Abstract
Purpose
Primary culture of nonpigmented ciliary epithelium (NPE) has proved difficult in the past. Here we report development of a method of growing and maintaining primary cultures of NPE from porcine eye. Studies were conducted to confirm that the cultured NPE expressed proteins characteristic of native NPE.
Methods
Intact rings of NPE were isolated from adult pig eyes. A mixture of hyaluronidase and collagenase was used to detach the cells from the basement membrane and vitreous. Dispersed cells were seeded at high density and grown in DMEM with 20% fetal bovine serum under 5% CO2 and 95% air. Protein expression was examined by immunohistochemistry and immunoblot analysis.
Results
NPE cells were grown in primary culture and maintained up to 10th passage. Analysis of the ciliary body showed three Na,K-ATPase isoforms (α1, α2, α3) and three nitric oxide synthase isoforms (eNOS, nNOS, iNOS) enriched in the NPE layer but weaker or absent in the PE layer. Each of these proteins as well as the tight junction–specific protein ZO-1 was detected in the cultured NPE.
Conclusions
We developed a simple and reliable way to isolate, culture, and maintain NPE cells from porcine eyes. Success of the method hinged on our ability to isolate pure NPE in large number, detach the cells from the vitreous, and seed the cells at high density. The cultured cells express several proteins that are characteristic of native NPE. NPE cells cultured in this way may prove to be valuable for the study of ciliary body function.
Keywords: immunoblot, immunohistochemistry, nonpigmented ciliary epithelium, porcine eye, primary culture
INTRODUCTION
Ciliary epithelium (CE) is the tissue responsible for the production of aqueous humor (AH), an important nutritive fluid within the eye. The CE is composed of an outer layer of pigmented (PE) cells attached to the stroma of the ciliary body and an inner layer of nonpigmented (NPE) cells facing the AH of the posterior chamber. The two cell layers are arranged apex to apex and are connected by gap junctions.1 The basolateral membrane of NPE cells constitutes the final exit pathway for the solutes and water to form AH. There is general agreement that AH formation requires active ion transport and that the tight junctions at the apical membrane of the NPE cells acts as the barrier to limit free passage of solutes and water from the stromal plasma into the AH.2
The best accepted model for AH secretion is that the two cell layers work together as a syncytium.3,4 According to this model, solutes and water from the stroma enter first into the PE cells, pass through gap junctions into the NPE, and then exit through the NPE into the posterior chamber.5,6 However, it has also been suggested that NPE layer plays a dominant role in the secretion of AH and may alone be capable of secreting fluid.7 There are still questions about the exact contribution of the PE and NPE in the secretion of AH. Primary cultures of the two cell types could be useful for future investigations. Pigmented cells are comparatively easy to grow in primary culture and have long been used in numerous investigations.8–13 Until now, it has been quite difficult to obtain primary cultures of NPE cells. The few successful NPE culture methods required stringent growth conditions,14,15 probably because the cells display negligible turnover in vivo.16
Recently, we developed a technique for isolation of NPE cells from the pig eye in bulk quantities.17 In the current investigation, we report growth and culture of porcine NPE cells to confluence and maintenance of the cells up to 10th passage. We show that the cultured NPE cells express several proteins characteristic of native NPE, including three isoforms of Na,K-ATPase catalytic subunit and three isoforms of nitric oxide (NO) synthase. Previously, we reported that NO donors reduce AH formation in the isolated porcine eye.18 and that NO generated by freshly isolated NPE from porcine eye was able to inhibit Na, K-ATPase activity in an autocrine fashion.17
MATERIALS AND METHODS
NPE cells were dispersed using hyaluronidase and collagenase A (Sigma, St Louis, MO, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; D/6046, Sigma) with 20% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA) and 100 μg/ml gentamicin (Sigma). For immunohistochemical or im-munoblot localization of proteins, the following primary antibodies were used: anti-eNOS/NOS III rabbit polyclonal antibody (Upstate, Lake Placid, NY, USA) (5 μg/ml); anti-iNOS/NOS II rabbit polyclonal antibody (Upstate) (5 μg/ml); anti-nNOS/NOS I rabbit polyclonal antibody (Upstate) (1:200); mouse mono-clonal anti-Na, -ATPase α-1 antibody (Sigma), (1:100 for immunohistochemistry and 1:1000 for immunoblot analysis); rabbit polyclonal anti-Na, K-ATPase α-2 antibody (Upstate) (1:100 for immunohistochemistry and 1:5000 for immunoblot); mouse monoclonal anti-Na,K-ATPase α-3 antibody (Sigma) (1:100 for im-munohistochemistry and 1:1000 for immunoblot); rabbit anti-occludin polyclonal antibody (Invitrogen, Carlsbad, CA, USA) (2.5 μg/ml); rabbit anti-ZO-1 poly-clonal antibody (Zymed Laboratories Inc., South San Francisco, CA, USA) (1.25–2.50 μg/ml).
Secondary antibodies used in immunohistochem-istry were Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, 2.0 mg/ml) and Alexa Fluor 546 goat anti-mouse IgG (Invitrogen, 2.0 mg/ml). Both secondary antibodies were diluted to 1:200 in PBS containing 10% goat serum before use. The two secondary antibodies used for immunoblot analysis were IRDye 800 goat anti-rabbit IgG (Rockland) and Alexa Fluor 680 goat anti-mouse IgG (Invitrogen Carlsbad, CA, USA) (1:20,000).
