Abstract
BACKGROUND
Image-guided radiation therapy (IGRT) is a novel array of in-room imaging modalities that are utilized for tumor localization and patient set-up in radiation oncology. The prevalence of IGRT use among U.S. radiation oncologists is unknown.
METHODS
A random sample of 1600 radiation oncologists was surveyed by internet, email and fax regarding frequency of IGRT use, clinical applications, and future plans for use. The definition of IGRT included imaging technologies used for set-up verification or tumor localization during treatment.
RESULTS
Of 1089 evaluable respondents, 393 responses (36.1%) were received. The proportion of radiation oncologists using IGRT was 93.5%. When the use of MV portal imaging was excluded from the definition of IGRT, the proportion using IGRT was 82.3%. The majority used IGRT rarely (in <25% of their patients) (28.9%) or infrequently (25–50% of their patients) (33.1%). The percentage using ultrasound, video, megavoltage (MV) planar, kilovoltage (kV) planar, and volumetric technologies were 22.3%, 3.2%, 62.7%, 57.7% and 58.8%, respectively. Among IGRT users, the most common disease sites treated were genitourinary (91.1%), head and neck (74.2%), central nervous system (71.9%), and lung (66.9%). 59.1% of IGRT users planned to increase use, while 71.4% of non-users planned to adopt IGRT in the future.
CONCLUSIONS
IGRT is widely used among radiation oncologists. Based on prospective plans of responders, its use is expected to increase. Further research is required to determine the safety, cost-efficacy, and optimal applications of these technologies.
Keywords: image-guided radiation therapy (IGRT), in-room, set-up, survey, radiation oncology
INTRODUCTION
Image-guided radiation therapy (IGRT) consists of an array of imaging technologies designed to improve target localization and patient set-up. In recent years, new in-room technologies have provided the opportunity for unprecedented accuracy in radiation therapy (RT) delivery. The concomitant expanding use of intensity modulated RT (IMRT) 1 and hypofractionated stereotactic techniques 2 has required improved accuracy, providing a strong impetus to adopt IGRT.
Numerous IGRT technologies have been applied to treat cancer over the last half century. Early technologies to improve patient set-up included kilovoltage (kV) planar x-ray-based 3 and video-based systems 4–6. Subsequently, megavoltage (MV) planar imaging technologies were developed, notably electronic portal imaging devices (EPID) 7, 8. Various types of floor-mounted 9–12 or gantry mounted 13–16 kV planar imaging technologies have also been implemented over the years. Ultrasound 17–24 and EPID with implanted radio-opaque (fiducial) markers 25–33 were relatively early developments to improve target localization. Recently, in-room volumetric imaging systems, such as MV computed tomography (CT) 34, 35 and MV 36–38 or kV 16, 39–43 cone-beam computed tomography (CBCT) have been introduced, providing greater soft tissue definition and improved target localization. Collectively, these IGRT technologies provide the potential to escalate target doses while decreasing normal tissue doses, thereby improving the therapeutic ratio of RT.
Although there is considerable interest in IGRT technologies, little is known about their use in the radiation oncology community. It is unclear how many radiation oncologists currently use these technologies, which technologies are used and to what extent, and how they are being applied. To answer these questions, we conducted a nationwide survey of practicing radiation oncologists.
MATERIALS AND METHODS
Sample
We randomly selected 1600 out of approximately 5000 radiation oncologists listed in the 2008 American Society for Radiation Oncology (ASTRO) directory. All physicians designated as active and allied members were included. Emeritus professors and radiation oncologists practicing outside of the United States were excluded. The survey was sent in three forms: as an email attachment, as a link to an online survey, and via fax. We attempted to contact each physician using the listed email address or fax number. If neither were valid, we searched for updated contact information in the 2009 ASTRO on-line directory. If no information could be found or if the fax or e-mail information were invalid, the physician was designated as uncontactable and excluded from further analysis. Those who had retired were also excluded. Physicians who returned the survey blank were counted as non-respondents.
Survey
A 10-question survey was designed to collect demographic information and address the use of IGRT technologies in patients undergoing RT (Table 1). This survey was conducted between February 1 and March 31, 2009 as part of a larger, comprehensive survey on IGRT, including the use of advanced imaging modalities to augment target delineation. The results of other aspects of the IGRT survey are the subject of a separate report.
Table 1.
