Abstract
We conducted a prospective, multicenter investigation of human-leukocyte antigen (HLA) identical sibling bone marrow transplantation (BMT) in children with severe sickle cell disease (SCD) between 1991 and 2000. To determine if children were protected from complications of SCD after successful BMT, we extended our initial study of BMT for SCD to conduct assessments of the central nervous system (CNS) and of pulmonary function 2 or more years after transplantation. In addition, the impact on gonadal function was studied. After BMT, patients with stroke who had stable engraftment of donor cells experienced no subsequent stroke events after BMT, and brain magnetic resonance imaging (MRI) exams demonstrated stable or improved appearance. However, 2 patients with graft rejection had a second stroke after BMT. After transplantation, most patients also had unchanged or improved pulmonary function. Among the 11 patients who had restrictive lung changes at baseline, 5 were improved and 6 had persistent restrictive disease after BMT. Of the 2 patients who had obstructive changes at baseline, 1 improved and 1 had worsened obstructive disease after BMT. There was, however, significant gonadal toxicity after BMT, particularly among female recipients. In summary, individuals who had stable donor engraftment did not experience sickle-related complications after BMT, and were protected from progressive CNS and pulmonary disease.
Keywords: Sickle cell anemia, Bone marrow transplantation, Long-term follow-up
INTRODUCTION
More than a decade ago, several groups showed in prospective clinical trials that it was possible to eliminate sickle erythropoiesis by human leukocyte antigen (HLA)- identical bone marrow transplantation (BMT) in symptomatic children [1–3]. After establishing a high probability of event-free survival (EFS) in most children after HLA-identical sibling donor BMT, the research focus appropriately has shifted to characterizing the quality of cure produced by successful transplantation and to expand the availability of transplantation to those who lack sibling donors. Previously, we showed that children who had stable engraftment of donor cells no longer experienced sickle-related clinical events after transplantation, and also that stable donor mixed chimerism was sufficient for this clinical benefit [4]. We have extended these initial findings by conducting routine evaluations of organ function after BMT that might detect subclinical evidence of progressive organ damage. In addition, these studies have generated longitudinal information based on extended follow-up about the toxicity of transplantation. Together, these assessments offer reassurance about the durable benefits of transplantation in children who have sickle cell disease (SCD) and more clearly define the long-term toxicities. The findings also provide additional information to families and their physicians about the long-term risks and benefits of this therapeutic option for children with SCD.
METHODS
Patients <16 years of age with symptomatic SCD (SS, SC, or Sβ°-thalassemia) who had an HLA-identical family member donor (Hb, AA, or AS) were considered for BMT. All individuals were required to meet eligibility criteria as reported earlier [1]. The initial study design included follow-up through 2 years after enrollment. A supplemental long-term follow-up study was initiated after patient accrual was completed in 2000. This extended study collected additional results of the long-term impact of transplantation 2 years and longer after initial enrollment, as described later. Patients were enrolled from 27 centers in the United States and Europe (see the Appendix for collaborating centers). The study was approved by the institutional review board of Children’s Hospital and Research Center, Oakland, and by institutional review boards or their equivalents at each of the collaborating sites. All patients and/or their parents or guardians gave written informed consent for their participation.
The National Heart, Lung, and Blood Institute appointed a data safety and monitoring board (DSMB) for the purpose of monitoring patient safety and the ethical conduct and progress of this investigation. The board consisted of 5 hematologists, a clinical statistician, and a patient advocate.
Treatment Regimen
Patients were prepared for transplantation with a combination of oral busulfan (Bu, 14 mg/kg), cyclophosphamide (Cy, 200 mg/kg) and horse antithymocyte globulin (ATG, 90 mg/kg) or CAM-PATH Immunoglobulin (10 mg for 5 days) in lieu of ATG.
Three patients (patients 9–11) received Bu (500 mg/m2), Cy (200 mg/kg), and rabbit ATG (20 mg/kg), and another (patient 6) received Bu (16 mg/kg), Cy (200 mg/kg), and ATG (80 mg/kg), as previously reported [1]. After November 1994, all North American patients had Bu pharmacokinetics performed with targeted steady-state concentrations adjusted to 400–600 ng/mL. Patients received a combination of methotrexate (MTX) and cyclosporine (CsA) for the prevention of acute graft-versus-host disease (aGVHD). Prophylaxis with CsA was given for 6 months following transplantation. Definition and grading of aGVHD and chronic GVHD (cGVHD) have been described [5,6]. Before transplantation, patients not receiving chronic transfusion therapy underwent a partial exchange transfusion to achieve a fraction of Hb S ≤30%. All patients received supportive care for infection prevention after transplantation, according to local institutional practice. In response to an apparent increased incidence of neurologic complications after transplantation [7,8], preventive measures were employed since June, 1993: anticonvulsant prophylaxis with phenytoin initiated with Bu dosing and continued for 6 months following transplantation (or until CsA was discontinued), strict control of hypertension, prompt repletion of magnesium deficiency, and maintenance of hemoglobin concentrations between 9 and 11 g/dL and platelet counts >50,000/mm3, as previously described.
