Abstract
Purpose
To better understand how nitric oxide (NO) alters the function of the nonpigmented ciliary epithelium (NPE), studies were performed to determine the influence of NO on sodium-hydrogen exchanger (NHE) activity.
Methods
Cytoplasmic pH (pHi) was measured in cultured porcine NPE loaded with BCECF (2′,7′-bis(2-carboxyl)-5(6)-carboxyfluorescein-acetoxyethyl ester). Na-H exchanger (NHE) was examined by immunolocalization.
Results
In cells acidified by 5 minutes of exposure to 20 mM ammonium chloride, pHi recovery was partially inhibited by sodium nitroprusside (SNP), an NO donor, and l-arginine, the endogenous substrate for NO synthase. SNP and dimethyl amiloride (DMA), an NHE inhibitor, inhibited pHi recovery to a similar degree. In bicarbonate-free buffer SNP+DMA elicited no additional change in pHi recovery beyond that elicited by DMA alone. This suggests that SNP causes NHE inhibition. the SNP's effect on pHi recovery was mimicked by 8-pCPT-cGMP but suppressed by ODQ and H-8. Ouabain alone reduced pHi recovery, but SNP+ouabain caused significant further reduction. Immunolocalization studies revealed NHE1 and -4 in native and cultured NPE.
Conclusions
NHE1 and -4 are expressed at the NPE basolateral margin. The findings suggest the NHE is inhibited by NO which acts via a cGMP and protein kinase G signaling pathway. The NHE response does not appear to be the consequence of NO-induced Na,K-ATPase inhibition. Because NO synthases are expressed in porcine NPE, NO could act as an autocrine regulator of NHE activity. Although NHE inhibitors are known to lower intraocular pressure (IOP), further studies are needed to understand whether changes in NHE activity contribute to the IOP-lowering effect of NO donors.
The ciliary processes are bounded by an epithelial bilayer that consists of the pigmented ciliary epithelium (PE) facing the stromal blood supply and the nonpigmented ciliary epithelium (NPE) that faces the aqueous humor (AH). The two layers are joined at their apical surfaces by gap junctions1 forming a functional syncytium.2 AH formation is the result of active solute transport that causes osmotic flux of water across the bilayer.3,4 The process is thought to require the coordinated action of several different ion transport mechanisms and ion channels at the NPE and PE basolateral surfaces, together with apical gap junctions that form a conduit between the PE and NPE.5,6
Previous studies point to the ability of nitric oxide (NO) donors to slow the rate of AH formation in different species including the porcine.7,8 This raises mechanistic questions regarding the effect of NO on the ciliary epithelium. At high concentration—for example, in inflammatory responses—NO causes cell damage but in many instances NO has a signaling role in which it acts to modulate cell function.9,10 Nitric oxide synthase (NOS) isoforms are expressed in the ciliary processes and NOS appears particularly abundant in the NPE,11,12 which suggests that the NPE is able to synthesize NO, a notion supported by the detection of nitrite in the bathing solution into which isolated porcine NPE is placed.13
It has been shown that NO donors cause Na,K-ATPase inhibition in ciliary processes14 and in isolated NPE.13 As in other tissues where NO has a signaling role, NO donors raise the concentration of cGMP in the porcine iris-ciliary body.15 Commonly, it is the cGMP increase elicited by NO that brings about a change in cell function.16,17 This seems to be the case in the NPE where there is strong evidence that the Na,KATPase inhibition response is cGMP-dependent.13 cGMP is a key second messenger, and an increase in cGMP may cause a variety of different responses in the NPE. Of importance, NO is not alone in its ability to increase cGMP. In cultured human fetal NPE, Crook and Chang18 reported a cGMP increase in response to the natriuretic peptides ANP, BNP, and CNP. In bovine and rat ciliary processes, there is evidence that natriuretic peptides elevate cGMP and the rise of cGMP appears to lead to inhibition of the sodium hydrogen exchanger (NHE).19 This fits with reports that NO donors causes cGMP-dependent NHE inhibition in the kidney20 and in myocytes.21 It is also interesting to note that NO reduces aqueous humor secretion and IOP in the porcine eye,8 and local application of NHE1 inhibitors in anesthetized mouse reduces IOP.22 Recent reports show that NO donors inhibit Na,K-ATPase in the bovine ciliary processes14 and choroid plexus23 and in the freshly isolated porcine NPE13 by a cGMP-mediated mechanism. Thus, in the present study, we sought to examine whether NO donors alter NHE function in porcine NPE and whether the NO effect on NHE is simply the reflection of Na,K-ATPase inhibition. We found evidence of a robust expression of NHE1 and -4 isoforms at the basolateral surface of the porcine NPE.
Materials and Methods
Isolation and Culture of Porcine NPE
Fresh pig eyes were kindly provided by the University of Arizona, Department of Animal Sciences. NPE was established in primary culture using a modification of our previous methodology.12 In brief, the entire ring of NPE, attached to the vitreous, was dissected from each eye, leaving the pigmented cell (PE) layer attached to the ciliary body. Cut pieces (~2 mm) of the isolated NPE rings from 5 to 10 eyes were pooled and treated for 5 to 7 minutes with 0.015% collagenase A and 500 U/mL of hyaluronidase (Sigma-Aldrich, St. Louis, MO) in a buffer containing (in mM): NaCl 66.7, KCl 13.4, HEPES 3.8, and CaCl2 4.8 (pH 7.4). Then, the collagenase and hyaluronidase were neutralized by adding an excess amount of 1:1 mixture of newborn calf serum (NCS) and fetal bovine serum (FBS). NPE cells were dispersed by gentle trituration with a Pasteur pipette and then pelleted by centrifugation. The cells were incubated for 2 to 3 days in a small volume of Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) containing 10% FBS and 100 IU/mL gentamicin at 37°C in 5% CO2/95% air. Thereafter, the medium was changed on alternate days. The cells attained confluence in 7 to 10 days then were trypsinized and seeded at a density of 2 × 104 to 5 × 104 cells · cm2 for subsequent passages. In the studies reported herein, 4th-passage cells were used.
Immunolocalization
NHE localization in the ciliary body was examined in fresh porcine eyes. The cornea was removed, and then part of the anterior sclera (~one third) was carefully dissected from the choroid. The whole iris-ciliary body along with the lens and anterior vitreous was removed from the globe and placed in ice-cold buffer solution comprising (mM) NaCl 113, KCl 4.6, NaHCO3 21.0, MgSO4 0.6, d-glucose 7.5, glutathione (reduced form) 1.0, Na2HPO4 1.0, HEPES 10.0, and CaCl2 1.4, pH adjusted to 7.4. The lens was removed by cutting the zonules, the vitreous was carefully trimmed away, and the iris was dissected to leave the ciliary body, which was fixed in formalin and used to prepare 5-to 7-μm paraffin-embedded sections. Cultured NPE cells also were used for immunolocalization. The cells, grown on specially designed chamber slides (Laboratory-Tek II Chamber Slides; Nalge Nunc, Roskilde, Denmark), were washed with PBS containing 1.0 mM MgCl2 and 0.1 mM CaCl2 then fixed in acetone for 2 minutes.