Image-guided radiation therapy survey
| 1. Type of center at which you practice? | |||||||||||
| _____Academic _____Private Practice | |||||||||||
| 2. Total number of radiation oncologists in your practice? _____ | |||||||||||
| 3. What is your gender? | |||||||||||
| _____Male _____Female | |||||||||||
| 4. What year did you graduate from residency? _____ | |||||||||||
| 5. Do you limit your practice predominantly to select disease sites? If so, which sites? (If you don’t limit your practice to select sites, skip to the next question) | |||||||||||
| _____CNS _____Breast _____Lung _____GU/Prostate | |||||||||||
| _____Other (please specify): ___________________________ | |||||||||||
| 6. Which of the following in-room IGRT technologies have you used for patient setup and/or tumor localization? For each technology you have used, please specify when you adopted it (including experience in residency, if any) and whether you currently still use it. If you have never used a specific technology, leave the corresponding answer space(s) blank. | |||||||||||
| Technology | Year Adopted | Currently Use? (Yes/No) |
If NO, what year did you stop? |
||||||||
| Ultrasound | |||||||||||
| Video | |||||||||||
| EPID without implanted fiducial markers |
|||||||||||
| EPID with implanted fiducial markers | |||||||||||
| On-Board Kilovoltage Imaging without implanted fiducial markers |
|||||||||||
| On-Board Kilovoltage Imaging with implanted fiducial markers |
|||||||||||
| Novalis | |||||||||||
| Cyberknife | |||||||||||
| Tomotherapy | |||||||||||
| CBCT (MV or kV) | |||||||||||
| CT-on-Rails | |||||||||||
|
If you do not CURRENTLY use any of the in-room IGRT technologies listed in question 6, skip to question 10 | |||||||||||
| 7. Mark the disease site(s) with an “x” in which you CURRENTLY use any of the following in- room IGRT technologies. (If you don’t use a specific technology in a particular disease site, leave the corresponding answer space(s) blank). | |||||||||||
|
Technology |
CNS | H/N | Breast | Lung | GI | GU | Gyn | Ped | Lym | Pall | Other (specify): |
| Ultrasound | |||||||||||
| Video | |||||||||||
| EPID without implanted fiducial markers |
|||||||||||
| EPID with implanted fiducial markers |
|||||||||||
| On-Board kV Imaging without fiducial markers |
|||||||||||
| On-Board kV Imaging with implanted fiducial Markers |
|||||||||||
| Novalis | |||||||||||
| Cyberknife | |||||||||||
| Tomotherapy | |||||||||||
| CBCT (MV or kV) | |||||||||||
| CT-on-Rails | |||||||||||
| 8. Approximately what percentage of your current patients are treated using any of the in-room IGRT technologies specified in question 7? | |||||||||||
| _____None ____<25% _____25–50% _____51–75% _____>75% | |||||||||||
| 9. Which of the in-room IGRT technologies listed in question 7 do you most commonly use currently? ___________________ | |||||||||||
| 10. What are your future plans for in-room IGRT technologies? | |||||||||||
| _____Do not plan to adopt _____Maintain current use _____Start using / Increase use Stop using / Decrease use | |||||||||||
IGRT: image-guided radiation therapy; CNS: central nervous system; H/N: head and neck; GI: gastrointestinal; GU: genitourinary; Gyn: gynecological; Ped: pediatric; Lym: lymphoma; Pal: palliative; EPID: electronic portal imaging device; kV: kilovoltage, MV: megavoltage, CT: computed tomography, CBCT: cone-beam computed tomography.
Survey responses were considered evaluable if the survey was at least partially completed. For the purposes of this survey, we defined IGRT as the use of any of the following imaging modalities: ultrasound, video, planar, and volumetric imaging performed in the treatment room to aid in patient set-up or tumor localization. Each of the four categories included home-grown and multiple commercial systems (Table 2). Accompanying the survey was a cover letter outlining the goals of the project and confidential nature of the results obtained. In particular, it was stressed that the findings were to be used for academic purposes only and that company-specific data would not be disclosed or presented.
Table 2.
In-room image-guided radiation therapy technologies
| Category | Product |
|---|---|
| Ultrasound | B-mode acquisition and targeting (BAT) system |
| I-Beam | |
| SonArray | |
| Restitu/Clarity system | |
| Video | AlignRT |
| Sentinel | |
| Planar (MV) (EPID) | iView |
| Beamview | |
| PortalVision | |
| Planar (kV) | Cyberknife |
| Novalis | |
| Elekta XVI | |
| OBI | |
| TomoTherapy | |
| Volumetric (MV) | MVision |
| Volumetric (kV) | Elekta XVI |
| OBI | |
| CT-on-rails | Primatom |
| EXaCT |
MV: megavoltage, kV: kilovoltage, OBI: On-board-imaging, CBCT: cone-beam computed tomography
In addition to inquiring about practice type (academic vs. private practice) and size of practice group, physicians were asked about the type(s) of IGRT technologies used, the year they had adopted them, percentage of patients in their practice they currently treat with IGRT, disease sites treated, and future plans for IGRT use. Nonusers were asked whether or not they intended to adopt IGRT technologies in the future.
Statistical Analysis
Survey results are presented as a percentage of evaluable responses. Differences in proportions between various groups were analyzed using the chi-square and Fisher’s exact test. The Holm step-down method was used to adjust the p values for multiple comparisons 44.
RESULTS
Of 1600 randomly selected physicians, 1089 physicians (68.1%) were contactable (Figure 1). From these, we received a total of 393 responses (36.1%). Of the 393 respondents, 7 were retired and 1 returned the survey blank, thus a total of 385 responses were evaluable.
Figure 1.