Late Effects Evaluation
All 55 long-term survivors had late effects evaluations performed. Brain magnetic resonance imaging (MRI) examinations were requested in all patients before, 1, and 2 years after transplantation, and reports by the local radiologist and the MR images were collected centrally. The MRI examinations were performed for follow-up purposes only, and were not obtained in response to new neurologic examination findings or clinical complications. The local radiology reports were compiled to create a summary of the posttransplantation central nervous system (CNS) outcomes for this analysis. In the long-term follow-up phase of the study, brain MRI exams were requested through 4 years after transplantation. To test the validity of the local MRI reports submitted by individual participating centers, a subset of 7 brain MRI pre-and post-BMT exams was evaluated in a blinded fashion by 1 neuroradiologist (J.B.). This blinded assessment included evaluation of the size (in millimeters), location, and number of cortical and white matter abnormalities, and whether these had changed since the prior scans. The scans had axial T1-weighted and T2-weighted (intermediate echo and long echo) images through the entire brain. The lesions were bright on T2-weighted images and dark on T1-weighted images compared to normal brain. For each set of images, it was determined if the lesions were new or old and, if old, whether they were larger than on the prior study. The signal intensity and degenerative character of any abnormalities were also noted, and the scoring was compiled using a standardized worksheet to generate an overall comparison of the blinded pre- and posttransplant MRI exams. An examination was scored as improved if the number of lesions was fewer and/or if the diameter of the cortical or white matter abnormalities was smaller compared to the baseline examination.
Similarly, pulmonary function tests (PFTs; total lung capacity [TLC], forced vital capacity [FVC], residual volume [RV], and the ratio of forced expiratory volume to FVC [FEV/FVC]) were measured before and at annual intervals after transplantation. Spirometry and/or lung volumes (either by Nitrogen washout and/or by body plethysmography) were measured. Diffusion capacity was measured in 14 patients by the single-breath diffusion technique. PFT usage was based upon common methods for comparison of reference values [9]. The raw data were converted to a single set of predicted values according to the method by Polgar [10]. The following parameters were analyzed: FVC, FEV1, FEV1%, TLC, RV, and carbon monoxide diffusing capacity (DLCO).
PFT results were segregated into 3 distinct categories of lung function: restrictive, obstructive, and normal. Two standards were considered in defining these categories: the American Thoracic Society (ATS) 2007 guideline suggests that restrictive disease is defined by having a TLC <80%, or by having an FVC <75% if no TLC result is available. Obstructive disease is defined by having a FEV1/FVC ratio <80%. Lacking any accepted parameters for significant change after BMT, we made the assumption that a change was significant if it exceeded the percent predicted measure by >10%, a magnitude of change that is not likely to be caused by measurement variability, according to current published guidelines [11–13].
Finally, endocrine function tests (thyroid function tests and luteinizing hormone [LH], follicle stimulating hormone [FSH], and estradiol or testosterone assays) in serum were measured before and at annual intervals after transplantation.
Statistical Analysis
Statistical analyses were performed to summarize results. The method of Kaplan and Meier was used to estimate survival and EFS (where an event was defined as death, graft rejection, or return of SCD) [14]. A cumulative incidence curve for graft rejection was also calculated [15]. EFS was defined as survival in the absence of clinical vasoocclusive complications typical of SCD with evidence of sustained donor engraftment. Student’s t-test was applied to each of 5 PFT variables to evaluate for any significant changes after BMT. Descriptive statistics were computed for each pulmonary function variable.
RESULTS
Outcome after Transplantation
The outcome after HLA-identical sibling BMT has been updated from previous reports [4]. Fifty-seven children with SCD (Hb SS), 1 with sickle β+-thalassemia and 1 with sickle/O-Arab disease from 27 transplant centers in the United States, South America, and Europe were enrolled in this collaborative study between September 1991 and April 2000, 3 of whom were included in a previous publication [3]. Fifty-five patients survive, and 50 survive free of the underlying disease. Four patients died of transplantation-related complications that included intracranial hemorrhage or complications related to GVHD. The median times to engraftment with an absolute neutrophil count (ANC) >500/mm3 and platelet count >20,000/mm3 were 19 and 25 days, respectively. Five patients experienced graft rejection with recurrent SCD. The Kaplan-Meier probabilities of survival and EFS are 93% and 85%, respectively, with a median follow-up of 6.5 years (range: 3.0–12.4) (Figure 1). The cumulative incidence of recurrent SCD was 9%. Most patients had stable engraftment with >95% donor cells after BMT. However, 8 patients (15%) had <95% donor cells, of whom 6 had ≤70 % donor cells (Table 1) in this update of an earlier report [16]. None of these 6 patients experienced sickle-related complications after BMT and 1 patient received a single red blood cell (RBC) transfusion beyond 90 days after transplantation. Long-term follow-up evaluations were performed in all survivors, including 4 patients who had graft rejection accompanied by return of SCD and patients with stable mixed chimerism. Engrafted patients were defined as having full donor chimerism or having stable persistence of donor cells sufficient to eliminate clinical and laboratory signs of SCD.
Figure 1.
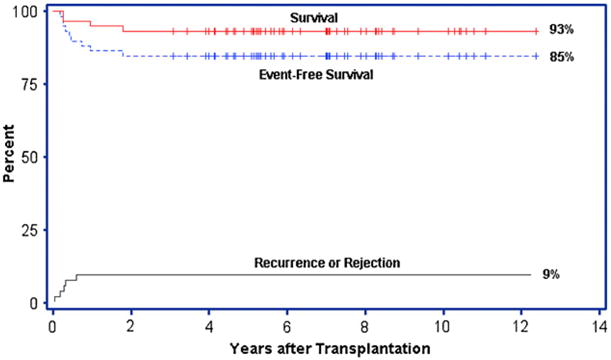
Survival probabilities and the cumulative incidence of graft rejection after BMT for sickle cell disease. Kaplan-Meier probabilities for survival and EFS after BMT for SCD are shown. An event was defined as death, graft rejection or return of SCD. A cumulative incidence curve for graft rejection/disease recurrence is also depicted.
