Abstract
A number of human immunodeficiency virus type-1 (HIV) positive subjects are also opiate abusers. These individuals are at high risk to develop neurological complications. However, little is still known about the molecular mechanism(s) linking opiates and HIV neurotoxicity. To learn more, we exposed rat neuronal/glial cultures prepared from different brain areas to opiate agonists and HIV envelope glycoproteins gp120IIIB or BaL. These strains bind to CXCR4 and CCR5 chemokine receptors, respectively, and promote neuronal death. Morphine did not synergize the toxic effect of gp120IIIB but inhibited the cytotoxic property of gp120BaL. This effect was blocked by naloxone and reproduced by the μ opioid receptor agonist DAMGO. To examine the potential mechanism(s) of neuroprotection, we determined the effect of morphine on the release of chemokines CCL5 and CXCL12 in neurons, astrocytes and microglia cultures. CCL5 has been shown to prevent gp120BaL neurotoxicity while CXCL12 decreases neuronal survival. Morphine elicited a time-dependent release of CCL5 but failed to affect the release of CXCL12. This effect was observed only in primary cultures of astrocytes. To examine the role of endogenous CCL5 in the neuroprotective activity of morphine, mixed cerebellar neurons/glial cells were immunoneutralized against CCL5 prior to morphine and gp120 treatment. In these cells the neuroprotective effect of opiate agonists was blocked. Our data suggest that morphine may exhibit a neuroprotective activity against M-tropic gp120 through the release of CCL5 from astrocytes.
Keywords: Astroglia, gp120BaL, DAMGO, CXCL12, CXCR4, CCR5
INTRODUCTION
Heroin abusers are at high risk for contracting human immunodeficiency virus type-1 (HIV) infection and for undergoing an accelerated rate of progression to Acquired Immune Deficiency Syndrome (AIDS) related to the drug-injecting lifestyle (Alonzo and Bayer 2002). In addition, there is an increased incidence for these individuals to develop brain abnormalities (Anthony et al. 2008) that lead to AIDS dementia complex (ADC), a neurological disorder characterized by neuronal loss, synaptic simplification, motor and cognitive impairments (Gonzalez-Scarano and Martin-Garcia 2005). Thus, the characterization of how opiates modulate neuronal degeneration caused by HIV is crucial to develop a therapy that limits ADC.
Various experimental data have suggested that opioid-HIV interactions in the central nervous system (CNS) may occur at several levels. ADC opiate abusers exhibit a trend toward increased microglia in the thalamus and hippocampus (Arango et al. 2004). Microglia can release inflammatory cytokines. Thus, morphine may exacerbate the toxic effect of these cytokines believed to mediate HIV neurotoxicity (Persidsky and Gendelman 2003). On the other hand, opiates have been shown to increase the expression of chemokine receptors CXCR4 and CCR5 in the immune system (Steele et al. 2003; Suzuki et al. 2002). These receptors are crucial for HIV infection. Indeed, the tropism of HIV is predominantly determined by the sequential interaction of the surface envelop protein gp120 with these receptors and CD4 (Berger et al. 1999). Macrophage-tropic (M-tropic) gp120/viruses primarily bind to CCR5, whereas T-cell tropic (T-tropic) gp120/viruses use CXCR4 as co-receptor (Dittmar et al. 1997; Weissman et al. 1997). T-tropic viruses occur in about 50% of infected individuals later in the course of HIV infection and indicate progression to AIDS (Pierson et al. 2004; Simmons et al. 1996). Dual-tropic viruses can use both co-receptors and appear to be specialized in replicating within the CNS (Gray et al. 2009). However, dual-tropic viruses that use primarily CXCR4 have also been isolated (Gray et al. 2009). Thus, opiates may be a risk factor for ADC by modifying HIV progression through increasing the expression of chemokine receptors crucial for HIV infection.