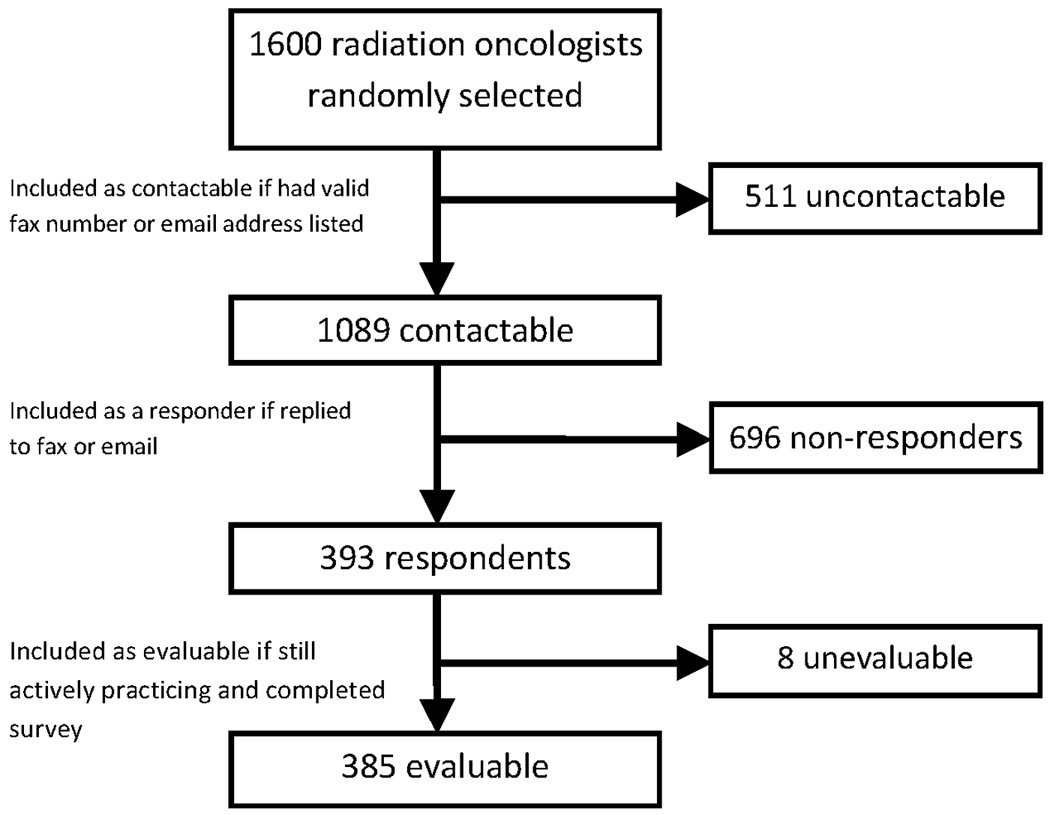
Survey Flow Chart
Responses were received from physicians in 45 states (Table 3). One-hundred thirty-three responses were from academic physicians (34.5%), and 252 responses were from private practice physicians (65.5%). Three respondents returned the survey with incomplete demographic information.
Table 3.
Characteristics of respondents
| Number of Physicians | 385 |
| Sex, n (%) | |
| Male | 287 (74.5) |
| Female | 97 (25.2) |
| Unknown | 1 (0.3) |
| Geographic location a, n (%) | |
| Midwest | 112 (29.1) |
| South | 104 (27.0) |
| East | 88 (22.9) |
| West | 79 (20.5) |
| Unknown | 2 (0.5) |
| Practice type, n (%) | |
| Academic | 133 (34.5) |
| Private | 252 (65.5) |
| Specialist b, n (%) | 107 (27.7) |
| Years in practice, median (range) | 16 (1–44) |
| Number of physicians per practice, median (range) |
5 (1–55) |
EAST: CT, DC, DE, MA, MD,ME,NH, NJ, NY, PA, RI, VT, WV; SOUTH: AL, AR, FL, GA, LA,MS, NC, SC, TN, TX, VA; MIDWEST: IA, IL, IN, KS, KY, MI, MN, MO, ND, NE, OH, OK, SD, WI; WEST: AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY.
Specialty categories: central nervous system, breast, lung, prostate, other.
Of 385 evaluable respondents, 360 respondents (93.5%; 95% confidence interval, 91.0–96.0%) reported having used IGRT technologies in their practices. When the use of MV portal imaging was excluded from the definition of IGRT, the proportion using IGRT was 82.3%. The majority reported using such technologies rarely (in <25% of their patients) (28.9%) or infrequently (25–50% of their patients) (33.1%). The percentages of physicians who reported using IGRT frequently (51–75% of their patients) or routinely (>75% of their patients) were 18.7% and 19.3%, respectively.
The most commonly used IGRT modalities were MV planar (62.7%), volumetric (58.8%) and kV planar imaging (57.7%). The percentage of respondents using at least one or more of these technologies was 89.4%. Ultrasound and video technologies were used by 22.3% and 3.2% of physicians, respectively.
IGRT was applied in all disease sites, most commonly genitourinary (GU) (91.1%), head and neck (74.2%), and the central nervous system (CNS) (71.9%) (Table 4). Volumetric-based technologies were the most commonly used modalities in lung (59.3%), head and neck (56.9%), gastrointestinal (56.9%), and GU (55.3%) tumors, while kV planar-based technologies were the most commonly used in CNS tumors (62.6%). Ultrasound (with the exception of GU tumors) and video were less commonly used in all sites (Figure 2).
Table 4.
Proportions of radiation oncologists using in-room image-guided radiation therapy to treat various disease sites.
| Disease Site | Number of users (% of all users) |
|---|---|
| Genitourinary | 328 (91.1) |
| Head and Neck | 267 (74.2) |
| Central Nervous System | 259 (71.9) |
| Lung | 241 (66.9) |
| Gastrointestinal | 216 (60.0) |
| Gynecologic | 209 (58.1) |
| Palliative | 164 (45.5) |
| Breast | 160 (44.4) |
| Lymphoma | 144 (40.0) |
| Pediatrics | 86 (23.9) |
Figure 2.