Table 1.
Characteristics of Patients Who Had Stable Mixed Chimerism (≤70% Donor Chimerism) after BMT for Sickle Cell Disease
| Patient No. | % Donor | %S | Hgb | HCT | Txn > D+90 | Sickle Symptoms |
|---|---|---|---|---|---|---|
| #13 | 13% | 6% | 10.8 | 33 | Yes | No |
| #16 | 70% | 2% | 13.1 | 39 | No | No |
| #18 | 60% | 0 | 16.1 | 48 | No | No |
| #38* | 20–30% | 36.3% | 11.6 | 33.4 | No | No |
| #49 | 62% | 0 | 11.3 | 33.6 | No | No |
| #52* | 55% | 39% | 11.7 | 34 | No | No |
HCT indicates hematocrit; Hgb, hemoglobin; S, hemoglobin S; Txn, RBC transfusion.
Hb AS (trait) donor.
Seven patients (12%) developed grades II-IV aGVHD after transplantation. The clinical grading, sites of involvement and treatment of GVHD are summarized in Table 2. Eight patients (14%) developed cGVHD that was limited in 4, and extensive in 4. Three patients with extensive cGVHD died of this complication, and the other patients with cGVHD recovered completely and are no longer receiving immunosuppressive therapy.
Table 2.
GVHD after HLA-Identical Sibling BMT for Sickle Cell Disease
| Patient No. | aGVHD (Grade/Site) | cGVHD (Grade/Site) | Treatment | Outcome |
|---|---|---|---|---|
| 1 | II/skin | Limited/skin | CsA/pred | resolved |
| 9 | III/skin | Extensive/lung, liver | CsA/Pred | Died of GVHD |
| 27 | None | Limited/skin | none | resolved |
| 30 | III/skin, GI, liver | Extensive/GI, liver | CsA/Pred/ATG/thalidomide | Died of GVHD |
| 34 | III/skin, GI, liver | Limited/oral | CsA/Pred | resolved |
| 35 | III/skin, GI, liver | Extensive/skin, liver, GI | CsA/tacrolimus/MMF/ATG/OKT3/sirolimus | resolved |
| 41 | III/skin | Extensive/skin, lung, oral | Tacrolimus/pred/MMF/azathioprine | Died of GVHD |
| 51 | II/skin | Limited/skin | CsA/pred | resolved |
aGVHD indicates acute graft-versus-host disease; ATG, antithymocyte globulin; BMT, bone marrow transplantation; cGVHD, chronic graft-versus-host disease; CsA, cyclosporine; GI, gastrointestinal; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; Pred, prednisone.
CNS Disease
Twenty-nine of 59 patients had stroke or other significant CNS disease as an indication for transplantation and 28 survive after BMT. Surviving patients with stroke who also had stable engraftment of donor cells (N = 25) experienced no subsequent stroke events after BMT. However, patients who had graft rejection after BMT were not protected from stroke. One patient with graft rejection experienced a second stroke when the Hb S fraction reached 60% after BMT and another patient with graft rejection had a subarachnoid hemorrhage after BMT. In total, 28 of 29 (97%) patients with stroke survive after transplantation, and 26 of 29 (90%) survive stroke-free after BMT.
Most patients who survived after BMT also had stabilization of the underlying cerebral vascular disease, as evaluated by brain imaging studies (Table 3). Forty-six of 55 surviving patients who were enrolled in the multicenter study had a brain MRI performed after transplantation. These studies were compared to pretransplantation baseline exams in all but 4 of the patients. Of the 28 patients with stroke studied after transplantation, except in the 2 patients with graft rejection noted earlier, there was stable appearance or lesions that had evolved to a smaller size by brain MRI, documented by studies that were performed a median of 3.2 (range: 0.6–7.3) years after BMT. Ten patients had evidence of silent cerebral infarction before transplantation. Of these 10, 8 patients had post-BMT studies performed a median of 1.7 (range: 0.5–4.9) years after BMT, which were stable in 3 patients and 4 patients had lesions that had evolved to a smaller size by brain MRI. In 1 patient who was enrolled for recurrent episodes of acute chest syndrome and who had silent cerebral infarction before BMT, additional small watershed lesions were noted 1 month after transplantation, but this individual had a stable brain appearance in subsequent exams through 5 years after BMT. There were no clinical strokes after BMT in this group.
Table 3.
Neurological Outcomes among Surviving Patients (N = 55)
| Pre-BMT Status with Duration Follow-up (median, Range) | Ischemic Stroke after BMT | Cerebral Hemorrhage (ICH) after BMT | Seizure after BMT | MRI Appearance after BMT (Median Duration, Range in Years after BMT) |
|---|---|---|---|---|
| “Clinical” stroke [n = 29] (7.0, 3.4–12.4 yrs) | N = 1 | N = 1 (SAH) | N = 7 | 27/28 studied had stable or improved MRI; 1 not studied (3.2 years, 0.6–7.3) |
| “silent” stroke [n = 10]* (6.7, 3.4–10.6 years) | N = 0 | N = 0 | N = 4 | All 8 studied had stable [4] or improved [4] MRI; 2 not studied (1.7 years, 0.6–4.9) |
| Normal (N = 16)† (6.6, 3.1–11.1 years) | N = 0 | N = 0 | N = 5 | All 10 studied had normal MRI; 6 not studied (3.1 years, 0.5–5.2) |
BMT indicates bone marrow transplantation; CNS, central nervous system; ICH, intracranial hemorrhage; MRI, magnetic resonance imaging; SAH, subarachnoid hemorrhage.
Including 4 with no baseline exam.