Tissue sections or cultured NPE cells were incubated at room temperature for 90 minutes in blocking buffer (10% goat serum in PBS) then primary antibodies directed against NHE1, -2, -3, or 4 were added for 24 hours at 4°C. Control specimens were incubated in blocking buffer alone. The specimens were washed with PBS and exposed for 24 hours at 4°C to a fluorescent secondary antibody (AlexaFluor 488 or 546, conjugated to either goat anti-rabbit or anti-mouse IgG, 1:200 dilution).
Measurement of Cytoplasmic pH
Cytoplasmic pH was measured by imaging microscopy using the pH-sensitive dye BCECF (2′,7′-bis(2-carboxyl)-5(6)-carboxyfluorescein-acetoxyethyl ester). To load the cells with BCECF, NPE cells grown to semiconfluence on a 35-mm plastic dish (Corning Costar, Corning, NY) were incubated for 10 minutes with BCECF-AM (5.0 μM) as described earlier.24 Then, the cells were washed five times with the Krebs’ solution and incubated for another 10 minutes in Krebs’ solution to allow de-esterification of the dye. De-esterification transforms BCECF-AM (the ester form) to membrane-impermeable BCECF (acid form), which is trapped in the cytoplasm. The cells were then washed again several times to remove any traces of external dye. The dish containing the cells was then placed in a temperature-controlled per-fusion microincubator (PDMI-2; Harvard Biosciences, Holliston, MA) on the stage of an upright epifluorescence microscope (Eclipse; Nikon, Tokyo, Japan) where the preparation was superfused (3.0 mL · min−1) with Krebs’ buffer containing (in mM): NaCl, 117; KCl, 4.5; NaHCO3, 20; d-glucose, 6.0; MgCl2, 1.0; CaCl2, 1.5; and HEPES, 10.0, adjusted to pH 7.35 and equilibrated by gassing with 5% CO2 and 95% air. In specified experiments, a bicarbonate/CO2-free or a sodium-free buffer was used. The bicarbonate/CO2-free buffer contained (in mM) NaCl, 137; KCl, 4.5; d-glucose, 6.0; MgCl2, 1.0; CaCl2, 1.5 and HEPES, 10.0 adjusted to pH 7.35. The sodium-free buffer contained (in mM) choline chloride, 117; choline bicarbonate, 20; KCl, 4.5; d-glucose, 6.0; MgCl2, 1.0; CaCl2, 1.5; and HEPES 10, adjusted to pH 7.35. The inflow and outflow was achieved by using peristaltic pumps (520S; Watson-Marlow, Falmouth, UK). The microscope was fitted with a high-resolution video camera (DVC 340M-00-CL; Applied Scientific Instrumentation, Eugene, OR) to continuously monitor and record the BCECF fluorescence intensity in the cells. Fluorescence intensity was measured at an emission wavelength of 535 nm, with alternating excitation wavelengths of 488 and 460 nm, programmed by an imaging system (InCyt Im2; Intracellular Imaging Inc., Cincinnati OH 45,219). The fluorescence intensity ratio I488/I460 was calibrated by titrating BCECF free acid with a range of buffers with defined pH values (5.49 – 8.5). Calibration was performed in vitro, either in the cell perfusion dish or the calibration chamber supplied by the manufacturer (Intracellular Imaging Inc.). The camera and the microscope settings for the calibration and for conducting experiments were the same. Studies were conducted to determine whether the resting pHi and the pHi recovery rate obtained by the in vitro calibration method are comparable to the values obtained in experiments using in-cell calibration in nigericin-treated cells. Control experiments were performed using the in-cell calibration method described earlier25 in which the relationship between pH and the 488/460 fluorescence ratio was established at the end of each experiment by exposing the cells to a series of potassium-rich pH buffers containing 10 μM nigericin added to equilibrate cytoplasmic pH with the superfusate. This in-cell calibration method showed the resting pHi and the pHi recovery rate to be 7.18 ± 0.04 (n = 8) and 0.0049 ± 0.0006 (n = 8) unit · s-1 respectively. The values are similar to the values obtained from experiments in which the in vitro calibration was used.
The experimental protocol was as follows. Cells were superfused with Krebs’ solution for 5 minutes to obtain a stable baseline pHi. The superfusate was then switched for 5 minutes to an ammonium chloride-containing buffer of the above composition but with an equivalent reduction of sodium chloride. The addition of ammonium chloride caused a rapid rise in pHi. Subsequent replacement of the ammonium chloride with Krebs’ solution caused a rapid decrease in pHi followed by a gradual recovery toward baseline. The pHi recovery rate was measured as the slope of the straight line obtained by plotting pHi values against time (in seconds). To focus on the initial linear phase of pHi recovery, the pHi recovery slope for just the initial 256 seconds of recovery was analyzed. The slope was calculated from the regression equation of the straight line (Excel; Microsoft, Redmond, WA, and the statistical program Minitab; MiniTab, State College, PA).
Reagents
Mouse anti-NHE1 monoclonal antibody was obtained from BD Biosciences, Franklin Lakes, NJ), and rabbit anti-NHE2, -3, and -4 polyclonal antibodies were purchased from Alpha Diagnostic International (San Antonio TX). Secondary antibodies, Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 546 goat anti-mouse IgG were obtained from Invitrogen (Carlsbad, CA). Dimethylamiloride (DMA), 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich. BCECF was obtained from Invitrogen. All other chemicals were obtained from Sigma-Aldrich. Stock solutions of test compounds were prepared in DMSO or in Krebs’ buffer before addition to the cell superfusate. Control solutions received the vehicle alone.
Statistical Analysis
A paired t-test was used to compare paired samples and a two-sample t-test was used to analyze unpaired data. One-way analysis of variance (ANOVA) followed by Bonferroni's post hoc multiple comparison tests was applied to compare differences between more than two groups of data. P <0.05 was considered significant.