Opioid receptor agonists have also been shown to affect the expression of chemokines (Szabo et al. 2002) and chemokine receptors (Chen et al. 2007) in the CNS. CXCR4 receptors, which are present in neurons and glia (Banisadr et al. 2002; Westmoreland et al. 2002), promote neuronal survival and differentiation (Imitola et al. 2004; Tran and Miller 2005). However, CXCR4 is also directly involved in HIV-associated neuronal damage and apoptosis by mediating the neuropathological action of T-tropic gp120 in vitro (Hesselgesser et al. 1998; Kaul and Lipton 1999; Meucci et al. 1998) and in vivo (Toggas et al. 1994). Neuronal apoptosis is also induced by CXCL12 (Bachis et al. 2003; Kaul et al. 2007), the natural ligand of CXCR4. Similarly, CCR5 mediates the toxic effect of M-tropic gp120 but paradoxically its endogenous ligand CCL5 exhibits neuronal protection (Bachis and Mocchetti 2005; Kaul et al. 2007). Previous data have shown that morphine exacerbates the pro-apoptotic property of T-tropic gp120 in human fetal cells (Hu et al. 2005). Moreover, systemic morphine treatment has been shown to synergize with another HIV protein, Tat, in causing neuronal loss (Bruce-Keller et al. 2008; Gurwell et al. 2001) as well as in increasing the number of macrophages/microglia (El-Hage et al. 2006). Thus, opiates may enhance the toxic response of viral proteins by promoting an environment that can be conducive for neuronal apoptosis. Nevertheless, depending upon the experimental conditions, opiates can also be neuroprotective (Polakiewicz et al. 1998). Thus, it is still unclear whether the consequences of opiate abuse in HIV subjects can be always deleterious. In this study, we used primary culture of mixed neuronal/glial cells to analyze the ability of morphine to synergize or prevent the neurotoxic effect of M- and T-tropic gp120s.
MATERIALS AND METHODS
Cerebellar granule cells
Cerebellar granule cells (CGC) were prepared as described previously (Brandoli et al., 1998; Bachis et al. 2003) from cerebella of 7–8-day-old Sprague Dawley (SD) rats (Charles River, Germatown, MD). Neurons were plated at a density of 5×105 cells per ml onto poly-L-lysine (1%) pre-coated plates (Sigma-Aldrich, St Louis, MO) and were grown in Basal Medium Eagle (BME) (Invitrogen, Grand Island, NY) containing 10% fetal bovine serum, 0.5 mM L-glutamine 25 mM KCl, and 1% antibiotic-antimycotic (Invitrogen). Cells were grown for 7–8 days at 37°C in 5% CO2 95% air.
Mix Cortical cultures
Mixed cortical cultures were prepared from 1–2-day-old SD rats as previously described (Bachis et al. 2009). Cells were recovered by centrifugation. Neurons were plated onto poly-L-Lysine pre-coated plates at a density of 0.5 × 106 cells/ml, and grown in BME containing glutamine (2 mM), fetal calf serum (10%), KCl (5 mM) and antibiotic-antimycotic (Invitrogen). The medium was replaced 24 hr later by fresh medium composed of 50% BME and 50% Neurobasal medium (Invitrogen). Cells were maintained for 7–8 days at 37°C in 5% CO2/95% air.
Embryonic neuronal cultures
Pure cortical or hippocampal neuronal cultures were prepared from the cortex or hippocampus of embryonic (E17–18) SD rats (Charles River) following an established protocol (Dichter 1983) with minor modifications. In brief, cortices were cleaned from blood vessels in Krebs-Ringers bicarbonate buffer containing 0.3% bovine serum albumin (BSA), hippocampi were dissected and processed separately. Cortices and hippocampi were minced and dissociated in the same buffer with 1800 U/ml trypsin at 37°C for 20 min. Trypsin was inactivated by the addition of soybean trypsin inhibitor and DNase. The combined supernatants from dissociated cortices or hippocampi were centrifuged through a 4% BSA layer, and the cell pellet was re-suspended in Neurobasal medium containing 2% B-27 supplement, 25 mM glutamate, 0.5 mM L-glutamine and 1% antibiotic-antimycotic solution (Invitrogen). Cells were seeded at density of 0.5×106 cells per ml onto poly-L-lysine pre-coated plates. Cultures were grown for 8 days at 37°C in 5% CO2/95% air for 7–8 days.