“Silent stroke” refers to individuals with MRI evidence of cerebrovascular disease without clinical manifestations.
Sixteen patients had no documented CNS disease before BMT (Table 3). All 10 studied in this group had normal MRI appearance a median of 3.1 (range: 0.5–5.2 years) after BMT. In the 7 cases for which a blinded adjudication was performed, the blinded assessments were concordant with the MRI reports submitted for review. Together, these observations show that most patients had stabilization of cerebral vasculopathy after BMT.
Initially in this cohort, seizures were observed commonly after BMT [7]. As a result, anticonvulsant therapy was routinely administered throughout the duration of CSP use to reduce the risk of seizures. Overall, 19 individuals had a neurogic event after transplantation, including 2 episodes of intracranial hemorrhage that was fatal in 1, a second thrombotic stroke after graft rejection, and 16 with seizures. These events occurred a median of 90 days after BMT (range: 6–1488 days). As noted previously, the episodes were associated with hypertension, thrombocytopenia, or relative polycythemia (hemoglobin level >11 g/dL), and seizures were commonly associated with absent or inadequate anticonvulsant blood levels. Generally, these occurred in the first 6 months after transplantation; however, seizures occurred >6 months after BMT in 2 patients with graft rejection and a history of stroke. After the routine institution of preventive guidelines to prevent neurologic events, 14 of 40 patients (35%) had events, although these were not associated with long-term sequelae, and there were no additional episodes of intracranial hemorrhage. Of interest, these events occurred at a similar frequency in patients who had or did not have preexisting neurovascular disease, as shown in Table 3.
Pulmonary Function after Transplantation
At the baseline, 25 of the 55 surviving patients had pulmonary function abnormalities, with restrictive pulmonary disease being the most frequent finding. Pulmonary function testing was performed in 45 of 55 (82%) surviving patients after BMT, and of these, 26 had baseline studies for comparing pre and post-BMT pulmonary function results (Table 4). Twenty-three patients had baseline and post-BMT evaluations that included spirometry measurements. Thirteen patients had diffusion capacity measured before and after BMT. Of the 10 patients who had no testing performed after BMT, 5 had normal pulmonary function before BMT, 4 had restrictive disease, and 1 had no testing before or after BMT. The longest interval between baseline and post-BMT pulmonary function testing was utilized for this analysis. The time interval to testing after BMT ranged from 0.2 to 10.6 years, with a median of 3.2 years.
Table 4.
Summary of Pulmonary Function Test (PFT) Results before and after BMT for SCD
| Patient No. | Baseline Pattern | Post-BMT | FVC Change† | FEV1/FVC Change† | Baseline DLCO % | Post-BMT DLCO | Years Post-BMT |
|---|---|---|---|---|---|---|---|
| 1 | R | R | +12% (58% → 70%) | +9% | 78 | 72 | 5 |
| 2 | N | N | +4% | +1% | 64 | 64 | 1 |
| 6 | N | N | +2% | −8% | 110 | 98 | 10 |
| 14 | N | N | +6% | +4% | 46 | 86 | 2 |
| 15 | R | N* | +12% (74% →86%) | −2% | 58 | 78 | 3 |
| 18 | R | R | +7% | −7% | — | — | 3 |
| 19 | N | N | −41% (126% →85%) | +4% | — | — | 3 |
| 21 | N | N | −2% | +4% | — | — | 5 |
| 23 | R | R | +2% | −39% (121% →82%) | 59 | 78 | 5 |
| 25 | N | R* | −13% (78% →65%) | +10% (87% →97%) | — | — | 1 |
| 27 | R | R | +6% | −14% (85% →69%) | — | — | 3 |
| 31 | R | R | −5% | +9% | — | — | 1 |
| 32 | O | O | −6% | −15% (66% →51%) | 90 | 99 | 6 |
| 33 | N | N | +23% (84% →107%) | +8% | 88 | 118 | 5 |
| 42 | R | N* | +27% (66% →93%) | −16% (100% →84%) | — | — | 1 |
| 44 | R | R | −6% | +4% | 116 | 79 | 1 |
| 45 | N | R* | −8% | −3% | 93 | 72 | 2 |
| 48 | N | N | +7% | +8% | — | — | 2 |
| 51 | O | N* | −8% | +13% (75% →88%) | 127 | 95 | 3 |
| 52 | R | N* | +27% (56% →83%) | +12% (88% →100%) | — | — | 2 |
| 53 | R | R | −11% (71% →60%) | −10% (92% →82%) | 108 | 103 | 1 |
| 56 | R | N* | +13% (77% →90%) | +3% | 100 | 103 | 2 |
| 58 | N | N | +6% | −2 | — | — | 2 |
DLCO indicates carbon monoxide diffusing capacity; FEV1/FVC, ratio of forced expiratory volume to forced vital capacity; FVC, forced vital capacity; N, normal; O, obstructive; R, restrictive; SCD, sickle cell disease.
Changed pattern after BMT.
When the difference between the pre- and post-BMT result was >10%, the value of the pre- and post-BMT measurement expressed as a percentage of normal is shown in parentheses.
Of the 23 patients who were evaluable for comparison of spirometry and lung volumetrics before and after BMT, the mean predicted FEV1 changed from 88% ± 10% to 86% ± 11% (P = .59) and the mean predicted FVC changed from 78% ± 16% to 81% ± 12% (P = .45) after transplantation. Before transplantation, 11 had restrictive changes, 2 had obstructive changes, and 10 patients had normal lung function at baseline (Tables 4 and 5). Among the 11 patients who had restrictive changes at baseline, 4 had normal function after BMT and 7 had persistent restrictive changes. Of these 7, 1 was improved but continued to have a restrictive pattern, 1 had more restrictive disease, and 5 were unchanged after BMT. Of the 2 patients who had obstructive changes at baseline, 1 had normal pulmonary function after BMT, and 1 had worsened obstructive disease after BMT. Of the 10 patients who had normal lung pattern at baseline, 8 remained normal after BMT and 2 developed a restrictive pattern after BMT.