Results
The baseline cytoplasmic pH (pHi) measured in cultured porcine NPE under control conditions in bicarbonate-containing buffer was 7.12 ± 0.08 (n = 13) and in bicarbonate-free HEPES buffer was 7.09 ± 0.03 (n = 27). There is no significant difference between the two values. After a stable baseline was established, the cells were subjected to an acid load by temporary (5 minutes) exposure to 20 mM ammonium chloride. When ammonium chloride was removed, pHi decreased sharply and then recovered toward the original baseline. The control rate of pHi recovery in bicarbonate-containing buffer was 0.0044 pH unit · s−1 and in bicarbonate-free buffer was 0.0016 pH unit · s−1.
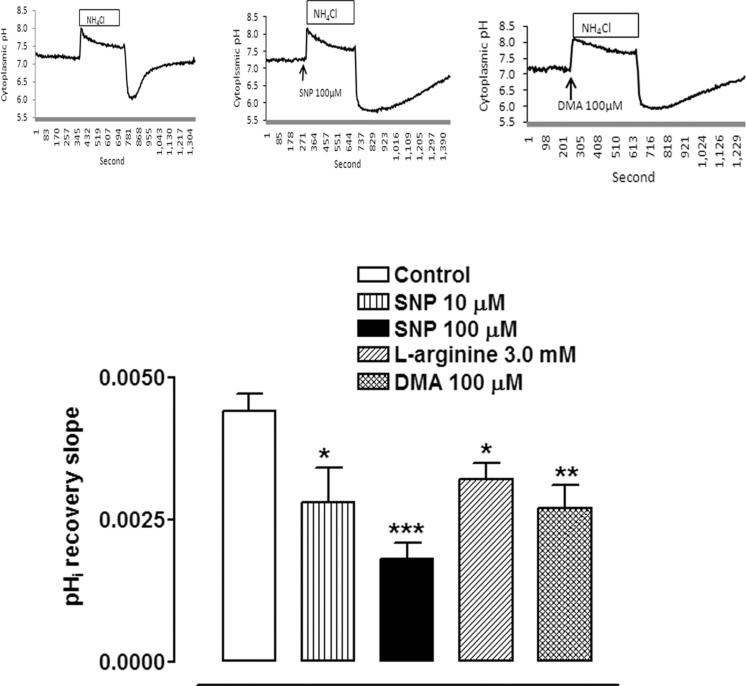
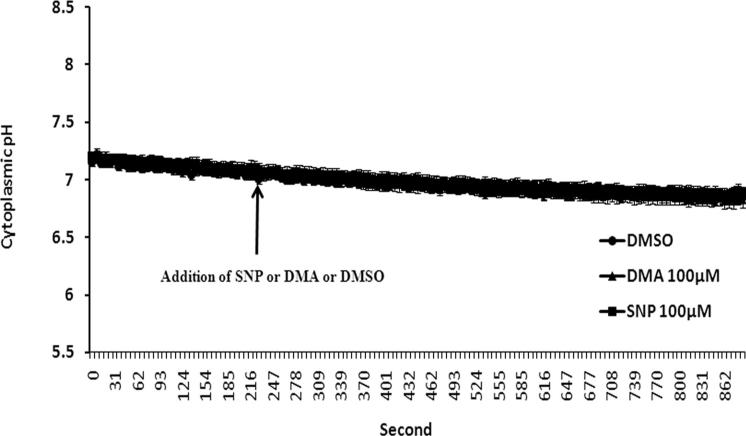
Some cells in bicarbonate/CO2 buffer were exposed to l-arginine, the physiological substrate for nitric oxide synthase, added 5 minutes before ammonium chloride removal. In the presence of 3.0 mM l-arginine the rate of pHi recovery was reduced by approximately 30% (Fig. 1). The rate of pHi recovery also was significantly reduced in the presence of sodium nitroprusside (SNP), a well-known NO donor (Fig. 1). A typical response to SNP is shown in Figure 1. At a concentration of 100 μM, SNP more than halved the rate of pHi recovery. In a few cells, SNP or l-arginine caused a small detectable reduction in baseline pHi. However, not all cells responded and for pooled data there was no significant change in mean baseline pHi either in bicarbonate buffer (data not shown) or in bicarbonate-free HEPES buffer (Fig. 2).
Figure 1.
The effect of SNP (10 or 100 μM) or l-arginine (3 mM) or DMA (100 μM) on the rate of pHi recovery toward baseline after the rapid decrease in pHi caused by the removal of ammonium chloride. SNP or l-arginine or DMA was added 5 minutes before ammonium chloride removal. The results are the mean ± SEM of data from 6 or 13 independent experiments. A significant difference from control is indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. Insets: typical experiments of control, and DMA- and SNP-treated cells.
Figure 2.
The effect of SNP or DMA on baseline cytoplasmic pH in bicarbonate-free HEPES buffer. The cells were loaded with BCECF superfused with bicarbonate-free HEPES buffer, and baseline pHi was recorded for 5 minutes. Then, 100 μM DMA or 100 μM SNP was added to the superperfusate. Control cells received an equivalent amount of DMSO. Results are shown as the mean ± SEM of five to seven individual experiments. The slow, continuous drift of baseline pHi is probably due to dye bleaching.
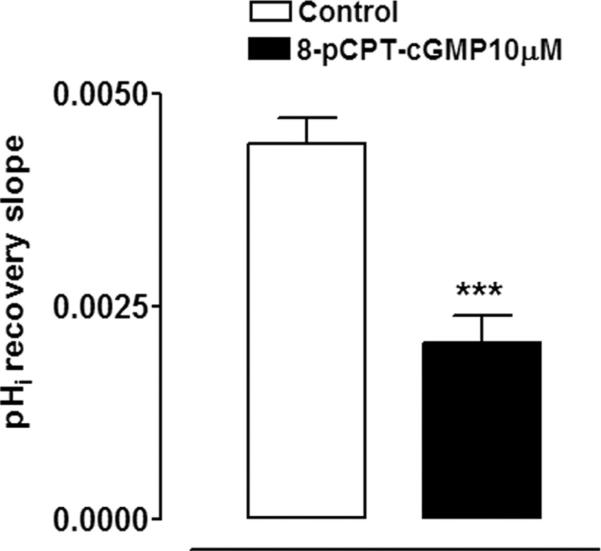
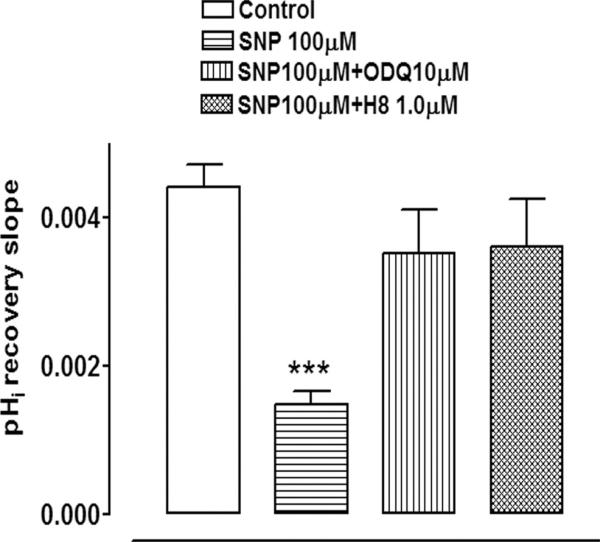
Since NO is reported to activate soluble guanylate cyclase and thus increase cGMP, studies were conducted to test whether rate of pHi recovery is altered by 8-pCPT c-GMP, a cell permeable and phosphodiesterase-resistant cGMP analogue. In the presence of 10 μM 8-pCPT c-GMP, added 5 minutes before ammonium chloride removal, the rate of pHi recovery observed on ammonium chloride removal was reduced by >50% (Fig. 3). The findings support the notion that the diminished rate of pHi recovery in SNP-treated cells could be dependent on an increase of cGMP. To further test this idea, some cells were exposed to SNP in the presence of ODQ, a soluble guanylate cyclase inhibitor. ODQ (10 μM) abolished the inhibitory effect of SNP on the rate of pHi recovery (Fig. 4).