Isolation of NPE Cells
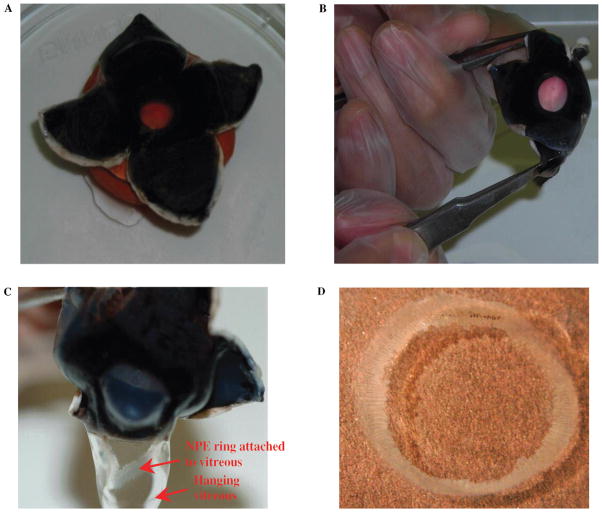
Porcine eyes from 6- to 8-month old animals were obtained from a local abattoir and transported to the laboratory on ice. First, extraocular tissues were removed, and the eyes were washed with phosphate-buffered saline (PBS) containing 200 μg/ml gentamicin. All subsequent operations were performed in sterile condition in a biological safety cabinet. Each eye was then placed, with the cornea facing downward, on a support made of modeling clay. The eye was then dissected open at the posterior pole by two incisions made at right angles using a scalpel. The incisions, which were made with minimum disturbance to the vitreous, enabled the globe to be opened by folding back the resultant four flaps of sclera (Fig. 1A). The cornea was everted by pulling the cut flaps of the sclera backward while at the same time gently pushing the cornea inward with the index finger (Fig. 1B). This causes the vitreous to protrude with its base still attached to the pars plana of the ciliary body. One side of the vitreous was then pushed aside from the pars plana using curved forceps, and the vitreous body was gently separated from the eye (Fig. 1C). The entire ring of NPE remains attached to the vitreous and is thus separated from the eye (Fig. 1D). The pigmented ciliary epithelial layer remains attached to the ciliary body. The ring of NPE shows no evidence of pigment. The NPE ring was then cut from the vitreous using fine scissors. With a small amount of the vitreous still attached, the cell ring was placed in an ice-cold centrifuge tube.
FIGURE 1.
Dissection technique of porcine eye to isolate intact NPE layer. (A) Cross section of the sclera at the posterior pole, which allows the eye to open by folding the resultant four flaps. (B) The technique of everting the cornea by pulling the cut flaps of the sclera backward and at the same time pushing the cornea inward with the index finger. (C) The method of separation of NPE layer along with vitreous. (D) The isolated intact ring of NPE layer. Please note that the NPE ring isolated from the porcine eye showed no detectable contamination by the PE or pigment granules.
Dispersion and Culturing of NPE
The isolated NPE ring was cut into 1- to 2-mm pieces and placed in an ice-cold centrifuge tube containing serum-free DMEM and 50 μg/ml gentam-icin. Pieces from 5–7 intact NPE rings were pooled and transferred to a 90-mm Petri dish containing 15 ml of 0.015% collagenase A and 300 U/ml of hyaluronidase (Sigma) in a collagenase buffer com-prising (mM) NaCl 66.7, KCl 6.7, HEPES 10, CaCl2 4.8 with the pH adjusted to 7.4. The Petri dish was then placed on a rotary shaker in an incubator at 37°C and subjected to gentle shaking for 5–7 min. The Petri dish was then removed from the shaker, and the collagenase and hyaluronidase were neutralized using excess (5–7 ml) of a 1:1 mixture of newborn calf serum (NCS) and fetal bovine serum (FBS). The purpose of the collagenase/hyaluronidase treatment was to cause the attached vitreous and the collagen fibers at the basement membrane of the NPE to dissolve and loosen enough to disperse the cells. Using a round-tipped Pasteur pipette, the loosened fragments of the tissue were transferred to two centrifuge tubes each containing 10 ml of DMED containing 20% of a mixture of NCS and FCS (1:1). The fragments were gently triturated with a round-tipped Pasteur pipette. The dispersed cells were centrifuged at 670 × g for 10 min at 4°C. The supernatant was then discarded, and the pellet was redispersed in complete medium (DMED+20% FBS + 100 IU gentamicin/ml). The cells were incubated in scant amount of DMEM with 20% fetal bovine serum at 37°C in 5% CO2/95% humidified air for 4–5 days without changing the medium. The medium was then changed every alternate day. In 10–15 days there were many isolated colonies of confluent cells. At this stage, the cells were trypsinized and propagated. The first batch of propagated cells thus obtained was designated as first passage cells, and so on.
At confluence, the cells were washed twice with 20 ml of a sterile calcium-free buffer comprising (mM) NaCl 142.0, KCl 6.7, HEPES 10.0, and EDTA 0.25 and the pH adjusted to 7.4. Washed cells were incubated with 5 ml (Corning cell culture flask, 25 cm2) of trypsin EDTA solution (1X) (Sigma) at 37°C for 4 min. The cells were easily detached by gentle shaking of the flask 3–4 times. The trypsin was immediately neutralized by adding 3–4 ml of FBS. The cells were centrifuged, washed with DMEM, and finally resuspended in 5 ml of complete medium. Cells were propagated in 25 cm2culture flasks each containing ~5 ml of complete medium. Conflu-ence was usually achieved in 7–10 days. We propagated the cells up to the 10th passage without any apparent signs of changing cell morphology as observed under light microscope and growth characteristics in terms of the time taken to reach confluence. In the current study, we used cells only from first through seventh passages.