Table 5.
Pulmonary Function after BMT According to pre-BMT Performance Status
| Post-BMT Pulm Function | Normal (N = 10) | Obstructive (N = 2) | Restrictive (N = 11) |
|---|---|---|---|
| Unchanged pattern | 8 | 0 | 5 |
| Improved | N/A | 1 (normal) | 5 (normal in 4) |
| More restrictive | 2 | — | 1 |
| More obstructive | — | 1 | — |
BMT indicates bone marrow transplantation.
Compared to pre-BMT measurements, the RV (P = .02) and RV/TLC (P = .03) were significantly decreased after BMT. The observed difference in RV and in RV/TLC suggests that there was decreased air trapping and therefore improved lung function after BMT. The DLCO was improved in 3 of 13 patients after BMT, but this was not statistically significant. Overall, patients who had normal baseline pulmonary function were likely to remain normal (8 of 10) after BMT. Patients who had restrictive disease at baseline were likely to have improved or persistent, but not progressive restrictive disease (10 of 11). Taken together, these data show that most patients had stable pulmonary function after BMT.
Posttransplantation Gonadal Function
The observations of gonadal function in males and females after transplantation for SCD are presented in Tables 6 and 7. All the survivors (33 male and 22 female) who were enrolled in protocol 610 are greater than 14 years of age. The current median ages are 21.6 (range: 16.1–28.5 years) and 21.7 (range: 14.1–27.8 years) among the males and females, respectively. Among these, 13 males (39%) and 14 females (64%) had posttransplantation endocrine studies reported. The LH and FSH levels were normal in 9 males and less than normal in 4 after transplantation. However, only 3 of 13 had normal testosterone levels (Table 6), consistent with hypogonadotrophic hypogonadism in most of the pubertal males. In contrast, 8 of 14 females had increased gonadotropin levels and/or below normal estradiol levels, a finding consistent with primary ovarian failure in the majority of postpubertal females. Only 4 of 14 females had normal estradiol levels (Table 6). However, 1 female had a successful pregnancy 13 years after BMT and another female with graft rejection gave birth to a healthy baby following preimplantation genetic diagnosis 14 years after BMT, although she had experienced 2 previous spontaneous abortions several years earlier.
Table 6.
Gonadotropin and Testosterone Levels in Males after BMT for SCD
| Patient No. | Age at BMT | Current Age | Age at Last Testing | LH | FSH | Testosterone |
|---|---|---|---|---|---|---|
| 2 | 10.6 | 25.4 | 12.9 | Nl | Nl | Below normal |
| 6 | 12.2 | 26.2 | 13.2 | Nl | Nl | Below normal |
| 14 | 12.3 | 22.2 | 13 | Nl | Nl | Below normal |
| 15 | 13.3 | 23.3 | 18.4 | Nl | Nl | Below normal (Nl at 16.7 years) |
| 16 | 10.1 | 20.1 | 13.7 | Nl | Nl | Below normal at 12.5 years |
| 18 | 8.2 | 17.7 | 11.7 | Nl | Nl | Below normal |
| 28 | 8.5 | 20.0 | 10.5 | Below normal | Below normal | Below normal |
| 32 | 7.4 | 18.5 | 13.7 | Below normal | Below normal | Below normal at 10.5 years |
| 33 | 12.8 | 23.4 | 18 | Nl | Nl | Nl at 19 years |
| 36 | 15.9 | 25.8 | 18.4 | Nl | Nl | Not tested |
| 40 | 12.5 | 21.9 | 16.7 | Below normal | Below normal | Nl on HRT |
| 56 | 10.8 | 15.3 | 11.9 | Below normal | Below normal | Not tested |
| 59 | 11.8 | 15.8 | 12.8 | Nl | Nl | Nl |
BMT indicates bone marrow transplantation; FSH, follicle stimulating hormone; HRT, hormone replacement therapy; LH, Luteinizing Hormone; Nl, normal; No., number; SCD, sickle cell disease.
Table 7.
Gonadotropin and Estradiol Levels in Females after BMT for SCD
| Patient No. | Age at BMT | Current Age | Age at Last Testing | LH | FSH | Estradiol | Amenorrhea |
|---|---|---|---|---|---|---|---|
| 1 | 10.5 | 26.5 | 16 | — | Increased | Receiving HRT | — |
| 3* | 10.2 | 25 | 17.3 | Nl | Nl | Not tested | Yes |
| 8 | 14 | 27.8 | 25.4 | Increased | Increased | Decreased | Yes |
| 10 | 8.3 | 23.3 | 12.3 | Increased | Nl | Decreased | Yes |
| 11 | 8.2 | 22.3 | 14.2 | Increased | Increased | Decreased | Yes |
| 12* | 13.7 | 27.6 | 18.8 | Nl | Nl | Nl | No |
| 20 | 12.2 | 24.8 | 15.5 | Increased | Increased | Decreased | — |
| 23 | 8.6 | 20.6 | 14.2 | Nl | Nl | Decreased | Yes |
| 26 | 12.4 | 24 | 21.1 | Increased | Increased | Decreased | Yes |
| 27 | 6.0 | 17.6 | 11 | Nl | Nl | Nl | — |
| 29 | 9.7 | 21.1 | 14.5 | Nl | Nl | Nl | No |
| 38 | 4.3 | 14.1 | 10.5 | — | — | — | Yes |
| 39 | 13.7 | 23.3 | 14.9 | Increased | Increased | — | — |
| 45 | 9.5 | 18.7 | 14.4 | Increased | Increased | Receiving HRT | — |
| 47 | 10.3 | 19.5 | 12.9 | Nl | Nl | Nl | Yes |
| 51 | 9.6 | 18.3 | 14.5 | Nl | Nl | Decreased | No |
| 57 | 11.2 | 19.3 | 16.5 | — | — | Receiving HRT | Yes |
BMT indicates bone marrow transplantation; FSH, follicle stimulating hormone; HRT, hormone replacement therapy; LH, Luteinizing Hormone; Nl, normal; No., number; SCD, sickle cell disease.