Figure 3.
The effect of 10 μM 8-pCPT-cGMP on the rate of pHi recovery after removal of ammonium chloride. 8-pCPT-cGMP was added 5 minutes before the removal of ammonium chloride. The results are the mean ± SEM of data from 5 or 13 independent experiments. ***Significant difference from the control (P < 0.001).
Figure 4.
The effect of SNP (100 μM) on the rate of pHi recovery after the removal of ammonium chloride in the presence of either ODQ (10 μM), an inhibitor of soluble guanylate cyclase, or H8 (1 μM), a protein kinase G inhibitor. SNP, ODQ, or H8 was added 5 minutes before the removal of ammonium chloride. The results are the mean ± SEM of data from 6 or 13 independent experiments. ***Significant difference from the control (P < 0.001).
The findings point to a role for cGMP in the response of the cultured NPE to SNP. For this reason, studies were conducted with H-8, a specific inhibitor of the well-known cGMP-activated protein kinase, protein kinase G. At a concentration of 1 μM, H8 effectively blocked the SNP-induced inhibition of pHi recovery (Fig. 4).
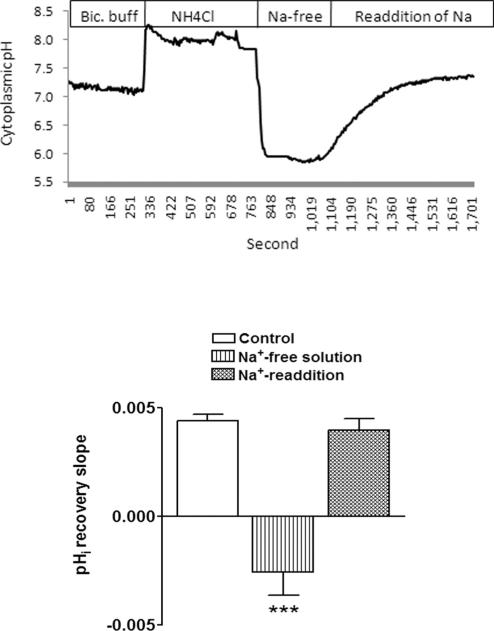
Studies were conducted to examine the mechanism responsible for pHi recovery. In sodium-free bathing solution, pHi after ammonium chloride removal caused cytoplasmic acidification, but pHi recovery did not occur. On readdition of sodium the rate of recovery returned to normal (Fig. 5). The potent NHE inhibitor, dimethylamiloride (DMA), reduced the rate of pHi recovery from 0.0044 to 0.0027 pH unit · s−1 (P > 0.01). When SNP and DMA were added together, the rate of pHi recovery was 0.0021 ± 0.0002 pH unit · s−1 which was not significantly different from the rate measured in either DMA or SNP alone. To examine the DMA-resistant component, some cells were exposed to the anion transport inhibitor DIDS (100 μM) together with DMA. DIDS + DMA reduced the recovery rate almost to zero (0.0004 ± 0.0002; Table 1). DIDS also inhibited the component of pHi recovery that persisted in the presence of SNP. DIDS+SNP reduced the recovery rate to 0.0008 ± 0.0002 (Table 1). The findings suggest bicarbonate transporters could contribute to pHi recovery, and, in keeping with this notion, bicarbonate-free buffer reduced pHi recovery to ~36% of the control rate (Table 1). In bicarbonate-free conditions, when NHE is likely to be the main mechanism responsible for pHi recovery, SNP and DMA inhibited the rate of pHi recovery to a similar degree. Of importance, in the presence of 100 μM DMA, the exposure of the cells to 100 μM SNP caused no further detectable reduction in the rate of pHi recovery (Table 1).
Figure 5.
The influence of sodium-free buffer on the rate of pHi recovery after the removal of ammonium chloride. Replacement of ammonium chloride with a sodium-free buffer acidified the cells but did not permit pHi recovery toward baseline. pHi recovery was observed on readdition of sodium-containing buffer. Result shows the mean ± SEM of data from 6 or 10 independent experiments. ***Significant difference from the control (P < 0.001). Inset: a typical record of pHi measured in porcine NPE cells subjected to removal and readdition of sodium from the bathing solution.
Table 1.
The Influence of 100 μM SNP, DMA, and DIDS on the Rate of Cytoplasmic pH Recovery in Bicarbonate-Containing and Bicarbonate-Free Buffers
| Drug | pHi Recovery (pH unit · s–1) | n | Significance of Difference (P) |
|---|---|---|---|
| Bicarbonate | 0.0044 ± 0.0003 | 13 | – |
| Bicarbonate + SNP | 0.0020 ± 0.0002 | 7 | >0.001* |
| Bicarbonate + DMA | 0.0027 ± 0.0004 | 5 | >0.01* |
| Bicarbonate + DMA + SNP | 0.0021 ± 0.0002 | 9 | >0.001* |
| Bicarbonate + DIDS + SNP | 0.0008 ± 0.0002 | 5 | >0.001* |
| Bicarbonate + DIDS + DMA | 0.0004 ± 0.0002 | 6 | >0.001* |
| Bicarbonate-free | 0.0016 ± 0.0002 | 9 | >0.01* |
| Bicarbonate-free + SNP | 0.0006 ± 0.0002 | 7 | >0.001*† |
| Bicarbonate-free + DMA | 0.0006 ± 0.0002 | 3 | >0.001*† |
| Bicarbonate-free + SNP + DMA | 0.0007 ± 0.0001 | 10 | >0.001*† |
Significant difference from bicarbonate buffer.