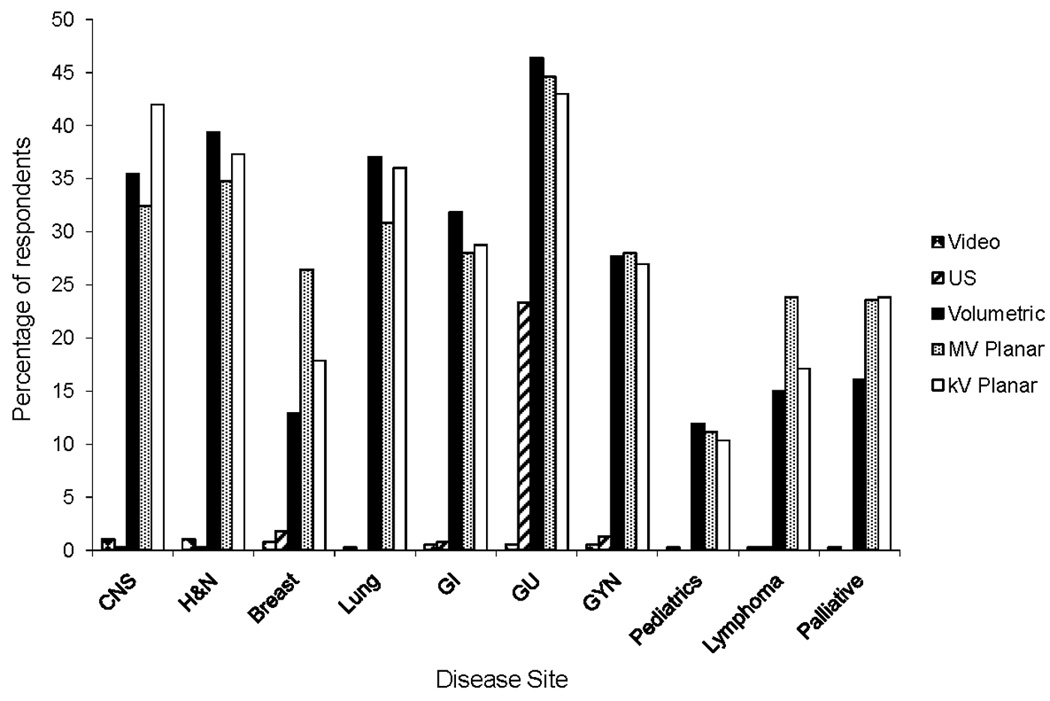
Utilization of individual image-guided radiation therapy modalities, by disease site. MV, megavoltage; kV, kilovoltage; CNS, central nervous system; H&N, head and neck; GI, gastrointestinal; GU, genitourinary; GYN, gynecology.
A similar proportion of academic and private practice radiation oncologists used IGRT overall (94.7% and 94.8%, p = 0.78). As shown in Figure 3, there was no difference observed in the proportion of academic and private practice physicians using video, ultrasound, or MV planar modalities. However, academic physicians were more likely to use volumetric techniques (75.2% vs. 50.8%, p <0.001) and kV planar techniques (72.2% vs. 50.0%, p <0.001) than private practice physicians. In addition, academic physicians were more likely to use IGRT frequently or routinely (>50% of their patients) in their practice, compared to private practice physicians (47.5% vs. 31.8%, p<0.01).
Figure 3.
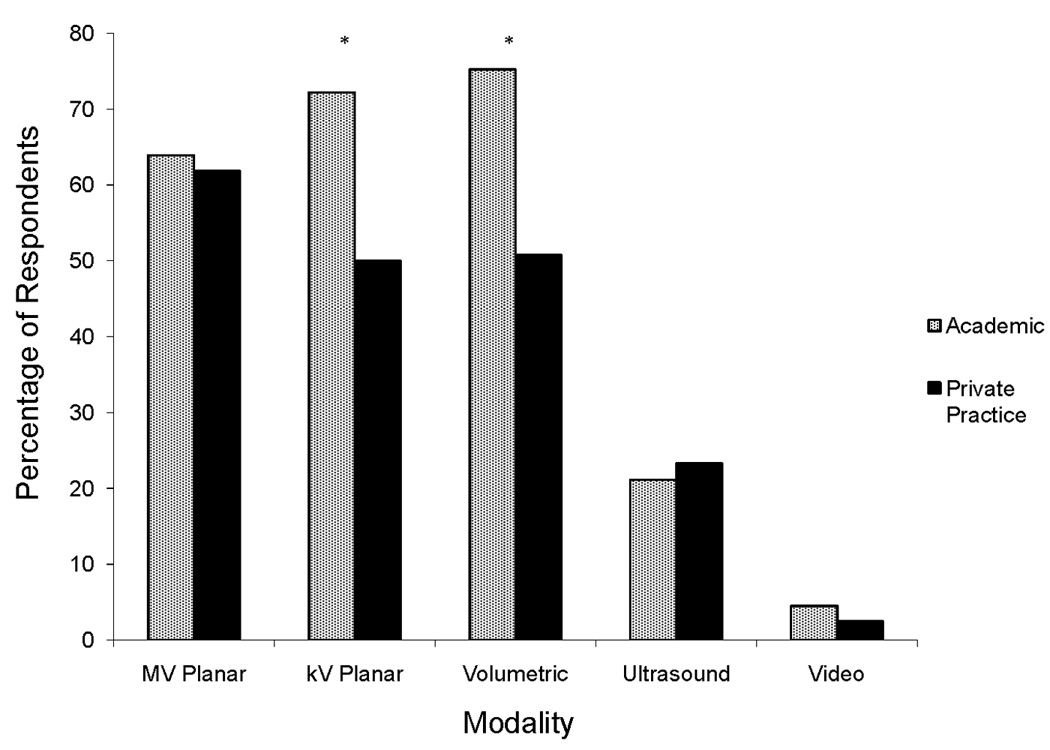
Percentage of academic versus private practice physicians using image-guided radiation therapy technologies. MV, megavoltage; kV, kilovoltage. *p<0.05.