Preparation of Ciliary Body for Immunohistochemistry
The fat and connective tissue around the globe were cut away with scissors. The cornea was cut circumferentially and removed. After removal of the cornea, 4–6 mm of the anterior sclera was carefully peeled from the choroid all around the globe using a pair of curved scissors. The incision ran tangentially to the globe beginning at the anterior chamber angle. The corneal remnant at the limbus was used as the handle to facilitate this dissection. The whole iris–ciliary body (I-CB) along with the lens and anterior vitreous was then carefully bisected out leaving behind the posterior part of the globe. The separated I-CB was immediately placed (corneal face down) on a Petri dish containing ice-cold Ringer solution comprising (mM) NaCl 113, KCl 4.6, NaHCO3 21.0, MgSO4 0.6, D-glucose 7.5, glutathione (reduced form) 1.0, Na2HPO4 1.0, HEPES 10.0, and CaCl2 1.4, pH adjusted to 7.4. The intact lens along with the capsule was removed from the I-CB by cutting the zonules. Vitreous was carefully removed and finally the iris was separated from the CB with a pair of scissors. The intact ciliary body ring (Fig. 2A), thus isolated, was cut radially to wedges (see Fig. 2B), fixed in appropriate fixatives, and stored to obtain thin sections. The fixed radial wedges were then cut perpendicularly to obtain frozen or paraffin sections for immunohistochemical analysis (Fig. 2B).
FIGURE 2.
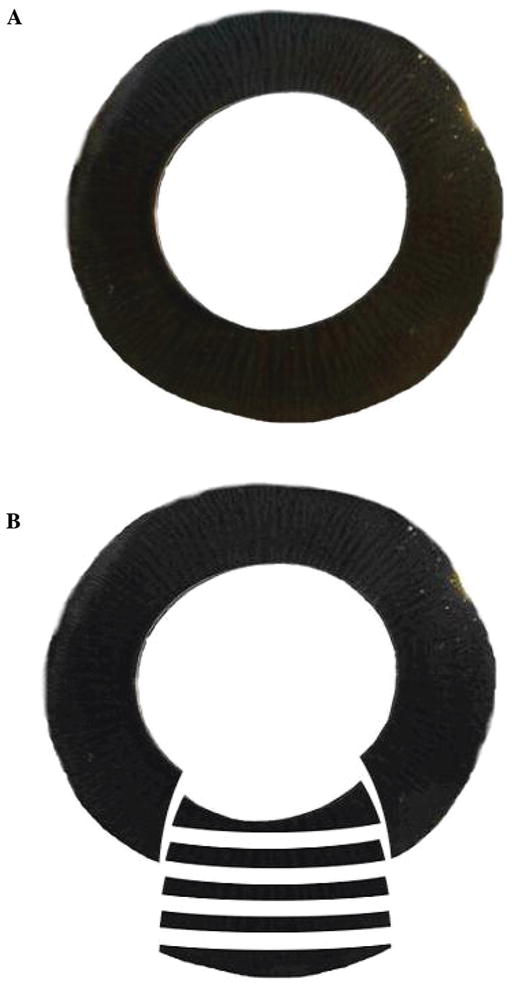
(A) Porcine ciliary body ring, with intact PE and NPE layers. Please note the heavy pigmentation compared with the isolated NPE layer shown in Figure 1D. (B) Diagram showing radial dissection of the isolated porcine ciliary body into wedges for fixing and perpendicular sectioning of the fixed radial wedges for immunohistochemical analysis.
Immunostaining
Paraffin or frozen sections were obtained either from the ciliary body or NPE ring, and the cultured NPE were treated with monoclonal/polyclonal antibodies to NOS isoforms, Na, K-ATPase isoforms, and ZO-1. Bound primary antibody was then detected by treating the tissue with fluorescent secondary antibodies. Fluorescence of the localized antibodies was visualized under a Zeiss Microscope (Axiovert 200M) and photographed using a Hamamatsu digital camera (C4742-95).
Immunoblotting
Cultured NPE cells were lysed in RIPA buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 10 mM sodium pyrophosphate, 2 mM sodium ortho-vanadate, 10 mM sodium fluoride, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, and protease inhibitors (2 μM antipain, 2 μM leupeptin, 1 μM pep-statin, 1 μM phenylmethylsulfonylfluoride [PMSF], and 2 μg/ml aprotinin). Immunoblot analysis of the cell membrane preparation was performed according to a published procedure,19 briefly summarized here. Membrane preparations obtained from rat kidney medulla, smooth muscle, and brain were used as controls. The proteins were separated by electrophoresis on a 7.5% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred electrophoretically to a nitrocellulose membrane. The nitrocellulose membrane was then blocked in blocking buffer (Pierce, Rockford, IL, USA) for an hour. Blocking was followed by 1 hr incubation with primary antibody. Then after 5 washes in TTBS for 5 min each, the nitrocellulose membrane was incubated with the secondary antibody for 1 hr. After three washes with Tween Tris buffered saline (TTBS) for 5 min each, and five washes in PBS, the gel was scanned using an Odyssey Western blot analyzer (Licor, Lincoln, NE, USA).
RESULTS
NPE Isolation and Culture
Freshly isolated NPE rings (Fig. 1D) showed no pigment under light microscope examination. In contrast, the isolated ciliary body ring containing both pigmented and nonpigmented CE layers showed heavy pigmentation (Fig. 2A). Using the ring of single layer cells (Fig. 1D) as a source of NPE, cells were cultured and maintained routinely without addition of any supplement or special media. When confluent, the cells demonstrated a characteristic cobblestone appearance at first and later passages. Photographs of confluent cells of passage 1 (Fig. 3A) and passage 7 (Fig. 3B) have been shown.
FIGURE 3.