Primary cultures of glia
Astrocytes and microglia were prepared from the cerebral cortex of 1–2 day-old SD rats according to an established protocol (Jakovcevski et al. 2007) with some modifications. In brief, the cortex was dissected and cleaned from the meninges. The tissue was mechanically dissociated by trituration. Cells were seeded on poly-L-lysine pre-coated tissue culture flasks in Dulbecco's Modified Eagle Medium (DMEM)/F12 containing 10% fetal bovine serum, 2% antibiotic–antimycotic (Invitrogen) and grown at 37°C in 5% CO2/95% relative atmosphere. The culture medium was replaced twice a week.
To obtain microglia, on day 6 in culture, the flasks were shaken at 200 rpm for 1 hr and the supernatants collected and centrifuged at 400×g for 8 min. Cells were resuspended and replated, and grown to 80% confluence. To grow astrocytes, fresh DMEM/F12 was added to the flasks which then were continually shaken for 4 more days. Cells were then trypsinized for 5 min, collected and centrifuged at 400×g for 8 min. Pellet was re-suspended in the proper amount of DMEM/F12 containing 10% fetal bovine serum, 2% antibiotic-antimycotic. Cells were then plated and grown as described above for three more days.
Characterization of cultures
The following antibodies were used overnight at 4°C: class III β-tubulin (1:1000; Covance, Emeryville, CA) glial fibrillary acidic protein (GFAP; 1:250, Abcam Inc., Cambridge, MA) ionized calcium binding adaptor molecule 1 (Iba1, 1: 500; Wako, Japan) to visualize neurons, astrocytes and microglia, respectively. Coverslips were then incubated for 1 hr at room temperature with the corresponding secondary antibody (AlexaFluor®, 1:1000, Invitrogen) followed by 4',6'-diamidino-2-phenylindole (DAPI) (1:5000, Invitrogen) for 5 min, washed and mounted using ProLong® gold antifade reagent (Invitrogen). Cells were imaged using an FV300 laser confocal scanning system attached to an Olympus IX-70 upright microscope. Immunoreactive cells were counted using a 20× objective. The amount of neurons, astrocytes or microglia per coverslip was calculated in ten randomly selected fields by counting the number of DAPI positive cells expressing specific cell markers.
Cell survival
The activity of mitochondrial dehydrogenases [3(4,5-dimethylthiazol-2-yl)-2.5-etrazolium bromide (MTT assay) was used to determine cell death/survival. This assay was carried out according to the manufacturer's specifications (MTT Cell Grow Assay Kit, Millipore, Temecula, CA) as described previously (Bachis et al. 2001; Bachis et al. 2003).
Enzyme-linked immunosorbent assay (ELISA)
Levels of CCL5 and CXCL12 were determined in the culture medium using the DuoSet ELISA Development System Kits (R&D, Minneapolis, MN), according to the manufacturer’s instructions.
Western blot
Cells lysates were prepared in RIPA buffer (Millipore) with protease inhibitors (Thermo Fisher Scientific, Pittsburgh, PA) at 4°C. Proteins were separated by NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to PVDF membranes using the iBlot device (Invitrogen). Membranes were blocked in 5% skim milk in PBS-T, and were probed with rabbit polyclonal anti-CCR5 antibody (1:500, Abcam). The same membranes were stripped and re-probed with monoclonal mouse anti-β-actin (1:20000, Sigma-Aldrich). Immune complexes were detected with appropriate horseradish peroxidase-conjugated secondary antibodies and chemiluminescence reagents (Millipore).