Significant difference from bicarbonate-free buffer.
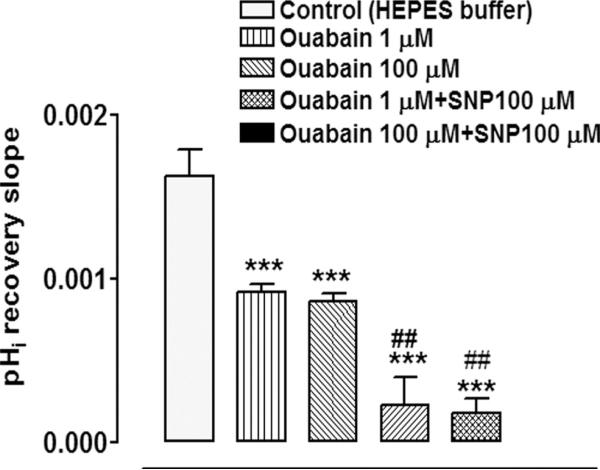
To examine the possible role of Na,K-ATPase-mediated sodium transport, some cells in bicarbonate-free buffer were exposed to the Na,K-ATPase inhibitor ouabain. Exposure to SNP in the presence of either 1 or 100 μM ouabain caused a significant decrease in the rate of pHi (Fig. 6). Added alone, ouabain reduced the rate of pHi recovery by approximately 50%.
Figure 6.
The effect of SNP (100 μM) on the rate of pHi recovery after the removal of ammonium chloride in the presence or absence of ouabain (1 or 100 μM), an Na,K-ATPase inhibitor. Ouabain was added 5 minutes before ammonium chloride removal and remained in the superfusate for the remainder of the experiment. Some cells received ouabain alone. The results are the mean ± SEM of data from 3 or 10 independent experiments. ***Significant difference from the control (P < 0.001); ##significant difference from either 1 or 100 μM ouabain alone (P < 0.01).
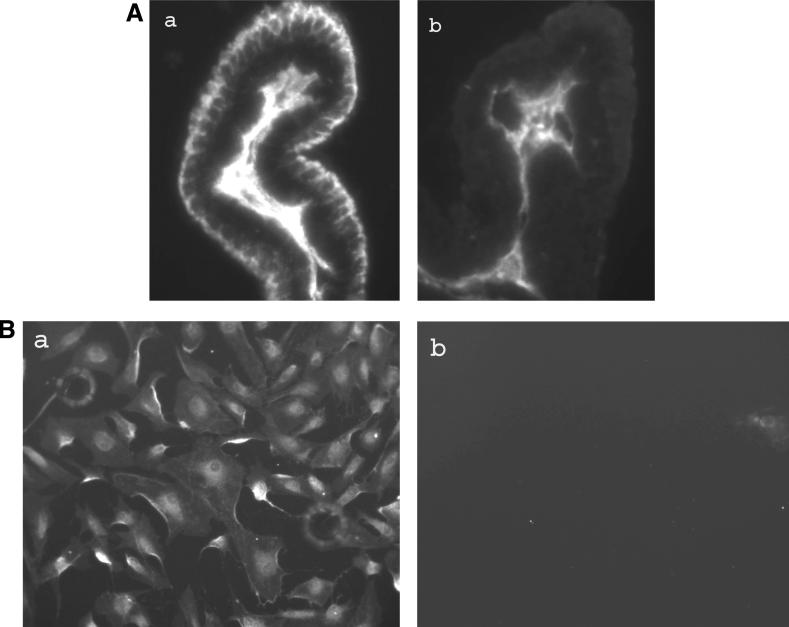
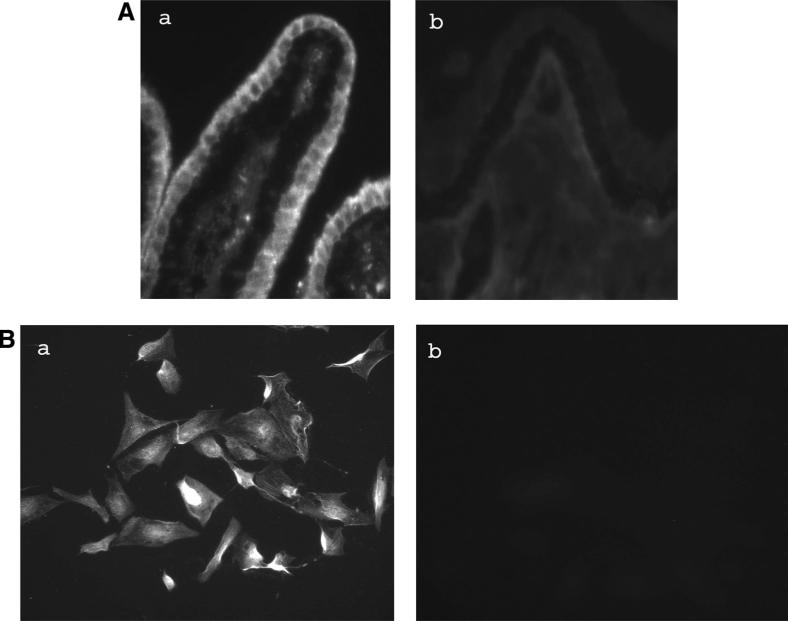
Immunolocalization studies were performed to probe for NHE1, -2, -3, and -4 in porcine ciliary processes. These are the NHE isoforms common in epithelial tissues. Two NHE iso-forms, NHE1 and -4, were detected in the NPE cell layer where staining appeared most abundant at the basolateral (aqueous humor-facing) surface, extending between adjacent cells almost to the NPE-PE junction (Figs. 7A, 8A). Although there is functional evidence for NHE in the PE,26,27 dense pigmentation tends to mask immunofluorescence, making it difficult to draw conclusions from Figures 7 and 8 regarding NHE localization in the PE layer. NHE1 and -4 also were detected in primary cultured NPE (Figs. 7B, 8B). NHE2 was not detected in either the native or cultured NPE. NHE3 immunolocalization results were difficult to interpret because of a high degree of nonspecific antibody binding.
Figure 7.
Immunolocalization of NHE1 in the porcine ciliary body where it appears at the basolateral surface of the NPE layer, extending along the lateral sides of the cells (A). The presence of pigment makes NHE difficult to detect in the PE layer. NHE1 also was detected in cultured NPE (B). The negative controls are ciliary processes (Ab) and cultured NPE (Bb) specimens, respectively, in which the primary antibody was replaced by PBS. Magnification, ×200.