Successful pregnancy and live birth after BMT.
DISCUSSION
These updated results confirm that children who were treated by HLA-identical BMT have durable engraftment of donor cells and do not experience painful or other clinical events related to SCD. We have extended these observations with findings that strongly suggest that most individuals are also protected from subclinical progression of end-organ pulmonary and CNS dysfunction that is associated with vaso-occlusion or other sickle-related pathophysiology. Together, these observations reinforce the notion that conventional BMT offers a favorable risk-benefit balance in children who have severe SCD. Unfortunately, it was not possible to conduct a comprehensive cross-sectional evaluation of pulmonary, gonadal, and CNS status at regular intervals in all the subjects after transplantation because of constraints of the original study design, which was to demonstrate the safety and feasibility of conducting a multicenter investigation of HLA-identical sibling BMT for SCD. In the future, however, transplantation study design should include a systematic evaluation of the quality of survival after successful transplantation and routine assessments of organ function, particularly of the kidney, lungs, brain, and endocrine system.
Although children who had stroke as an indication for BMT were protected from clinical strokes after successful transplantation, the long-term natural history of neurovascular disease after transplantation remains somewhat uncertain. It was not possible to conduct routine neuropsychologic testing before and after transplantation as part of the long-term follow-up evaluations; thus, the impact of successful BMT on functional CNS status remains undefined in this cohort. Although most patients with stroke or silent cerebral infarction before BMT had no further changes after BMT, 1 patient in this study had progressive changes by MRI in the initial month after transplantation that did not change in subsequent examinations through 5 years after BMT. There is a possibility that the natural history of neurovascular injury might include reactive gliosis or other changes related to the initial event that are not altered by the transplantation itself. This possibility is also supported by another retrospective clinical survey in which radiographic changes were noted in some patients with SCD who underwent transplantation for stroke [17]. Five of 9 patients had either new or slightly increased size of cerebral lacunae or leukoencephaly that stabilized 2–7.5 years after transplantation. Thus, it is possible that these observations reflect the evolution of cerebrovascular disease that existed before BMT. Of interest, these brain MRI changes were not associated by progressive neurocognitive deficits.
This study shows that children with stroke are protected from a second stroke after BMT, when there is durable engraftment of donor cells. Thus, it is clear that transfusions for stroke prophylaxis can be stopped after BMT. This contrasts sharply with the current practice of regular RBC transfusions for stroke prophylaxis that remains the standard of care outside successful BMT. In 1 series of 137 pediatric patients from 14 centers (mean age at first stroke was 6.3 years) for whom there was a mean follow-up of 10.1 years, 31 (22%) patients had a second stroke (2.2 per 100 patient years) while receiving regular RBC therapy [18]. There is also a risk of intracranial hemorrhage in adults who had a childhood stroke [19]. We observed significant CNS events (stroke or intracranial hemorrhage) in 3 of the 59 patients enrolled in this trial, and 1 patient died of intracranial hemorrhage after BMT. Thus, it appears that the intention to treat by transplantation provides a similar level of protection from stroke, as do regular transfusions. However, BMT is associated with a risk of mortality and other significant morbidities such as GVHD in the short-term, although these risks are balanced by the elimination of chronic RBC transfusions and iron chelation therapy in the long term. Whether transplantation might also be a suitable alternative in preventing a first stroke is less certain, although in the series reported here, individuals with silent cerebral infarction were protected from progressive changes by brain MRI, and in some, the pre-BMT changes resolved. Thus, it might also be appropriate to consider BMT in children who have a high risk for stroke and progressive neurologic injury [20–23].
Pulmonary function after BMT was, for the most part, little changed after transplantation. Primarily, this was interpreted to indicate that there was no additive functional impairment caused by the conditioning regimen. However, in other pediatric series, progressive obstructive spirometric changes were observed more frequently after transplantation with Bu than after total body irradiation (TBI) [24]. However, there is a growing body of evidence to indicate that the pulmonary toxicity of SCD is progressive, and may be expressed as either restrictive or obstructive pulmonary disease [25,26]. In a series from the Cooperative Study of Sickle Cell disease, only 10% of adults had normal PFT results, with restrictive pulmonary disease (74%) accounting for the most frequent abnormality among 310 adult SCD individuals tested [27]. The rate of progression of pulmonary function abnormalities is not certain, but in 1 series of children and adolescents tested over 42 ± 23 months, 56% had normal PFTs at the baseline, and only 29% remained normal at the second testing, with obstructive patterns being observed more frequently than restrictive [28]. In another series of 413 children with SCD who had pulmonary function testing results analyzed by a linear mixed effects model, showed significant serial decline in the percent predicted values for FEV1, FVC, and FEF25-75 across age [29]. Thus, these data strongly suggest that the negative impact of sickle vasculopathy on pulmonary function is progressive. This type of pulmonary injury appears to be halted by the establishment of donor erythropoiesis and cessation of acute chest episodes that we observed in this transplant cohort. We conclude, therefore, that successful transplantation protects recipients from this effect of the underlying disease.