Results were also compared based on geography, years of experience, size of practice, and specialization. The percentages of radiation oncologists using IGRT in the East, South, Midwest, and West were 92.6%, 93.1%, 94.4%, 97.4%, respectively (p=0.56). No difference was seen in utilization by years in practice; the percentages of users with 1–10, 11–20, and > 20 years in practice were 96.1%, 93.9% and 96.5% (p = 0.38), respectively. However, a difference in the type of IGRT used was seen, with physicians with ≤ 10 years in practice less likely to use MV planar-based technologies than those with > 10 years in practice (50.0% vs 65.0%, p=0.02). The percentages of respondents in practices with 1, 2–10, and >10 physicians who reported using IGRT were similar (90.7%, 94.8%, and 97.2%, respectively, p=0.31) Overall, utilization was similar for specialists and non-specialists. However, specialists were more likely to use them frequently or routinely (in >50% of their patients) compared to non-specialists (47.1% vs. 19.1%, p<0.001).
Figure 4 illustrates the cumulative adoption of each IGRT modality, based on reported years of adoption and cessation. Ultrasound and MV planar-based systems were adopted earliest. The majority of respondents using ultrasound (54.5%) reported having adopted it by 2001. However, the percentage of respondents adopting ultrasound peaked in 2006, then declined. The majority of respondents using MV planar technologies (53.4%) reported having implemented them by 2004. Adoption of kV planar-based modalities followed, with the majority of users (54.3%) having adopted them by 2006. Volumetric-based imaging modalities were implemented more recently, with the majority of users (67.1%) having adopted them by 2007. Of responders using IGRT, 40.6% planned to maintain their current level of use, while 59.1% planned to increase use. Among rare or infrequent users (≤ 50% of their patients), 65.8% planned to increase their use, while 47.0% of frequent or routine users (> 50% of their patients) users planned to increase use. One current user planned to decrease use. Among non-users, 71.4% planned to adopt IGRT technologies in the future.
Figure 4.
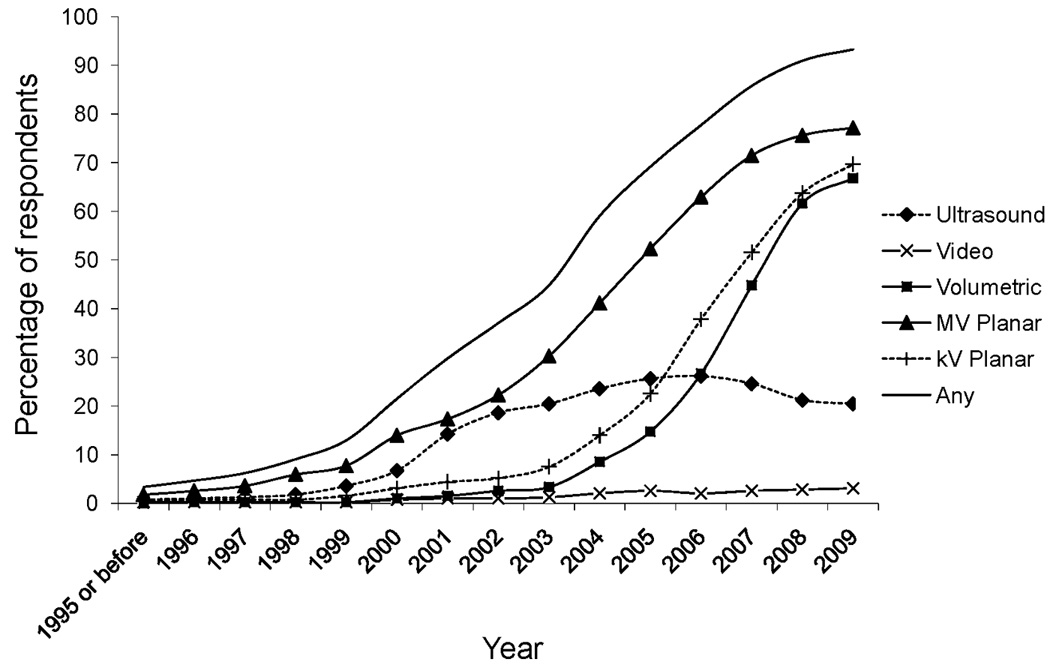
Cumulative adoption of image-guided radiation therapy (IGRT) technologies. The total percentage of respondents adopting or discontinuing IGRT utilization is plotted by year. MV: megavoltage, kV: kilovoltage.
DISCUSSION
Our aim in this study was to assess the utilization of IGRT technologies among radiation oncologists in the United States. We found that the great majority of practicing radiation oncology physicians currently use IGRT, with more than 90% of respondents using at least one form of IGRT in their practice. However, the majority of users implemented IGRT in less than 50% of their patients.
Although no overall difference in IGRT use existed between academic and private practice physicians, academic physicians tended to use IGRT in a larger proportion of their patients. Moreover, we found that certain modalities, notably kV planar and volumetric imaging, were used more commonly among academic physicians. The reason underlying such differences is uncertain, but may be due to different levels of access to these technologies, greater use by specialists, or use of volumetric-based imaging for research trials at academic centers.