qPCR
Total RNA was extracted using RNeasy Plus Mini Kit and reverse transcribed using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA) according to manufacturer’s specifications. The qPCR reaction mixture contained the Platinum SYBR Green qPCR SuperMix (Invitrogen), cDNA and primers. Primers for CCL5 were 5-ACCACTCCCTGCTGCTTTG-3, 5-ACACTTGGCGGTTCCTTCG-3 (forward, reverse). For CCR5, 5-TTTTCCAGCAAGTCAATCC-3, 5-CTCTACCCTCAAGCTACAT-3 (forward, reverse). Housekeeping gene primers were obtained from RealTimePrimers.com. All primer pairs were selected that displayed linear amplification. The specificity of the qPCR was determined by the analysis of the dissociation curves. The 7900HT Fast Real-Time PCR System (Applied Biosystems Inc., Foster City, CA) was used. Data were analyzed using SDS v2.3 software (Applied Biosystems Inc.).
Reagents
Morphine and naloxone were obtained through NIDA, NIH, Division of Neuroscience & Behavioral Research. Gp120BaL was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Gp120IIIB was purchased from Immunodiagnostics Inc. (Woburn, MA) and D-Ala2-N-Me-Phe4-glycol5-enkephalin (DAMGO) from Sigma. Lypopolisaccharide (LPS) was from EMD/Calbiochem, (Gibbston, NJ). The CCL5 blocking antibody was from R&D.
Statistical Analysis
Statistical analysis was performed using ANOVA and Scheffe’s test for multiple comparisons.
RESULTS
Morphine prevents gp120Bal toxicity
Gp120IIIB and gp120BaL bind to CXCR4 or CCR5, respectively. Thus, to examine whether morphine synergizes with gp120 in promoting cell death, we used CGC that are known to express both CXCR4 and CCR5 (Bachis et al. 2003). CGC were prepared to contain a mixture of neurons and non-neuronal cells by omitting cytosine arabinoside (Fig. 1A). CGC were exposed to medium control or medium containing morphine 48 hr prior to gp120s. A group of cells were exposed to morphine together with gp120s. Morphine alone did not affect cell survival (data not shown). Both gp120s decreased cell survival by 24 hr (Fig. 1B). Gp120IIIB was more potent than gp120BaL, confirming previous results (Bachis and Mocchetti 2005). Morphine did not synergize with gp120s in decreasing cell survival, either when added 10 min after gp120 (data not shown) or 48 hr prior to gp120s (Fig. 1B). Intriguingly, morphine blocked the toxic effect of gp120BaL when added 48 hr before the envelop protein (Fig. 1B). Time course experiments revealed that neuroprotection was obtained when morphine was added at least 6 hr prior to gp120BaL. Indeed, pretreatment of neurons with morphine for 1 or 3 hr prior to gp120BaL failed to suppress gp120-mediated neurotoxicity (Fig. 2).
Figure 1. Morphine inhibits gp120BaL neurotoxicity.
CGC were grown in the absence of cytosine arabinoside for 7 days. A. Fixed cells were stained with a β-tubulin antibody (red) and counterstained with DAPI (blue). Bar=50 µm. B. CGC were exposed to morphine (10 µM) or phosphate buffer saline (PBS) 48 hr prior to gp120s (5 nM, each). Cell survival was determined 24 hr after gp120s by MTT assay. Comparable results were obtained with a lower concentration of morphine (1 µM). Neurons exposed to PBS alone or to morphine alone showed an equivalent survival rate (~95%). Data, expressed as % of control (cells exposed to heat inactivated gp120s) are the mean ± SEM of three separate experiments (n=6). ^p<0.05 vs control, *p<0.01 vs control.
Figure 2. Temporal profile of morphine-mediated neuroprotection.
