch S, Galal R, Kolios L, Tezval M, Dullin C, Zimmer S, Stuermer KM, Stuermer EK. Effects of low-magnitude, high-frequency mechanical stimulation in the rat osteopenia model. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flieger J, Karachalios T, Khaldi L, Raptou P, Lyritis G. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int. 1998;63:510–4. doi: 10.1007/s002239900566. [DOI] [PubMed] [Google Scholar]
- 38.Rubinacci A, Marenzana M, Cavani F, Colasante F, Villa I, Willnecker J, Moro GL, Spreafico LP, Ferretti M, Guidobono F, Marotti G. Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int. 2008;82:316–26. doi: 10.1007/s00223-008-9115-8. [DOI] [PubMed] [Google Scholar]
- 39.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–62. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 40.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–75. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 42.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–9. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104:17879–84. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de HA. The influence of stimulation frequency on force-velocity characteristics of in situ rat medial gastrocnemius muscle. Exp Physiol. 1998;83:77–84. doi: 10.1113/expphysiol.1998.sp004093. [DOI] [PubMed] [Google Scholar]
- 46.Wakeling JM, Nigg BM. Modification of soft tissue vibrations in the leg by muscular activity. J Appl Physiol. 2001;90:412–20. doi: 10.1152/jappl.2001.90.2.412. [DOI] [PubMed] [Google Scholar]
- 47.Ozcivici E, Garman R, Judex S. High-frequency oscillatory motions enhance the simulated mechanical properties of non-weight bearing trabecular bone. J Biomech. 2007;40:3404–11. doi: 10.1016/j.jbiomech.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated Resistance Training During Hindlimb Unloading Abolishes Disuse Bone Loss and Maintains Muscle Strength. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090811. [DOI] [PubMed] [Google Scholar]
- 49.Lam H, Qin YX. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordstrom P, Pettersson U, Lorentzon R. Type of physical activity, muscle strength, and pubertal stage as determinants of bone mineral density and bone area in adolescent boys. J Bone Miner Res. 1998;13:1141–8. doi: 10.1359/jbmr.1998.13.7.1141. [DOI] [PubMed] [Google Scholar]