We noted that among the various IGRT modalities, MV planar and ultrasound modalities were adopted earliest, followed later by kV planar and volumetric modalities. Utilization of ultrasound appears to be decreasing, likely due to decreasing use in favor of alternative technologies. This may be in part due to studies comparing ultrasound to other technologies, which have found planar imaging with implanted seeds and volumetric modalities to provide superior accuracy in terms of set-up and tumor localization 45–47. We also observed that physicians with fewer years in practice tended to use MV-planar modalities less commonly, possibly signifying a decline in their use in the future. Overall, however, based on future plans of both users and non-users, IGRT utilization is expected to increase. Notably, even physicians who reported using IGRT in the majority of patients planned to increase utilization within their practices.
This is the first study to assess the overall utilization of in-room IGRT in the radiation oncology community. We randomly sampled a large cohort of radiation oncologists representative of physicians with a wide range of characteristics. However, despite diligent attempts to collect responses from the sample, non-response and recall bias are potential limitations of this study. It is possible that IGRT non-users or users of specific technologies were less likely to respond, which would lead to biased estimates of the true prevalence of IGRT utilization. The survey was also brief and could not address questions concerning reasons for IGRT adoption. In order to address some of these limitations, we intend to conduct a follow-up survey in 2011.
Our study’s findings indicate a need for further research to assess the efficacy and safety of IGRT utilization 48. IGRT technologies come with added cost, time, and, in the case of some imaging modalities, dose delivered to patients during treatment 49–51. The majority of literature published on these technologies reports on dosimetric consequences and set-up accuracy, but there is limited data regarding clinical outcomes, such as disease recurrence and treatment toxicity. Given the widespread and apparently increasing use of IGRT, prospective studies on clinical outcomes are needed to assess its clinical impact, safety, and cost-efficacy.
Acknowledgments
Supported by NIH T32 grant RR023254
Footnotes
Conflicts of Interest Notification: None
To be presented at the 51st Annual Meeting of the American Society of Radiation Oncology (ASTRO), Chicago, IL, November, 2009
REFERENCES
- 1.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005 Sep 15;104(6):1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 2.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007 Dec;30(6):637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 3.Johns HE, Cunningham JR. A precision cobalt 60 unit for fixed field and rotation therapy. Am J Roentgenol Radium Ther Nucl Med. 1959 Jan;81(1):4–12. [PubMed] [Google Scholar]
- 4.Johnson LS, Milliken BD, Hadley SW, Pelizzari CA, Haraf DJ, Chen GT. Initial clinical experience with a video-based patient positioning system. Int J Radiat Oncol Biol Phys. 1999 Aug 1;45(1):205–213. doi: 10.1016/s0360-3016(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 5.Connor WG, Boone ML, Veomett R, et al. Patient repositioning and motion detection using a video cancellation system. Int J Radiat Oncol Biol Phys. 1975 Oct–Nov;1(1–2):147–153. doi: 10.1016/0360-3016(75)90023-1. [DOI] [PubMed] [Google Scholar]
- 6.Milliken BD, Rubin SJ, Hamilton RJ, Johnson LS, Chen GT. Performance of a video-image-subtraction-based patient positioning system. Int J Radiat Oncol Biol Phys. 1997 Jul 1;38(4):855–866. doi: 10.1016/s0360-3016(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 7.Lam KS, Partowmah M, Lam WC. An on-line electronic portal imaging system for external beam radiotherapy. Br J Radiol. 1986 Oct;59(706):1007–1013. doi: 10.1259/0007-1285-59-706-1007. [DOI] [PubMed] [Google Scholar]
- 8.Meertens H, van Herk M, Bijhold J, Bartelink H. First clinical experience with a newly developed electronic portal imaging device. Int J Radiat Oncol Biol Phys. 1990 May;18(5):1173–1181. doi: 10.1016/0360-3016(90)90455-s. [DOI] [PubMed] [Google Scholar]
- 9.Chang SD, Murphy M, Geis P, et al. Clinical experience with image-guided robotic radiosurgery (the Cyberknife) in the treatment of brain and spinal cord tumors. Neurol Med Chir (Tokyo) 1998 Nov;38(11):780–783. doi: 10.2176/nmc.38.780. [DOI] [PubMed] [Google Scholar]
- 10.Adler JR, Jr, Murphy MJ, Chang SD, Hancock SL. Image-guided robotic radiosurgery. Neurosurgery. 1999 Jun;44(6):1299–1306. discussion 1306-1297. [PubMed] [Google Scholar]
- 11.Yan H, Yin FF, Kim JH. A phantom study on the positioning accuracy of the Novalis Body system. Med Phys. 2003 Dec;30(12):3052–3060. doi: 10.1118/1.1626122. [DOI] [PubMed] [Google Scholar]