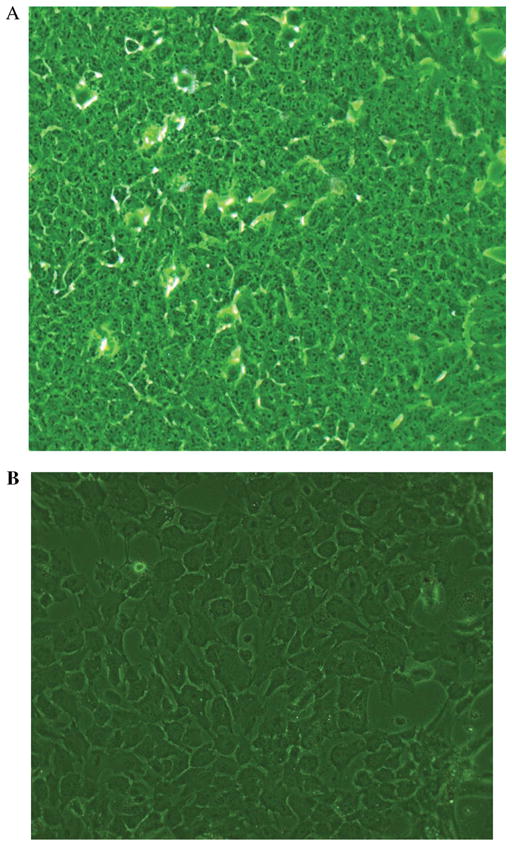
Photographs of porcine cultured NPE in confluent state. (A) First passage and (B) Seventh passage cells.
All three isoforms of nitric oxide synthase (NOS) (i. e., eNOS, nNOS and iNOS) were detected in the isolated native NPE layer (Fig. 4). eNOS, nNOS, and iNOS also were detected in the cultured NPE (Fig. 5). The negative controls, where the single layer of NPE or the cultured NPE cells had been treated similarly except that the primary antibody had been replaced by PBS, showed no staining (Figs. 4D and 5D).
FIGURE 4.
Immunolocalization of eNOS (A), nNOS (B), and iNOS (C) in the isolated NPE layer. The negative control, in which the primary antibody was replaced by PBS, shows no staining (D). Arrows indicate nonpigmented epithelium (NPE), pigmented epithelium (PE), and stroma (S).
FIGURE 5.
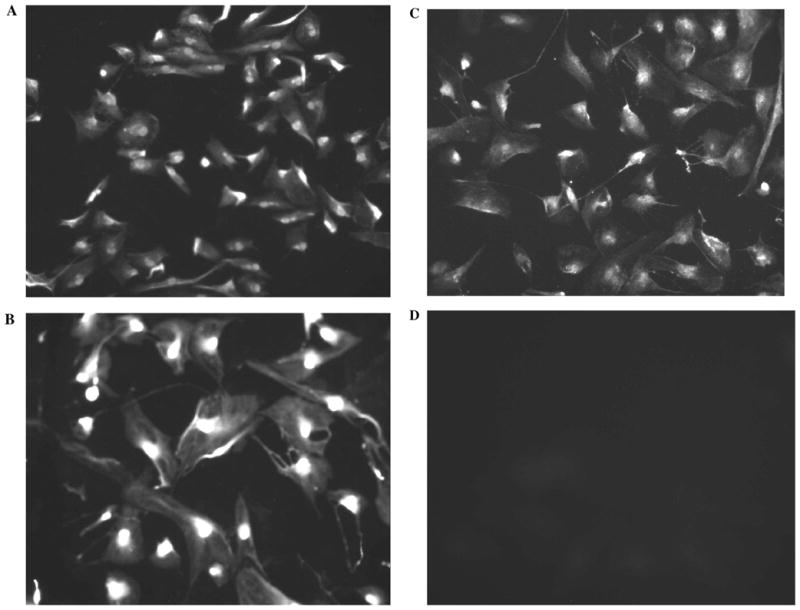
Immunolocalization of eNOS (A), nNOS (B), and iNOS (C) in preconfluent cultured NPE (fifth passage). The negative control, in which the primary antibody was replaced by PBS, shows no staining (D). Arrows indicate nonpigmented epithelium (NPE), pigmented epithelium (PE), and stroma (S)
Three α isoforms of Na, K-ATPase catalytic subunit were detected in the native NPE layer (Fig. 6). The negative controls, where the NPE layer was treated only with the secondary antibody, showed no staining (Fig. 6D). Using the same antibodies for immunoblot analysis, the three isoforms of Na, K-ATPase were also localized in cultured NPE (Fig. 7). Using the immunoblot approach enabled us to confirm Na, K-ATPase antibody selectivity by probing membrane preparations obtained from rat kidney medulla, skeletal muscle, and brain. α1 and α2, and α3 were detected in rat brain. α1 and α2 were detected in rat skeletal muscle. α1 alone was detected in the rat kidney.
FIGURE 6.
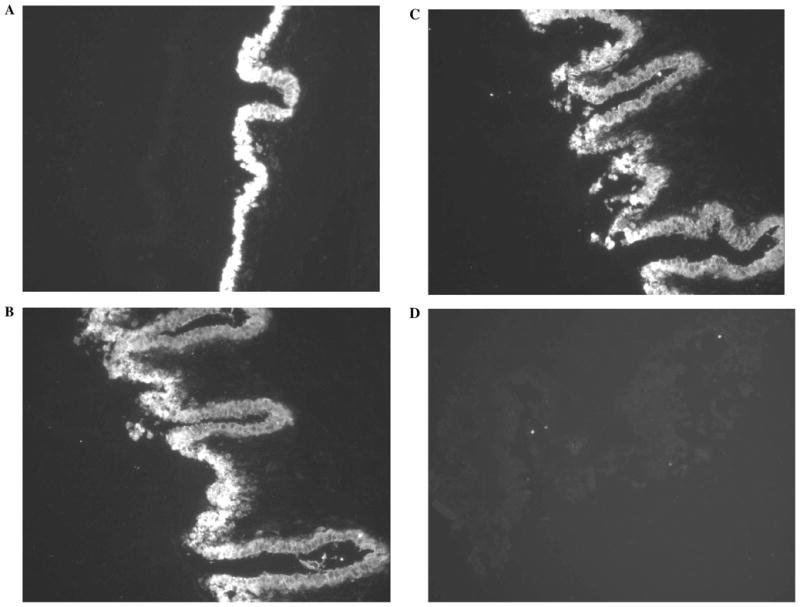
Immunolocalization of Na, K-ATPase α1 (A), α2 (B), and α3 (C) isoforms in the isolated NPE layer. The negative control, in which the primary antibody was replaced by PBS, shows no staining (D).