CGC were exposed to morphine (10 µM) for the indicated time points prior to gp120s (5nM, each). Control cells were exposed to heat inactivated gp120s. Cell survival was determined 24 hr after gp120s by MTT assay. Data, expressed as % of control, are the mean ± SEM of 6 samples each point from three separate experiments. ^p<0.05 vs control, *p<0.01 vs control.
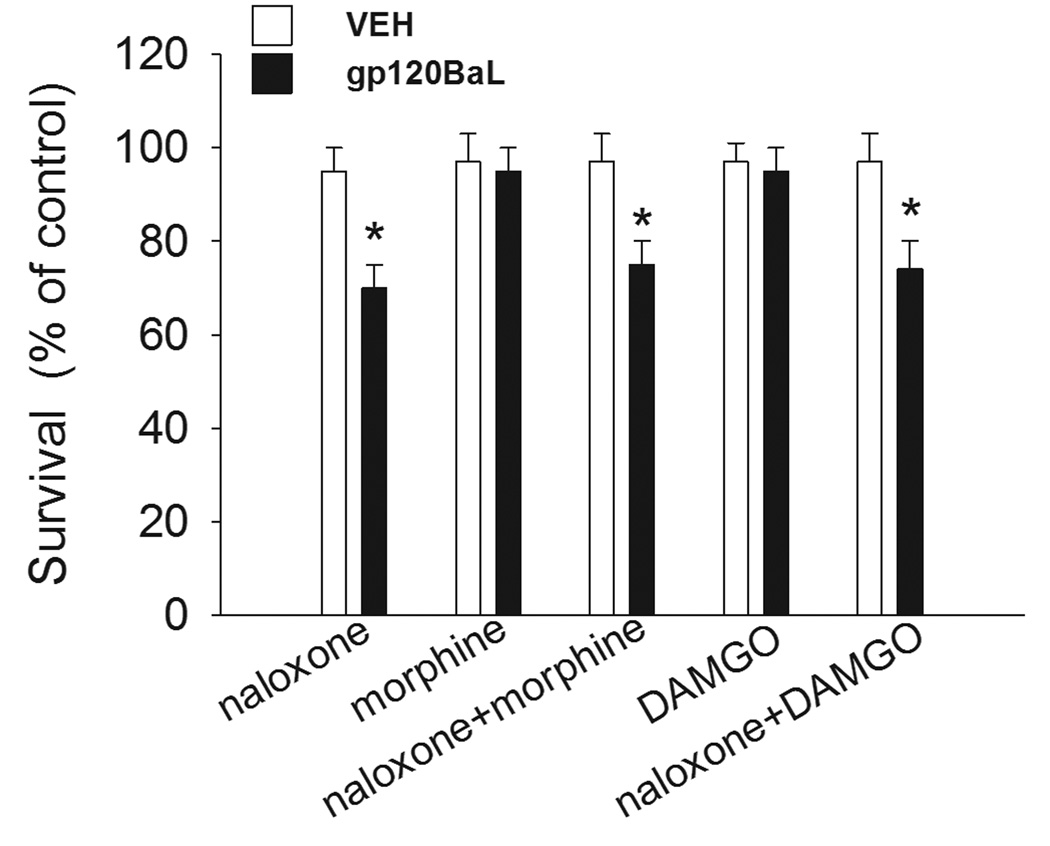
To examine whether the neuroprotective effect of morphine was due to opioid receptors, neurons were exposed to the opioid receptor antagonist naloxone prior to morphine, or to DAMGO, a selective μ receptor agonist. Gp120BaL was then added 48 hr later. DAMGO reproduced the effect of morphine (Fig. 3). Naloxone blocked the neuroprotective effect of both morphine and DAMGO (Fig. 3).
Figure 3. DAMGO inhibits gp120BaL neurotoxicity.