Another pulmonary consequence of SCD is a predilection for pulmonary hypertension, a condition that is linked to risk of sudden death in adulthood [30]. Hemolysis, which is a hallmark of SCD, appears to be mechanistically linked with this clinical complication [31]. This is because of impaired nitric oxide (NO) bio-availability, which appears to be the central feature of endothelial dysfunction in SCD [32]. Thus, one might predict that the abrogation of hemolysis through correction of SCD by successful BMT should also eliminate any risk of developing pulmonary hypertension as a consequence of hemolysis and NO depletion [33,34]. We have commenced a cross-sectional study of tricuspid regurgitant jet velocity in survivors of successful BMT to explore this prediction.
A significant fraction of individuals developed gonadal insufficiency after transplantation, and 3 post-pubertal females were treated with hormone-replacement therapy (Table 5). Together, these observations tend to confirm the gonadal toxicity that occurs after exposure to myeloablative (MA) doses of Bu, particularly in the females [35–38]. Although formalized studies of fertility per se were not conducted, many of these survivors would be predicted to have infertility. Of interest, many of the males exhibited a pattern of hypogondadotropic hypogonadism, which is often associated with a central etiology, and which has been observed in the setting of iron overload after transplantation for thalassemia major. However, a more likely explanation here is that the timing of puberty in males with SCD can be delayed compared to unaffected males [39], and that many of the males studied had not yet achieved postpubertal status when the posttransplant assessments were performed. Additional follow-up in this cohort, particularly with respect to fertility, will provide useful information to physicians and families.
In summary, we observe that HLA-identical BMT offers long-term protection from clinical and subclinical vaso-occlusion, and thus should be offered sooner and with greater frequency to families that might benefit from this curative therapeutic option. However, decision making must also take into account the risks of transplantation, which include a significant risk of infertility and gonadal dysfunction, and warrant the application of transplantation in those who are most likely to follow a morbid clinical course. Nonetheless, it is hoped that this report might support a change in the perception that only a very small fraction of children are appropriate for BMT. The unfolding story of SCD is that the scope of disease is far wider, rather than more narrow than initially believed, which lends considerable urgency to establishing and expanding curative therapies for this disorder.
Acknowledgments
The authors thank the patients, nurses, and coordinators of the participating sickle cell anemia and hematopoietic cell transplantation centers for their participation in the study. They also thank the Data Safety and Monitoring Board with Liana Harvath and Helena Mishoe at the NHLBI for their guidance in conducting this study.
APPENDIX
The following investigators and centers participated in this collaborative study: Ann Arbor, MI (J.E. Levine, Univ. of Michigan) Atlanta, GA (J.R. Eckman, A. Haight, T. Olson, P. Lane, A. Yeager, Emory University; T. Adamkiewicz, Morehouse School of Medicine); Birmingham, UK (P.J. Darbyshire); Chapel Hill, NC (R. Redding-Lallinger, E. Orringer); Cleveland, OH (M. Nieder, Rainbow Babies Hospital, Case Western University); Creteil, France (F. Bernaudin, G. Souillet, Hopital Henri Mondor); Dallas, TX (G.R. Buchanan, Z.R. Rogers, V. Aquino, University of Texas Southwestern Medical Center at Dallas); Denver, CO (R. Giller, University of Colorado); Durham, NC (J. Kurtzberg, P. Martin, K.M. Sullivan, Duke University Medical Center); Fort Worth, TX (G. Eames, D. Friedman); Houston, TX (K.W. Chan, University of Texas); Indiannapolis, IN (P. Haut, Riley Children’s Hospital); London, UK (S.C. Davies, I. Dokal, I.A.G. Roberts, Royal Postgraduate Medical School); Los Angeles, CA (R. Parkman, D. Powars, N. Kapoor, W.-Y. Wong, T. Coates, University of Southern California; T. Moore, S.A. Feig, University of California at Los Angeles); Miami FL (O. Alvarez, G. Kleiner, University of Miami); Milwaukee, WI (D. Margolis, J.P. Scott, J. Panepinto, Medical College of Wisconsin); Minneapolis, MN (P. Orchard, J. Wagner, University of Minnesota); New Haven, CT (B. Sleight, Yale University); Oakland, CA (L.A. Styles, K. Hardy, S. Ozdogan, E. Vichinsky, M.C. Walters, Children’s Hospital & Research Center, Oakland); Philadelphia, PA (K. Ohene-Frempong, K. Smith-Whitley, N. Bunin, University of Pennsylvania; L.L. Hsu, St. Christopher); Pittsburgh, PA (L. Krishnamurti); Saint Louis, MO (S. Shenoy, R. Hayashi, Washington University); Rochester, NY (J. Horan, R. Duerst, University of Rochester); Saint Petersburg, FL (M. Klemperer, University of S. Florida); Sao Paolo, Brazil (C. Bonfim, Federal University of Parana; R. Pasquini, University of Campinas); San Francisco, CA (W.C. Mentzer, M Cowan, J. Barkovich, University of California, San Francisco); St. Augustin, Germany (R. Dickerhoff, University of Bonn; T. Klingebiel, University of Frankfurt); Seattle, WA (A. Woolfrey, J.E. Sanders, Fred Hutchinson Cancer Research Center and the University of Washington); and Washington, DC (N. Kamani, C. Minniti, O. Castro, Children’s Hospital National Medical Center, George Washington University and Howard University).