FIGURE 7.
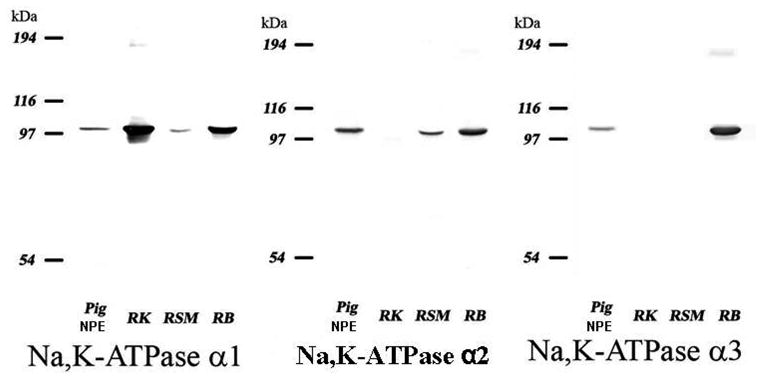
Immunoblot for Na, K-ATPase α1, α2, and α3 isoforms in the membrane preparation of cultured Fourth passage porcine NPE cells. Membrane preparations obtained from rat kidney (RK), rat skeletal muscle (RSM), and rat brain (RB) were probed to confirm selectivity of the antibodies.
The NPE layer is the site of tight junctions that form the blood-aqueous barrier.1 ZO-1, a tight junction–specific protein, was localized in cultured NPE, both in nonconfluent (Fig. 8A) and in confluent cells (Fig. 8B). It is interesting to note that in confluent cells, the ZO-1 is localized at the contact points between adjacent cells. In contrast, the negative controls (Fig. 8C) showed no staining. Occludin, an exclusively tight junction–specific protein, has also been localized in cultured NPE (seventh passage cells) (Fig. 9).
FIGURE 8.
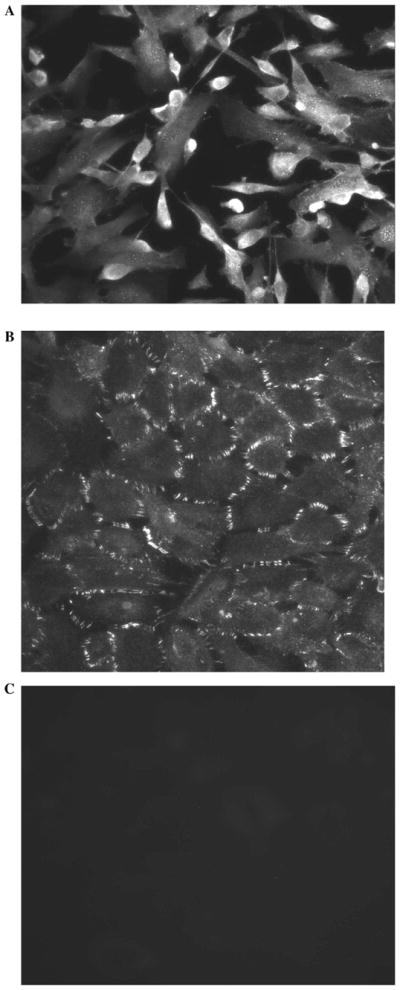
Immunolocalization of ZO-1 in cultured seventh passage porcine NPE. (A) Preconfluent cells. (B) Confluent cells. Note the meticulous orientation of ZO-1 along the borders of confluent NPE cells. The negative control (C), in which the primary antibody was replaced by PBS, showed no staining.
FIGURE 9.
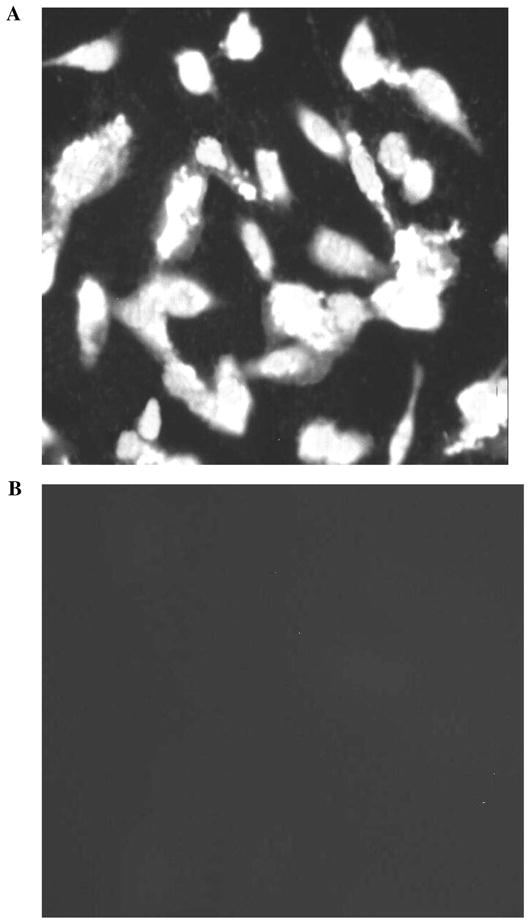
(A) Immunolocalization of occludin in cultured seventh passage porcine NPE. (B) The negative control, in which the primary antibody was replaced by PBS, showed no staining.