CGC were exposed to medium containing PBS (control), naloxone (3 µM), morphine (10 µM), naloxone and morphine, DAMGO (1 µM), naloxone and DAMGO for 48 hr prior to gp120BaL (5nM). VEH=heat inactivated gp120BaL. Cell survival was determined 24 hr after gp120BaL by MTT assay. Data, expressed as % of control (PBS), are the mean ± SEM of three separate experiments (n=6). *p<0.01 vs control.
We and others have previously shown that gp120 or HIV causes a time dependent decrease in cell survival in cortical neurons (Bachis et al. 2009; Kaul et al. 2007). Therefore, we used mixed cortical neurons to establish whether morphine is neuroprotective against gp120 in different neuronal populations. Cultures were exposed to gp120s for 24 hr prior to morphine or 48 hr after morphine. Cell survival was compromised in gp120-treated cells (Fig. 4), confirming previous results (Kaul et al. 2007). Morphine prevented gp120BaL but not gp120IIIB toxicity (Fig. 4). Thus, it appears that the neuroprotective action of morphine is not selective for CGC.
Figure 4. gp120BaL toxicity is prevented by morphine in cortical cultures.
Mix cortical neurons were prepared in the absence of cytosine arabinoside as described in Materials and Methods. At day 7 in vitro, cells were exposed to medium alone or medium containing morphine (10 µM) for 48 hr. Gp120s (5 nM, each) were then added and cell survival was determined 24 hr later by MTT assay. Control cells were exposed to heat inactivated gp120s. Data, expressed as % of control, are the mean ± SEM of two separate experiments (n=6). ^p<0.05 vs control, *p<0.01 vs control.
Morphine and chemokine expression
Binding of gp120 to CXCR4 and CCR5 is necessary for the neurotoxic activity. Moreover, morphine has been shown to modulate the expression and function of neuronal CXCR4 (Happel et al. 2008; Mahajan et al. 2002; Sengupta et al. 2009). Therefore, we examined whether morphine down-regulates CCR5. CGC were exposed to morphine for 24 hr, and then expression of CCR5 was examined by Western blot analysis (Fig. 5A) and qPCR (Fig. 5B). Morphine did not change the protein or mRNA levels of CCR5 (Fig. 5).
Figure 5. Morphine does not change CCR5 expression.
The effect of morphine on CCR5 protein and mRNA was examined in CGC. A. Example of a Western blot using a CCR5 antibody. Blot was stripped and re-probed with a β-actin antibody. The experiment was repeated twice with comparable results. B. CCR5 mRNA levels were determined by qPCR as described in Materials and Methods. Data are the mean ± SEM of 6 separate samples and are expressed as arbitrary units using the 60S ribosomal protein L13a (RPL13A) gene for normalization. RPL13A mRNA showed a stable expression across the samples.
Early data have shown that morphine increases the release of CCL5, the endogenous ligand for CCR5, from human peripheral blood mononuclear cells (Wetzel et al. 2000). Independent investigators have shown that CCL5 can prevent gp120 neurotoxicity (Bachis and Mocchetti 2005; Kaul et al. 2007). Therefore, morphine may promote the release of CCL5, which, in turn, inhibits the neurotoxic effect of gp120BaL. To test this hypothesis, CGC were exposed to morphine for various time-points and CCL5 was determined in the medium by ELISA. In control cells, the levels of CCL5 were near to the limit of detection of the assay (~10pg/ml) throughout the experiment. A similar result was observed in CGC exposed to morphine up to 3 hr (Fig. 6A). However, by 6 hr, morphine elicited a time-dependent increase of CCL5 levels in the medium that continued up to 48 hr (Fig. 6A). The accumulation of CCL5 in the medium was accompanied by a corresponding increase in CCL5 mRNA (~300 fold induction, data not shown). Thus, it appears that morphine enhances the synthesis and release of CCL5.
Figure 6. Morphine increases the release of CCL5.