- 12.Rahimian J, Chen JC, Rao AA, Girvigian MR, Miller MJ, Greathouse HE. Geometrical accuracy of the Novalis stereotactic radiosurgery system for trigeminal neuralgia. J Neurosurg. 2004 Nov;101 Suppl 3:351–355. [PubMed] [Google Scholar]
- 13.Fox T, Huntzinger C, Johnstone P, Ogunleye T, Elder E. Performance evaluation of an automated image registration algorithm using an integrated kilovoltage imaging and guidance system. J Appl Clin Med Phys. 2006 Winter;7(1):97–104. doi: 10.1120/jacmp.v7i1.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisani L, Lockman D, Jaffray D, Yan D, Martinez A, Wong J. Setup error in radiotherapy: on-line correction using electronic kilovoltage and megavoltage radiographs. Int J Radiat Oncol Biol Phys. 2000 Jun 1;47(3):825–839. doi: 10.1016/s0360-3016(00)00476-4. [DOI] [PubMed] [Google Scholar]
- 15.Perkins CL, Fox T, Elder E, Kooby DA, Staley CA, 3rd, Landry J. Image-guided radiation therapy (IGRT) in gastrointestinal tumors. JOP. 2006;7(4):372–381. [PubMed] [Google Scholar]
- 16.Sorcini B, Tilikidis A. Clinical application of image-guided radiotherapy, IGRT (on the Varian OBI platform) Cancer Radiother. 2006 Sep;10(5):252–257. doi: 10.1016/j.canrad.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Tome WA, Meeks SL, Orton NP, Bouchet LG, Bova FJ. Commissioning and quality assurance of an optically guided three-dimensional ultrasound target localization system for radiotherapy. Med Phys. 2002 Aug;29(8):1781–1788. doi: 10.1118/1.1494835. [DOI] [PubMed] [Google Scholar]
- 18.Fuss M, Salter BJ, Cavanaugh SX, et al. Daily ultrasound-based image-guided targeting for radiotherapy of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 2004 Jul 15;59(4):1245–1256. doi: 10.1016/j.ijrobp.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Morr J, DiPetrillo T, Tsai JS, Engler M, Wazer DE. Implementation and utility of a daily ultrasound-based localization system with intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002 Aug 1;53(5):1124–1129. doi: 10.1016/s0360-3016(02)02820-1. [DOI] [PubMed] [Google Scholar]
- 20.Chandra A, Dong L, Huang E, et al. Experience of ultrasound-based daily prostate localization. Int J Radiat Oncol Biol Phys. 2003 Jun 1;56(2):436–447. doi: 10.1016/s0360-3016(02)04612-6. [DOI] [PubMed] [Google Scholar]
- 21.Fung AY, Enke CA, Ayyangar KM, et al. Prostate motion and isocenter adjustment from ultrasound-based localization during delivery of radiation therapy. Int J Radiat Oncol Biol Phys. 2005 Mar 15;61(4):984–992. doi: 10.1016/j.ijrobp.2004.07.727. [DOI] [PubMed] [Google Scholar]
- 22.Langen KM, Pouliot J, Anezinos C, et al. Evaluation of ultrasound-based prostate localization for image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2003 Nov 1;57(3):635–644. doi: 10.1016/s0360-3016(03)00633-3. [DOI] [PubMed] [Google Scholar]
- 23.Little DJ, Dong L, Levy LB, Chandra A, Kuban DA. Use of portal images and BAT ultrasonography to measure setup error and organ motion for prostate IMRT: implications for treatment margins. Int J Radiat Oncol Biol Phys. 2003 Aug 1;56(5):1218–1224. doi: 10.1016/s0360-3016(03)00290-6. [DOI] [PubMed] [Google Scholar]
- 24.Trichter F, Ennis RD. Prostate localization using transabdominal ultrasound imaging. Int J Radiat Oncol Biol Phys. 2003 Aug 1;56(5):1225–1233. doi: 10.1016/s0360-3016(03)00269-4. [DOI] [PubMed] [Google Scholar]
- 25.De Neve W, Van den Heuvel F, De Beukeleer M, et al. Routine clinical on-line portal imaging followed by immediate field adjustment using a tele-controlled patient couch. Radiother Oncol. 1992 May;24(1):45–54. doi: 10.1016/0167-8140(92)90353-v. [DOI] [PubMed] [Google Scholar]
- 26.Gildersleve J, Dearnaley DP, Evans PM, Law M, Rawlings C, Swindell W. A randomised trial of patient repositioning during radiotherapy using a megavoltage imaging system. Radiother Oncol. 1994 May;31(2):161–168. doi: 10.1016/0167-8140(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 27.Bel A, Keus R, Vijlbrief RE, Lebesque JV. Setup deviations in wedged pair irradiation of parotid gland and tonsillar tumors, measured with an electronic portal imaging device. Radiother Oncol. 1995 Nov;37(2):153–159. doi: 10.1016/0167-8140(95)01627-s. [DOI] [PubMed] [Google Scholar]
- 28.Fein DA, McGee KP, Schultheiss TE, Fowble BL, Hanks GE. Intra- and interfractional reproducibility of tangential breast fields: a prospective on-line portal imaging study. Int J Radiat Oncol Biol Phys. 1996 Feb 1;34(3):733–740. doi: 10.1016/0360-3016(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 29.Vigneault E, Pouliot J, Laverdiere J, Roy J, Dorion M. Electronic portal imaging device detection of radioopaque markers for the evaluation of prostate position during megavoltage irradiation: a clinical study. Int J Radiat Oncol Biol Phys. 1997 Jan 1;37(1):205–212. doi: 10.1016/s0360-3016(96)00341-0. [DOI] [PubMed] [Google Scholar]
- 30.Van de Steene J, Van den Heuvel F, Bel A, et al. Electronic portal imaging with on-line correction of setup error in thoracic irradiation: clinical evaluation. Int J Radiat Oncol Biol Phys. 1998 Mar 1;40(4):967–976. doi: 10.1016/s0360-3016(97)00925-5. [DOI] [PubMed] [Google Scholar]