DISCUSSION
Primary Culture of NPE
We report a simple and reliable way to culture and maintain porcine NPE cells. Cells were maintained up to 10th passage without any apparent change in appearance or growth characteristics. Confluent cells showed an epithelial cobblestone appearance, and cells up to passage 10 maintained apparently similar growth rate as determined by the time to reach confluence after seeding. It has been difficult to obtain primary cultures of NPE cells,16 and to the best of our knowledge there are only a few published reports showing proliferation and growth of NPE cells in primary culture.14,15 In one study where NPE from rabbit eye was cultured,15 it was reported that in order for cell growth and proliferation, special conditioned medium harvested from confluent PE cells or a 20% extract of vitreous was necessary. In another study in which NPE from bovine eye was cultured,14 cells required hormonally defined serum-free medium as well as an artificial substrate with high binding affinity to attach cells to the culture dish. Proliferation in primary cultures of bovine NPE cells was also reported to be limited to 3–5 weeks. However, it was not clear in either report whether the investigators were able to passage or obtain confluent monolayer of NPE cells. In the current study, we were routinely able to obtain confluent monolayer and propagate the cells up to 10th passage using DMEM (Sigma) with 20% fetal calf serum and without the need for other supplements or special substrates. This argues against the proposal that NPE growth requires unknown factors that are expressed in PE or are present in the vitreous.15
We observed that if NPE cells were seeded sparsely, they proliferate slowly for 7–10 weeks without forming a confluent monolayer. Then the cells became stunted. It is possible that previous investigators had difficulty growing NPE because they used an insufficient number of cells for seeding. In our experience, obtaining sufficient quantity of NPE cells for initial seeding was the key to success of obtaining a confluent monolayer. Success therefore hinged on our ability to isolate the entire ring of NPE and also on the efficient use of hyaluronidase/collagenase to free the cells from the basement membrane and the vitreous. When seeded at a high density (approximately 5 × 104 to 5 × 10 cells/cm2), the cells attached rapidly and grew to confluence within 7–10 days. If the cells failed to reach confluence in 10 days, they seldom reached con-fluence. It also was important to begin the culture with NPE cells free from PE or other cell types. A relatively small number of PE cells can easily take over the NPE cells in culture, as the proliferation rate of PE cells in culture is much faster than NPE cells.20 Our culture starts from a sheet of pure NPE cells that are free from underlying pigmented ciliary epithelial cells. By histologic and electron microscopic method, we have recently shown17 that the NPE sheet thus isolated does not have any pigment or pigmented cells. Because fibroblasts or connective tissue cell lie beneath the still intact pigmented epithelium, the chance of contamination with those cells is low. Moreover, we have shown that the cultured NPE cells express ZO-1 and occludin, proteins that are not expressed in fibroblasts or connective tissue. At seventh passage, ZO-1 continued to be expressed by the cultured NPE, and at confluence, ZO-1 was found at the margins of confluent cells (Fig. 8B). The results from histology, immunohistochemistry, and electron microscopy led us to think that NPE cells isolated and cultured from porcine eyes have little or no contamination by PE or connective tissue cells. Several lines of evidence support identification of the cultured cells as NPE, not PE. First, we cultured cells that do not contain pigment. Second, the expression of occludin. Third, the three NOS proteins expressed in the cultured cells were characteristic of NPE layer.21,22 Fourth, the cultured cells expressed Na,K-ATPase isoforms α1, α2, and α3, which also were detected in the native NPE layer. The PE expresses only α1.23
Epithelial cells in culture may undergo epithelial to mesenchymal transformation (EMT). However, in the current study NPE cells even at late passage (seventh) express two important markers, namely ZO-1 and occludin. Mesenchymal cells rarely express ZO-1 or occludin. In addition, cultured NPE, even at the late passage, consistently shows characteristics of a typical epithelium: (1) typical cobblestone-shaped appearance, (2) cells never became elongated, (3) typical one-cell-thick monolayer, (4) individual cells were abutting each other in a uniform array, (5) cells were regularly spaced and joined together without any sign of individual cells moving away from the monolayer, (6) cells showed polarization as evidenced by the expression of ZO-1 on the borders of cells forming the monolayer. Together, these characteristics and the expression of ZO-1 and occludin support the notion that the cultured NPE cells have not undergone EMT.
The finding that all three nitric oxide synthase isoforms (eNOS, nNOS, and iNOS) were expressed in cultured NPE as well as in the native NPE of porcine eye points to maintenance of the NPE phenotype to some degree in the cultured cells. This finding differs from the previous report22 that only nNOS was consistently found in porcine NPE and that iNOS was found in only 50% of NPE cells. It is interesting to note that in another report all three NOS isoforms (eNOS, nNOS, and iNOS) were shown to express in porcine ciliary process.21 However, ciliary process is a tissue rich in vas-culature, and vascular endothelium is known to express eNOS. Thus, from that report it was not possible to pinpoint the exact source of NOS isoforms. In contrast, we have shown expression of all three NOS isoforms exclusively in the NPE. NOS is an enzyme involved in the generation of nitric oxide (NO) by metabolizing the amino acid L-arginine. NO acts as an important signaling molecule that regulates numerous functions in the body24 including the eye.25 Recently, we found that NO generated by porcine NPE could act in an au-tocrine manner to inhibit Na,K-ATPase activity.17 Using an arterially perfused porcine eye, it was shown that NO reduce AH secretion.18
Maintenance of the NPE phenotype in the cultured cells also was apparent from studies of Na,K-ATPase. Na,K-ATPase isoforms α1, α2, and α3 were observed both in native NPE and also in cultured NPE cells. Na, K-ATPase is a primary active transport system that establishes and maintains a low concentration of cytoplasmic sodium in NPE. The sodium gradient across the cell membrane is utilized to drive other secondary active transport system for solute transport. Na, K-ATPase activity is a prerequisite for aqueous humor formation.26 It has been reported that Na, K-ATPase activity is higher in the NPE than in the PE.27–29 The expression pattern of different Na, K-ATPase α isoforms are also found to be different in the two cell types. By immunochemi-cal localization, it has been shown that NPE expresses Na, K-ATPase α1, α2, and α3 isoforms while PE expresses only the α1 isoform.23 Thus, expression of Na,K-ATPase α1, α2, and α3 isoforms in cultured NPE in the current study is in agreement with the previous finding.23,30 It has been shown that NPE cells in the anterior or pars plicata region of the CE show very intense expression of α1, α2, and α3 Na,K-ATPase isoforms, whereas NPE in the middle region shows lower or no expression of these isoforms.30 In the current study, we did not culture cells from a selected region of the CE. We isolated entire ring of the NPE, dispersed, it and then cultured. Thus, it is a heterogeneous population of cells from all the regions, including pars plicata and pars plana.