Figure 8.
Immunolocalization of NHE4 in the porcine ciliary body where it appears at the basolateral surface of the NPE layer, extending along the lateral sides of the cells (A). The presence of pigment makes NHE difficult to detect in the PE layer. NHE4 also was detected in cultured NPE (B). The negative controls are ciliary processes (Ab) and cultured NPE (Bb) specimens, respectively, in which the primary antibody was replaced by PBS. Magnification, ×200.
Discussion
SNP causes inhibition of the rate of pHi recovery from an ammonium chloride-induced acid load. SNP is an NO donor and the response appears linked to NO since l-arginine, a NOS substrate, similarly inhibits pHi recovery. Earlier studies confirm NO generation in l-arginine-treated NPE, as evidenced by nitrite detection in the bathing medium.13 The ability of SNP and DMA, an NHE inhibitor, to decrease the rate of pHi recovery to a similar extent, points to the possible inhibition of NHE-mediated proton export in SNP-treated cells. Consistent with this line of thinking, SNP elicited no additional change of pHi recovery rate in cells that also were exposed to DMA in bicarbonate-free buffer. The findings fit reports in the kidney that NO causes functional NHE inhibition.20 Both NHE1 and -4 are expressed in porcine NPE, and it is not possible to specify whether the mechanisms respond differently to NO. In bicarbonate/CO2-rich buffer, SNP appeared to have a slightly larger effect than DMA, but the difference was not statistically significant. With bicarbonate present, the combined effect of SNP and DMA is equal to SNP alone. Although SNP has been reported to have an effect on bicarbonate transporters in kidney tubules28 the current data on NPE cells do not support the idea that SNP has an effect other than on NHE.
SNP-induced inhibition of pHi recovery was suppressed to a similar degree by ODQ, an inhibitor of soluble guanylate cyclase, and H8, an inhibitor of protein kinase G. Based on this evidence, we suggest generation of NO in SNP-treated cells causes activation of soluble guanylate cyclase, leading to a cGMP increase that activates protein kinase G which then elicits a reduction in NHE activity. It is noteworthy that cGMP-dependent inhibition of NHE has been reported under other conditions where generation of NO is not apparently stimulated.18 Consistent with the possible link between cGMP signaling and NHE inhibition observed in the present study, the pHi recovery response was significantly suppressed by 8-pCPT c-GMP which was added to the bathing medium to mimic a cytoplasmic cGMP increase. A similar cGMP signaling pathway has been determined to underlie NO response in a variety of tissues,21,29,30 including NPE.19
In NPE,13 ciliary body14 and choroid plexus23 NO-dependent Na,K-ATPase inhibition has been linked to a cGMP signaling pathway. Since NHE-mediated proton export derives its driving force from the plasma membrane sodium gradient, we considered the possibility that dissipation of the sodium gradient by NO-dependent Na,K-ATPase inhibition could underlie the observed inhibition of pHi recovery by SNP. This did not seem to be the case because inhibition of pHi recovery by SNP persisted in the presence of either 1 or 100 μM ouabain. At these ouabain concentrations, porcine Na,K-ATPase activity is abolished.
In various tissues, an increase in cGMP is elicited by atriopeptin or natriuretic peptide,31–33 arbachol,34,35 and shear stress.36–38 This raises the possibility of a variety of stimuli could potentially lead to inhibition of NHE function. In the intact eye, the significance of cGMP-mediated NHE inhibition in the NPE remains to be determined. It is known, however, that NHE inhibitors reduced IOP in mouse,22 and NO donors reduce IOP in normal39–41 and glaucomatous42 animals as well an in humans.43 There is direct evidence that NO donors SNP, SNAP, and l-arginine reduce aqueous formation.8 The evidence of an effect of NHE inhibitors DMA and EIPA on aqueous secretion is less direct,22 but McLaughlin et al.44 have reported clear effects of NHE inhibitors on the ciliary epithelial bilayer, as evidenced by changes in cytoplasmic ion levels detected by x-ray microanalysis.
Initially, NHE in the ciliary epithelium was thought to be localized to the basolateral surface of the PE layer.45 Later, McLaughlin et al.44 proposed functional NHE activity in both the PE the NPE layers. The NHE immunolocalization studies presented here provide the first direct evidence of the basolateral distribution of NHE in the NPE layer. NHE1 and -4 were detected at the aqueous-facing basolateral surface and extended between adjacent NPE cells. We detected no NHE immunostaining between adjacent cells in the PE layer. However, heavy pigmentation in these cells is likely to have masked fluorescence. The NHE gene family comprises nine members, designated NHE1 to -9,46,47 of which four isoforms (NHE1 to -4) are found in mammalian epithelial tissues.48,49 NHE1 is expressed ubiquitously in all mammalian cells and plays a housekeeping role in pH and cell volume regulation. We found abundant NHE1 and -4 at the basolateral aspect of the NPE, and this region thus appears specialized in terms of Na-H exchange. McLaughlin et al.44 suggest that this NHE pool could be used for vectorial ion transport that supports fluid movement in an aqueous-to-blood direction. If this was the case, NHE inhibition would tend to increase AH formation. It is also possible that the highly metabolic NPE cells require NHE mainly for the purpose of regulating pHi, on which basis reduced NHE activity might impair many aspects of NPE function. It should be understood that NHE is just one of several different mechanisms responsible to pHi homeostasis. The present data also show a very substantial bicarbonate-and DIDS-sensitive component of pHi recovery in porcine NPE. This may explain why, in the present studies, mean baseline pHi was not significantly altered by SNP or l-arginine, even though there is evidence of NHE inhibition. In many cells, including the NPE,24 NHE-mediated proton export acts mainly contribute to the process of recovery from large acid swings of pHi, whereas bicarbonate transporters cope with smaller pHi shifts.
It is interesting to note that in bicarbonate-rich buffer, the absolute rate of DMA-sensitive pHi change is greater than in bicarbonate-free medium. The additional buffering capacity in bicarbonate solution means that any given pHi change signifies a greater proton export compared to the same pH change in bicarbonate-free solution. Thus, the measured differences in pHi recovery rate underestimate the difference in DMA-sensitive proton flux in bicarbonate-containing and bicarbonate-free buffer. The results suggest that NHE-mediated transport activity is markedly diminished by elimination of bicarbonate buffer. It is also notable that bicarbonate-free conditions are nonphysiological.