CGC (grown in the absence of cytosine arabinoside) were exposed to medium control (PBS) or medium containing morphine (10 µM) for the indicated time points. The medium was collected and an aliquot used to determine CCL5 (A) or CXCL12 (B) by ELISA. Data are the mean ± SEM of 4 independent samples each point. ^p<0.05 vs control, #p<0.01 vs morphine 6 hr, *p<0.01 vs morphine 12 hr.
To determine the specificity of the effect we also measured the levels of CXCL12, the ligand for CXCR4 which promotes neuronal apoptosis in CGC (Bachis et al. 2003). Unlike CCL5, CXCL12 protein levels remained unchanged in both control and treated cells (Fig. 6B).
Morphine increases the release of CCL5 from astrocytes
CGC in vitro contain also glial cells. To determine which cell subtype releases CCL5 in response to morphine, we prepared astrocytes and microglia from P1 rats. The purity of cultures was assessed by immunocytological analysis of specific cellular markers, such as GFAP (Fig. 7A) and Iba1 (Fig. 8A) for astrocytes and microglia, respectively. The levels of CCL5 in the medium of untreated astrocytes were around the detection threshold. However, exposure to morphine for 6 and 24 hr promoted a robust increase in CCL5 levels (Fig. 7B). The effect was blocked by naloxone (data not shown), confirming an opioid receptor-mediated effect. In addition, morphine increased CCL5 mRNA in these cells (~300 fold induction, data not shown). Thus, morphine appears to increase synthesis and release of CCL5 in astrocytes.
Figure 7. Morphine increases the release of CCL5 in astrocytes.
Astrocytes were prepared from cortices as described in Materials and Methods. A. Representative image of cells grown on coverslips, fixed and stained with GFAP antibody (red) and counterstained with DAPI (blue). Bar=50 µm. B. CCL5 levels were determined in the medium of astrocytes exposed to medium control or medium containing morphine (10 µM) for 6 and 24 hr. Data are the mean ± SEM of five independent samples. *p<0.01 vs control, **p<0.01 vs morphine 6 hr.
Figure 8. Microglia increase CCL5 release in response to LPS.
Microglia cells were prepared as described in Materials and Methods. A. Example of cells grown on coverslips, fixed and stained with Iba1 antibody (red) and DAPI (blue). Bar=50 µm. B. Microglia cells were exposed to medium control or medium containing morphine or LPS (1 µM) for 24 or 48 hr. CCL5 levels were determined in the medium by ELISA. Data are the mean ± SEM of six independent samples from two separate preparations. *p<0.01 vs control, #p<0.05 vs control.
CCL5 levels were near the limit of sensitivity of the assay also in the medium of microglia in both control and morphine-treated cultures (Fig. 8B). To examine whether microglia can release CCL5 in response to other stimuli, cells were exposed to LPS for 24 and 48 hr. LPS induced a time-dependent release of CCL5 (Fig. 8B). Thus, these cells are capable of producing and releasing CCL5 under appropriate stimuli.
To determine whether pure neuronal cultures release CCL5 in response to morphine, we prepared CGC in the presence of cytosine arabinoside to inhibit proliferation of non neuronal cells, and cortical and hippocampal neurons from E17 embryos. The purity of these cultures was assessed by determining the amount of DAPI positive cells that were also β-tubulin. CGC (Fig. 9A), cortical (Fig. 9B) and hippocampal (Fig. 9C) cultures contained mostly neurons because 98% of cells were positive for β-tubulin. Morphine did not promote the release of CCL5 in any of these cultures (Fig. 9D). Overall, our data suggest that morphine affects the release of CCL5 only from astrocytes.
Figure 9. Morphine does not induce the release of CCL5 from neurons.
Pure neuronal cultures were obtained either from P8 rat cerebella in the presence of cytosine arabinoside (10 µM) added 24 hr after plating (A), E17–18 cortices (B) or hippocampi (C) as described in Materials and Methods. Cells were then fixed and stained with a β-tubulin antibody (red) and counterstained with DAPI (blue). Bars: A =50 µm; B= 50 µm, C= 100 µm. D. CCL5 levels were determined in the medium of indicated neuronal cultures by ELISA 24 hr after morphine treatment. Data are the mean ± SEM of six independent samples from two separate preparations.