In the current investigation, we have shown rich expression of both occludin and ZO-1 in cultured cells. Occludin and ZO-1 are integral components of the blood-aqueous barrier in the eye.31 Occludin is a trans-membrane protein specific only to tight junction.32 Oc-cludin and ZO-1 had previously been colocalized in the tight junction.33 In a previous report, it had been shown that in tissues where tight junctions are well developed, ZO-1 was exclusively concentrated at the site of tight junction.34 There is, however, a report that in the human eye, ZO-1 was found along the apical surfaces between the pigmented and nonpigmented ciliary epithelial cell layers.35 We observed ZO-1 expression in both nonconfluent and confluent cultured NPE. When cells were confluent, ZO-1 seems to be oriented along the borders of most adjacent cells. This pattern of ZO-1 expression together with rich expression of occluding in cultured NPE suggests that the confluent monolayer might form tight junctions. Tight junctions are found only in the NPE layer of the ciliary epithelium. It has been shown convincingly that passage of high-molecular-weight solutes from ciliary process stroma to the posterior chamber is blocked at the level of the NPE.2 It had also been shown that in tissues where tight junctions are well developed, ZO-1 is exclusively concentrated at the site of tight junction.34
In spite of the similar expression of several proteins of interest, we acknowledge that some differences are likely between the native and cultured NPE cells, both in terms of function and expression of proteins, as has been reported earlier.36 It is also possible that cells may vary from one passage to another. Here we have presented a reproducible method of culture and propagation of NPE cells. Moreover, the continued expression of several native key proteins, including three isoforms of Na, K-ATPase α catalytic subunit, three isoforms of NOS, tight junction–associated proteins occludin and ZO-1, suggests that the cultured NPE may be useful for studying some aspects of NPE function. Just how useful these cells will be in studying aqueous humor physiology/pharmacology clearly awaits further investigation.
In summary, we present here a reliable method for culturing NPE cells from the porcine eye. We were able to obtain and maintain cells in primary culture that continue to express several proteins characteristic of native NPE. We anticipate that the NPE cells cultured in this way will be a valuable in vitro model for use in future studies.
Acknowledgments
This work was supported by NIH grant EY06915 and the core facilities grant R24EY015636.
Contributor Information
Mohammad Shahidullah, Department of Physiology, University of Arizona, Tucson, Arizona, USA.
Shigeo Tamiya, Department of Ophthalmology, University of Louisville, Louisville, Kentucky, USA.
Nicholas A. Delamere, Department of Physiology, University of Arizona, Tucson, Arizona, USA
References
- 1.Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Investi Ophthalmol Vis Sci. 1978;17:958–981. [PubMed] [Google Scholar]
- 2.Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res. 2001;73:581–592. doi: 10.1006/exer.2001.1056. [DOI] [PubMed] [Google Scholar]
- 3.Civan MM. The Eye’s Aqueous Humor: From Secretion to Glaucoma. New York: Academic Press; 1998. Transport components of net secretion of the aqueous humour and their integrated regulation; pp. 1–24. [Google Scholar]
- 4.McLaughlin CW, Zellhuber-McMillan S, Macknight AD, et al. Electron microprobe analysis of ouabain-exposed ciliary epithelium: PE-NPE cell couplets form the functional units. Amer J Physiol Cell Physiol. 2004;286:C1376–C1389. doi: 10.1152/ajpcell.00248.2003. [DOI] [PubMed] [Google Scholar]
- 5.Edelman JL, Sachs G, Adorante JS. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. Ame J Physiol. 1994;266:C1210–1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]
- 6.Oh J, Krupin T, Tang LQ, et al. Dye coupling of rabbit ciliary epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1994;35:2509–2514. [PubMed] [Google Scholar]
- 7.Patil RV, Han Z, Yiming M, et al. Fluid transport by human non-pigmented ciliary epithelial layers in culture: a homeostatic role for aquaporin-1. Ame J Physiol Cell Physiol. 2001:281. doi: 10.1152/ajpcell.2001.281.4.C1139. [DOI] [PubMed] [Google Scholar]
- 8.Bode DC, Hamel LT, Wax MB. Distinct profiles of phosphodi-esterase isozymes in cultured cells derived from nonpigmented and pigmented ocular ciliary epithelium. J Pharmacol Exper Ther. 1993;267:1286–1291. [PubMed] [Google Scholar]
- 9.Hochgesand DH, Dunn JJ, Crook RB. Catecholaminergic regulation of Na-K-Cl cotransport in pigmented ciliary epithelium: differences between PE and NPE. Exp Eye Res. 2001;72:1–12. doi: 10.1006/exer.2000.0927. [DOI] [PubMed] [Google Scholar]
- 10.Coca-Prados M, Ghosh S, Gilula NB, et al. Expression and cellular distribution of the alpha 1 gap junction gene product in the ocular pigmented ciliary epithelium. Curr Eye Res. 1992;11:113–122. doi: 10.3109/02713689209000061. [DOI] [PubMed] [Google Scholar]
- 11.Helbig H, Korbmacher C, Kuhner D, et al. Characterization of Cl-/HCO3- exchange in cultured bovine pigmented ciliary epithe-lium. [erratum appears in Exp Eye Res 1989 Feb;48(2):319] Exp Eye Res. 1988;47:515–523. doi: 10.1016/0014-4835(88)90091-7. [DOI] [PubMed] [Google Scholar]