Although we cannot be certain that the findings in the primary culture NPE would be the same as in native NPE, the cultured NPE displays many characteristics of native tissue. So far, we have shown that ~20 key native proteins, including those known to be involved in aqueous humor secretion, are expressed by primary cultured NPE. Some of these findings have recently been published.12,24,50 The cultured NPE cells also form confluent monolayers and express tight junction proteins occluding and ZO-1 at their contact points.12 Our unpublished data show that when NPE is cultured on permeable support, the cells form a tight monolayer that restricts fluorescein dextran (MW 10 kDa) and has a resistance of 99.1 ± 5.8 Ω · cm2 (n = 25). The observed resistance compares well with values reported for other ciliary body preparations: rabbit ciliary epithelial bilayer (46 Ω · cm2),51 shark ciliary body (50 Ω · cm2),52 and bovine ciliary body (~100 Ω · cm2).53
In summary, we present evidence of robust expression of NHE at the basolateral margin of the ciliary body NPE layer. The findings point to inhibition of NHE activity by NO which acts via a cGMP and protein kinase G–signaling pathway. Since eNOS, nNOS, and iNOS are abundant in the porcine NPE,12 NO could potentially act as an endogenous regulator of NHE activity. The NHE response does not appear to be the consequence of NO-induced Na,K-ATPase inhibition. Although NHE inhibitors are known to lower IOP, further studies are needed if we are to understand whether changes of NHE activity in the NPE contribute mechanistically to the IOP-lowering effect of NO donors.
Acknowledgments
The authors thank Ryan Pelis for helpful discussion.
Supported by National Institutes of Health Grant EY006915.
Footnotes
Disclosure: M. Shahidullah, None; A. Mandal, None; N.A. Delamere, None
References
- 1.Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978;17:958–981. [PubMed] [Google Scholar]
- 2.Edelman JL, Sachs G, Adorante JS. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. Am J Physiol. 1994;266:C1210–C1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]
- 3.Burstein NL, Fischbarg J, Liebovitch L, Cole DF. Electrical potential, resistance, and fluid secretion across isolated ciliary body. Exp Eye Res. 1984;39:771–779. doi: 10.1016/0014-4835(84)90076-9. [DOI] [PubMed] [Google Scholar]
- 4.Jacob TJ, Civan MM. Role of ion channels in aqueous humor formation. Am J Physiol. 1996;271:C703–C720. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- 5.Civan MM. The Eye's Aqueous Humor: from Secretion to Glaucoma. Academic Press; San Diego, CA: 1998. Transport components of net secretion of the aqueous humour and their integrated regulation. pp. 1–24. [Google Scholar]
- 6.To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optom. 2002;85:335–349. [PubMed] [Google Scholar]
- 7.Millar JC, Shahidullah M, Wilson WS. Intraocular pressure and vascular effects of sodium azide in bovine perfused eye. J Ocul Pharmacol Therapeut. 2001;17:225–234. doi: 10.1089/108076801750295263. [DOI] [PubMed] [Google Scholar]
- 8.Shahidullah M, Yap MK, To CH. cGMP, sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br J Pharmacol. 2005;144:1–9. doi: 10.1038/sj.bjp.0706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection: how, why, when, and where? Nitric Oxide. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 10.Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog Hormone Res. 1998;53:43–59. discussion 59 – 60. [PubMed] [Google Scholar]
- 11.Meyer P, Champion C, Schlotzer-Schrehardt U, Flammer J, Haefliger IO. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999;18:375–380. doi: 10.1076/ceyr.18.5.375.5355. [DOI] [PubMed] [Google Scholar]
- 12.Shahidullah M, Tamiya S, Delamere NA. Primary culture of porcine nonpigmented ciliary epithelium. Curr Eye Res. 2007;32:511–522. doi: 10.1080/02713680701434899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the non-pigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis DZ, Nathanson JA, Rabe J, Sweadner KJ. Carbachol and nitric oxide inhibition of Na,K-ATPase activity in bovine ciliary processes. Invest Ophthalmol Vis Sci. 2001;42:2625–2631. [PubMed] [Google Scholar]
- 15.Kotikoski H, Kankuri E, Vapaatalo H. Incubation of porcine iris-ciliary bodies to study the mechanisms by which nitric oxide donors lower intraocular pressure. Med Sci Moni. 2003;9:BR1–BR7. [PubMed] [Google Scholar]
- 16.Feelisch M, Noack EA. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- 17.Murad F, Mittal CK, Arnold WP, Katsuki S, Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- 18.Crook RB, Chang AT. Differential regulation of natriuretic peptide receptors on ciliary body epithelial cells. Biochem J. 1997;324:49–55. doi: 10.1042/bj3240049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fidzinski P, Salvador-Silva M, Choritz L, Geibel J, Coca-Prados M. Inhibition of NHE-1 Na+/H+ exchanger by natriuretic peptides in ocular nonpigmented ciliary epithelium. Am J Physiol Cell Physiol. 2004;287:C655–C663. doi: 10.1152/ajpcell.00552.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Fluid Electrolyte Physiol. 2002;282:F777–F784. doi: 10.1152/ajprenal.00334.2001. [DOI] [PubMed] [Google Scholar]
- 21.Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation. 1997;95:2303–2311. doi: 10.1161/01.cir.95.9.2303. [DOI] [PubMed] [Google Scholar]
- 22.Avila MY, Seidler RW, Stone RA, Civan MM. Inhibitors of NHE-1 Na+/H+ exchange reduce mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:1897–1902. [PubMed] [Google Scholar]
- 23.Ellis DZ, Nathanson JA, Sweadner KJ. Carbachol inhibits Na(+)-K(+)-ATPase activity in choroid plexus via stimulation of the NO/cGMP pathway. Am J Physiol Cell Physiol. 2000;279:C1685–C1693. doi: 10.1152/ajpcell.2000.279.6.C1685. [DOI] [PubMed] [Google Scholar]
- 24.Shahidullah M, To C-H, Pelis RM, Delamere NA. Studies on bicarbonate transporters and carbonic anhydrase in porcine nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 2009;50:1791–1800. doi: 10.1167/iovs.08-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou Y, Delamere NA. Influence of ANG II on cytoplasmic sodium in cultured rabbit nonpigmented ciliary epithelium. Am J Physiol Cell Physiol. 2002;283:C552–C559. doi: 10.1152/ajpcell.00459.2001. [DOI] [PubMed] [Google Scholar]