Footnotes
Authorship
M.C.W., M.P., and K.M.S. designed the study, interpreted the data, and wrote the manuscript; K.H. and J.B. performed data analysis; S.E., T.A., F.B., G.R.B., N.B., R.D., R.G., P.R.H., J.H., L.L.H., N.K., J.E.L., D.M., K.O.-F., R.R.-L., I.A.G.R., Z.R.R., J.E.S., and J.P.S provided study materials or patients and reviewed the data; all authors gave final approval of the manuscript. The authors have no conflicts of interest to disclose.
Financial disclosure: This investigation was supported by NIH Grants HL 36444 and HL 68091.
References
- 1.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 2.Vermylen C, Cornu G, Ferster A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant. 1998;22:1–6. doi: 10.1038/sj.bmt.1701291. [DOI] [PubMed] [Google Scholar]
- 3.Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related, myeloablative stem cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–2756. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- 4.Walters MC, Storb R, Patience M, et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Blood. 2000;95:1918–1924. [PubMed] [Google Scholar]
- 5.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 6.Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 7.Walters MC, Sullivan KM, Bernaudin F, et al. Neurologic complications after allogeneic marrow transplantation for sickle cell anemia. Blood. 1995;85:879–884. [PubMed] [Google Scholar]
- 8.Ferster A, Christophe C, Dan B, Devalck C, Sariban E. Neurologic complications after bone marrow transplantation for sickle cell anemia. Blood. 1995;86:408–409. [PubMed] [Google Scholar]
- 9.Schoenberg JB, Beck GJ, Bouhuys A. Growth and decay of pulmonary function in healthy blacks and whites. Respir Physiol. 1978;33:367–393. doi: 10.1016/0034-5687(78)90063-4. [DOI] [PubMed] [Google Scholar]
- 10.Polgar G, Promadhat V. Pulmonary Function Testing in Children: Techniques and Standards. Philadelphia, PA: WB Saunders Co; 1971. [Google Scholar]
- 11.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 12.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley; 1980. [Google Scholar]
- 16.Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–673. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 17.Woodard P, Helton KJ, Khan RB, et al. Brain parenchymal damage after haematopoietic stem cell transplantation for severe sickle cell disease. Br J Haematol. 2005;129:550–552. doi: 10.1111/j.1365-2141.2005.05491.x. [DOI] [PubMed] [Google Scholar]
- 18.Scothorn DJ, Price C, Schwartz D, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140:348–354. doi: 10.1067/mpd.2002.122498. [DOI] [PubMed] [Google Scholar]
- 19.Powars D, Adams RJ, Nichols FT, Milner P, Charache S, Sarnaik S. Delayed intracranial hemorrhage following cerebral infarction in sickle cell anemia. J Assoc Acad Minor Phys. 1990;1:79–82. [PubMed] [Google Scholar]
- 20.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Enos L, Gallagher D, et al. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139:391–397. doi: 10.1067/mpd.2001.116935. [DOI] [PubMed] [Google Scholar]
- 23.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139:385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- 24.Bruno B, Souillet G, Bertrand Y, Werck-Gallois MC, So Satta A, Bellon G. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant. 2004;34:143–147. doi: 10.1038/sj.bmt.1704549. [DOI] [PubMed] [Google Scholar]
- 25.Field JJ, Glassberg J, Gilmore A, et al. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83:574–576. doi: 10.1002/ajh.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatr Pulmonol. 2008;43:1061–1066. doi: 10.1002/ppul.20883. [DOI] [PubMed] [Google Scholar]
- 27.Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koumbourlis AC, Lee DJ, Lee A. Longitudinal changes in lung function and somatic growth in children with sickle cell disease. Pediatr Pulmonol. 2007;42:483–488. doi: 10.1002/ppul.20601. [DOI] [PubMed] [Google Scholar]
- 29.MacLean JE, Atenafu E, Kirby-Allen M, et al. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178:1055–1059. doi: 10.1164/rccm.200708-1219OC. [DOI] [PubMed] [Google Scholar]
- 30.Gladwin M, Sachdev V, Jison M, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:22–31. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 31.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu LL, Champion HC, Campbell-Lee SA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor P, Veys P, Amrolia P, Haworth S, Ashworth M, Moledina S. Pulmonary hypertension in children with Evans syndrome. Pediatr Hematol Oncol. 2008;25:93–98. doi: 10.1080/08880010801888253. [DOI] [PubMed] [Google Scholar]
- 35.Grigg AP, McLachlan R, Zaja J, Szer J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26:1089–1095. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 36.Teinturier C, Hartmann O, Valteau-Couanet D, Benhamou E, Bougneres PF. Ovarian function after autologous bone marrow transplantation in childhood: high-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 1998;22:989–994. doi: 10.1038/sj.bmt.1701483. [DOI] [PubMed] [Google Scholar]
- 37.Brachet C, Heinrichs C, Tenoutasse S, Devalck C, Azzi N, Ferster A. Children with sickle cell disease: growth and gonadal function after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2007;29:445–450. doi: 10.1097/MPH.0b013e31806451ac. [DOI] [PubMed] [Google Scholar]
- 38.Cicognani A, Pasini A, Pession A, et al. Gonadal function and pubertal development after treatment of a childhood malignancy. J Pediatr Endocrinol Metab. 2003;16(Suppl 2):321–326. [PubMed] [Google Scholar]
- 39.Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311:7–12. doi: 10.1056/NEJM198407053110102. [DOI] [PubMed] [Google Scholar]