- 12.Fleisher LN, Mc GM, Ferrell JB. Rabbit pigmented ciliary epithelium produces interleukin-6 in response to inflammatory cytokines. Exper Eye Res. 2000;70:271–279. doi: 10.1006/exer.1999.0787. [DOI] [PubMed] [Google Scholar]
- 13.Shahidullah M, Wilson WS. Atriopeptin, sodium azide and cyclic GMP reduce secretion of aqueous humour and inhibit intracellular calcium release in bovine cultured ciliary epithelium. Bri J Pharmacol. 1999;127:1438–1446. doi: 10.1038/sj.bjp.0702681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coca-Prados M, Chatt G. Growth of non-pigmented ciliary epithelial cells in serum-free hormone-supplemented media. Exp Eye Res. 1986;43:617–629. doi: 10.1016/s0014-4835(86)80028-8. [DOI] [PubMed] [Google Scholar]
- 15.Smyth RJ, Kitada S, Lee DA. Growth of rabbit pigmented and nonpigmented ciliary body epithelium. Vision Res. 1994;34:137–141. doi: 10.1016/0042-6989(94)90326-3. [DOI] [PubMed] [Google Scholar]
- 16.McDonald TF, Green K. Cell turnover in ciliary epithelium compared to other slow renewing epithelia in the adult mouse. Curr Eye Res. 1988;7:247–252. doi: 10.3109/02713688809047029. [DOI] [PubMed] [Google Scholar]
- 17.Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the non-pigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahidullah M, Yap MK, To CH. cGMP, sodiun nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br J Pharmacol. 2005;144:1–9. doi: 10.1038/sj.bjp.0706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamiya S, Delamere NA. The influence of sodium-calcium exchange inhibitors on rabbit lens ion balance and transparency. Exp Eye Res. 2006;83:1–7. doi: 10.1016/j.exer.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Helbig H, Korbmacher C, Wohlfarth J, et al. Electrical membrane properties of a cell clone derived from human nonpigmented ciliary epithelium. Invest Ophthalmol Visual Sci. 1989;30:882–889. [PubMed] [Google Scholar]
- 21.Liu R, Flammer J, Haefliger IO. Forskolin upregulation of NOS I protein expression in porcine ciliary processes: a new aspect of aqueous humor regulation. Klinische Monatsblatter fur Augenheilkunde. 2002;219:281–283. doi: 10.1055/s-2002-30643. [DOI] [PubMed] [Google Scholar]
- 22.Meyer P, Champion C, Schlotzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999;18:375–380. doi: 10.1076/ceyr.18.5.375.5355. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S, Freitag AC, Martin-Vasallo P, et al. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J Biol Chem. 1990;265:2935–29340. [PubMed] [Google Scholar]
- 24.Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog Horm Res. 1998;53:43–59. discussion 59–60. [PubMed] [Google Scholar]
- 25.Chiou GC. Review: effects of nitric oxide on eye diseases and their treatment. J Ocul Pharmacol Ther. 2001;17:189–198. doi: 10.1089/10807680151125555. [DOI] [PubMed] [Google Scholar]
- 26.Cole DF. Location of Ouabain-sensitive adenosine Triphosphatase in ciliary epithelium. Expe Eye Res. 1964;3:72–75. doi: 10.1016/s0014-4835(64)80009-9. [DOI] [PubMed] [Google Scholar]
- 27.Riley MV, Kishida K. ATPases of ciliary epithelium: cellular and subcel-lular distribution and probable role in secretion of aqueous humor. Exper Eye Res. 1986;42:559–568. doi: 10.1016/0014-4835(86)90046-1. [DOI] [PubMed] [Google Scholar]
- 28.Usukura J, Fain GL, Bok D. [3H]ouabain localization of Na-K ATPase in the epithelium of rabbit ciliary body pars plicata. Invest Ophthalmol Visual Sci. 1988;29:606–614. [PubMed] [Google Scholar]
- 29.Dunn JJ, Lytle C, Crook RB. Immunolocalization of the Na-K-Cl co-transporter in bovine ciliary epithelium. Invest Ophthalmol Visual Sci. 2001;42:343–353. [PubMed] [Google Scholar]
- 30.Ghosh S, Hernando N, Martin-Alonso JM, et al. Expression of multiple Na+, K(+)-ATPase genes reveals a gradient of isoforms along the nonpigmented ciliary epithelium: functional implications in aqueous humor secretion. J Cell Physiol. 1991;149:184–194. doi: 10.1002/jcp.1041490203. [DOI] [PubMed] [Google Scholar]
- 31.Wu P, Gong H, Richman R, et al. Localization of occludin, ZO-1, and pan-cadherin in rabbit ciliary epithelium and iris vascular endothelium. Histoche Cell Biol. 2000;114:303–310. doi: 10.1007/s004180000195. [DOI] [PubMed] [Google Scholar]
- 32.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. [see comment] J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tserentsoodol N, Shin BC, Suzuki T, et al. Colocalization of tight junction proteins, occludin and ZO-1, and glucose transporter GLUT1 in cells of the blood-ocular barrier in the mouse eye. Histochem Cell Biol. 1998;110:543–551. doi: 10.1007/s004180050316. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson BR, Siliciano JD, Mooseker MS, et al. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonsino J, Gong H, Wu P, et al. Co-localization of junction-associated proteins of the human blood–aqueous barrier: occludin, ZO-1 and F-actin. Exp Eye Res. 2002;74:123–129. doi: 10.1006/exer.2001.1100. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Vasallo P, Ghosh S, Coca-Prados M. Expression of Na,K-ATPase alpha subunit isoforms in the human ciliary body and cultured ciliary epithelial cells. J Cell Phys. 1989;141:243–252. doi: 10.1002/jcp.1041410203. [DOI] [PubMed] [Google Scholar]