- 26.Helbig H, Korbmacher C, Berweck S, Kuhner D, Wiederholt M. Kinetic properties of Na+/H+ exchange in cultured bovine pigmented ciliary epithelial cells. Pflugers Archiv Eur J Physiol. 1988;412:80–85. doi: 10.1007/BF00583734. [DOI] [PubMed] [Google Scholar]
- 27.Helbig H, Korbmacher C, Stumpff F, Coca-Prados M, Wiederholt M. Na+/H+ exchange regulates intracellular pH in a cell clone derived from bovine pigmented ciliary epithelium. J Cell Physiol. 1988;137:384–389. doi: 10.1002/jcp.1041370225. [DOI] [PubMed] [Google Scholar]
- 28.Wang T. Nitric oxide regulates HCO3- and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. Am J Physiol Renal Physiol. 1997;272:F242–F248. doi: 10.1152/ajprenal.1997.272.2.F242. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Guo ZG. Nitric oxide derived from endothelial cells inhibits Na+/H+ exchange in rabbit platelets activated by thrombin. Zhongguo Yao Li Xue Bao/Acta Pharmacol Sinica. 1999;20:333–337. [PubMed] [Google Scholar]
- 30.Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol Renal Physiol. 1999;277:F377–F382. doi: 10.1152/ajprenal.1999.277.3.F377. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Levine YC, Golan DE, Michel T, Lin AJ. Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J Biol Chem. 2008;283:4439–4447. doi: 10.1074/jbc.M709439200. [DOI] [PubMed] [Google Scholar]
- 32.Diederen RMH, La Heij EC, Markerink-van Ittersum M, Hendrikse F, de Vente J. Two autocrine pathways to regulate cyclic GMP synthesis in cultured human retinal pigment epithelial cells. Ophthalmic Res. 2008;40:227–234. doi: 10.1159/000127829. [DOI] [PubMed] [Google Scholar]
- 33.Herring N, Zaman JA, Paterson DJ. Natriuretic peptides like NO facilitate cardiac vagal neurotransmission and bradycardia via a cGMP pathway. Am J Physiol Heart Circulatory Physiol. 2001;281:H2318–H2327. doi: 10.1152/ajpheart.2001.281.6.H2318. [DOI] [PubMed] [Google Scholar]
- 34.Borda E, Berra A, Saravia M, Ganzinelli S, Sterin-Borda L. Correlations between neuronal nitric oxide synthase and muscarinic M3/M1 receptors in the rat retina. Exp Eye Res. 2005;80:391–399. doi: 10.1016/j.exer.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Orman B, Reina S, Borda E, Sterin-Borda L. Signal transduction underlying carbachol-induced PGE2 generation and cox-1 mRNA expression of rat brain. Neuropharmacology. 2005;48:757–765. doi: 10.1016/j.neuropharm.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Dusserre N, L'Heureux N, Bell KS, et al. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arteriosclerosis Thrombosis Vasc Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 37.Hillsley MV, Tarbell JM. Oscillatory shear alters endothelial hydraulic conductivity and nitric oxide levels. Biochem Biophys Res Commun. 2002;293:1466–1471. doi: 10.1016/S0006-291X(02)00410-2. [DOI] [PubMed] [Google Scholar]
- 38.Qian X-x, Chen Y-m, Wu W-k, et al. Effects of external counter-pulsation on shear stress and production of nitric oxide and cGMP in canines with myocardial infarction (in Chinese). Nan Fang Yi Ke Da Xue Xue Bao/J Southern Med Univ. 2006;26:1003–1005. [PubMed] [Google Scholar]
- 39.Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996;37:1711–1715. [PubMed] [Google Scholar]
- 40.Kotikoski H, Vapaatalo H, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003;26:119–123. doi: 10.1076/ceyr.26.2.119.14511. [DOI] [PubMed] [Google Scholar]
- 41.Nathanson JA. Nitrovasodilators as a new class of ocular hypotensive agents. J Pharmacol Exp Therapeut. 1992;260:956–965. [PubMed] [Google Scholar]
- 42.Wang RF, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995;60:337–339. doi: 10.1016/s0014-4835(05)80116-2. [DOI] [PubMed] [Google Scholar]
- 43.Chuman H, Chuman T, Nao-i N, Sawada A. The effect of L-arginine on intraocular pressure in the human eye. Curr Eye Res. 2000;20:511–516. [PubMed] [Google Scholar]
- 44.McLaughlin CW, Zellhuber-McMillan S, Macknight ADC, Civan MM. Electron microprobe analysis of rabbit ciliary epithelium indicates enhanced secretion posteriorly and enhanced absorption anteriorly. Am J Physiol Cell Physiol. 2007;293:C1455–C1466. doi: 10.1152/ajpcell.00205.2007. [DOI] [PubMed] [Google Scholar]
- 45.Counillon L, Touret N, Bidet M, et al. Na+/H+ and CI-/HCO3-antiporters of bovine pigmented ciliary epithelial cells. Pflugers Archiv Eur J Physiol. 2000;440:667–678. doi: 10.1007/s004240000302. [DOI] [PubMed] [Google Scholar]
- 46.Fliegel L. The Na+/H+ exchanger isoform 1. Int J Biochem Cell Biol. 2005;37:33–37. doi: 10.1016/j.biocel.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Ann Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 48.Yun CH, Tse CM, Donowitz M. Chimeric Na+/H+ exchangers: an epithelial membrane-bound N-terminal domain requires an epithelial cytoplasmic C-terminal domain for regulation by protein kinases. Proc Natl Acad Sci U S A. 1995;92:10723–10727. doi: 10.1073/pnas.92.23.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol. 1995;269:G1–G11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- 50.Pelis RM, Shahidullah M, Ghosh S, Coca-Prados M, Wright SH, Delamere NA. Localization of multidrug resistance-associated protein 2 (MRP2) in the non-pigmented ciliary epithelium of the eye. J Pharmacol Exp Ther. 2009 May;329(2):479–485. doi: 10.1124/jpet.108.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui H, Murakami M, Wynns GC, et al. Membrane carbonic anhydrase (IV) and ciliary epithelium: carbonic anhydrase activity is present in the basolateral membranes of the non-pigmented ciliary epithelium of rabbit eyes. Exp Eye Res. 1996;62:409–417. doi: 10.1006/exer.1996.0046. [DOI] [PubMed] [Google Scholar]
- 52.Wiederholt M, Flugel C, Lutjen-Drecoll E, Zadunaisky JA. Mechanically stripped pigmented and non-pigmented epithelium of the shark ciliary body: morphology and transepithelial electrical properties. Exp Eye Res. 1989;49:1031–1043. doi: 10.1016/s0014-4835(89)80024-7. [DOI] [PubMed] [Google Scholar]
- 53.To CH, Do CW, Zamudio AC, Candia OA. Model of ionic transport for bovine ciliary epithelium: effects of acetazolamide and HCO. Am J Physiol Cell Physiol. 2001;280:C1521–C1530. doi: 10.1152/ajpcell.2001.280.6.C1521. [DOI] [PubMed] [Google Scholar]