- 31.Stroom JC, Olofsen-van Acht MJ, Quint S, et al. On-line set-up corrections during radiotherapy of patients with gynecologic tumors. Int J Radiat Oncol Biol Phys. 2000 Jan 15;46(2):499–506. doi: 10.1016/s0360-3016(99)00386-7. [DOI] [PubMed] [Google Scholar]
- 32.de Boer HC, van Sornsen de Koste JR, Creutzberg CL, Visser AG, Levendag PC, Heijmen BJ. Electronic portal image assisted reduction of systematic set-up errors in head and neck irradiation. Radiother Oncol. 2001 Dec;61(3):299–308. doi: 10.1016/s0167-8140(01)00437-6. [DOI] [PubMed] [Google Scholar]
- 33.Antonuk LE. Electronic portal imaging devices: a review and historical perspective of contemporary technologies and research. Phys Med Biol. 2002 Mar 21;47(6):R31–R65. [PubMed] [Google Scholar]
- 34.Tome WA, Jaradat HA, Nelson IA, Ritter MA, Mehta MP. Helical tomotherapy: image guidance and adaptive dose guidance. Front Radiat Ther Oncol. 2007;40:162–178. doi: 10.1159/000106034. [DOI] [PubMed] [Google Scholar]
- 35.Welsh JS, Lock M, Harari PM, et al. Clinical implementation of adaptive helical tomotherapy: a unique approach to image-guided intensity modulated radiotherapy. Technol Cancer Res Treat. 2006 Oct;5(5):465–479. doi: 10.1177/153303460600500503. [DOI] [PubMed] [Google Scholar]
- 36.Morin O, Gillis A, Chen J, et al. Megavoltage cone-beam CT: system description and clinical applications. Med Dosim. 2006 Spring;31(1):51–61. doi: 10.1016/j.meddos.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Mosleh-Shirazi MA, Evans PM, Swindell W, Webb S, Partridge M. A cone-beam megavoltage CT scanner for treatment verification in conformal radiotherapy. Radiother Oncol. 1998 Sep;48(3):319–328. doi: 10.1016/s0167-8140(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 38.Pouliot J, Bani-Hashemi A, Chen J, et al. Low-dose megavoltage cone-beam CT for radiation therapy. Int J Radiat Oncol Biol Phys. 2005 Feb 1;61(2):552–560. doi: 10.1016/j.ijrobp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Huntzinger C, Munro P, Johnson S, et al. Dynamic targeting image-guided radiotherapy. Med Dosim. 2006 Summer;31(2):113–125. doi: 10.1016/j.meddos.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Letourneau D, Martinez AA, Lockman D, et al. Assessment of residual error for online cone-beam CT-guided treatment of prostate cancer patients. Int J Radiat Oncol Biol Phys. 2005 Jul 15;62(4):1239–1246. doi: 10.1016/j.ijrobp.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Letourneau D, Wong JW, Oldham M, et al. Cone-beam-CT guided radiation therapy: technical implementation. Radiother Oncol. 2005 Jun;75(3):279–286. doi: 10.1016/j.radonc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Oelfke U, Tucking T, Nill S, et al. Linac-integrated kV-cone beam CT: technical features and first applications. Med Dosim. 2006 Spring;31(1):62–70. doi: 10.1016/j.meddos.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Guckenberger M, Meyer J, Vordermark D, Baier K, Wilbert J, Flentje M. Magnitude and clinical relevance of translational and rotational patient setup errors: a cone-beam CT study. Int J Radiat Oncol Biol Phys. 2006 Jul 1;65(3):934–942. doi: 10.1016/j.ijrobp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 45.Scarbrough TJ, Golden NM, Ting JY, et al. Comparison of ultrasound and implanted seed marker prostate localization methods: Implications for image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2006 Jun 1;65(2):378–387. doi: 10.1016/j.ijrobp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Gayou O, Miften M. Comparison of mega-voltage cone-beam computed tomography prostate localization with online ultrasound and fiducial markers methods. Med Phys. 2008 Feb;35(2):531–538. doi: 10.1118/1.2830381. [DOI] [PubMed] [Google Scholar]
- 47.Scarbrough TJ, Ting JY, Kuritzky N. Ultrasound for radiotherapy targeting. Int J Radiat Oncol Biol Phys. 2007 Aug 1;68(5):1579. doi: 10.1016/j.ijrobp.2007.04.010. author reply 1579–1580. [DOI] [PubMed] [Google Scholar]
- 48.Ting JY, Scarbrough TJ. Intensity-modulated radiation therapy and image-guided radiation therapy: small clinic implementation. Hematol Oncol Clin North Am. 2006 Feb;20(1):63–86. doi: 10.1016/j.hoc.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Ding GX, Coffey CW. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys. 2009 Feb 1;73(2):610–617. doi: 10.1016/j.ijrobp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Kan MW, Leung LH, Wong W, Lam N. Radiation dose from cone beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2008 Jan 1;70(1):272–279. doi: 10.1016/j.ijrobp.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 51.Amer A, Marchant T, Sykes J, Czajka J, Moore C. Imaging doses from the Elekta Synergy X-ray cone beam CT system. Br J Radiol. 2007 Jun;80(954):476–482. doi: 10.1259/bjr/80446730. [DOI] [PubMed] [Google Scholar]