Neuroprotection of morphine occurs via CCL5
The neuroprotective activity of morphine and its ability to induce the release of CCL5 follow a similar temporal profile, suggesting that CCL5 may mediate the neuroprotective action of morphine. To support this suggestion, CGC were immunoneutralized for CCL5 using a CCL5 blocking antibody (Chou et al. 2008). Cells were then exposed to medium control or medium containing morphine alone or in combination with gp120BaL. We observed that in neurons pretreated with the CCL5 antibody, the neuroprotective effect of morphine was blocked (Fig. 10). A similar result was obtained with DAMGO (Fig. 10). Thus, our data suggest that the neuroprotective effect of morphine may include the release of CCL5 from astrocytes.
Figure 10. CCL5 mediates the neuroprotective action of morphine.
Mixed CGC were exposed to morphine or DAMGO 48 hr prior to gp120BaL. A group of neurons was exposed to CCL5 antibody (Ab) 15 min prior to morphine or DAMGO. Cell survival was determined 24 hr after gp120BaL by MTT assay. Control cells were exposed to boiled gp120BaL. Data are the mean ± SEM of six independent samples. *p<0.05 vs control.
DISCUSSION
Opiates have been postulated to serve as cofactors in the progression of HIV infection and to play a role in the development of ADC (Nath et al. 2002). Yet, not all HIV drug abusers develop ADC, despite the fact that opiates alter their immune system. In this work, we examined the effect of morphine in mixed neuronal/glial cultures exposed to M- or T-tropic gp120 at concentrations known to promote neuronal apoptosis (Bachis & Mocchetti, 2005; Kaul et al., 2007). We have found that morphine does not change the ability of gp120IIIB to reduce cell survival but it prevents neuronal loss caused by gp120BaL. Thus, our data show that morphine may have a neuroprotective action against M-tropic gp120. This effect was blocked by the non-selective opioid receptor antagonist naloxone. Moreover, the neuroprotective effect of morphine was replicated with DAMGO, a μ receptor selective agonist. Thus, the effect of morphine appears to be related to its ability to activate μ receptors.
Both opioid and chemokine receptors belong to the superfamily of G protein coupled receptors that can influence each other’s functions by heterologous desensitization (Chen et al. 2004; Grimm et al. 1998). This mechanism may account for the neuroprotective action of morphine. Nevertheless, our results show that morphine does not affect gp120IIIB toxicity. Moreover, the neuroprotective property of morphine requires several hours, whereas heterologous desensitization can occur within minutes (Chen et al. 2004). Thus, it appears that a mechanism involving down-regulation of chemokine receptors alone may not be sufficient to explain the neuroprotective activity of morphine. Opiates have been shown to promote the release of various cytokines (Hauser et al. 2007) and to inhibit CXCL12-dependent survival pathway (Sengupta et al. 2009). In addition, CXCL12 has been shown to promote apoptosis of CGC whereas CCL5 has a neuroprotective activity (Bachis et al. 2003; Kaul et al. 2007). Therefore, to gain insight into the neuroprotective effect of morphine against gp120BaL, we tested the hypothesis that this opiate prevents gp120-mediated cell death by inducing the release of α chemokine CXCL12 and the β chemokine CCL5. We show that morphine induces a robust accumulation of CCL5 in the medium of astrocytes with a temporal profile that matches its ability to prevent gp120-mediated cell death. Moreover, morphine and DAMGO neuroprotection was blocked when the biological activity of released CCL5 was immunoneutralized. Thus, CCL5 rather than CCR5 appears to be a crucial component of the neuroprotection pathway.
The morphine-mediated release/synthesis of CCL5 is not due to a generalized effect of protein synthesis because the levels of CXCL12 did not change even after long-term exposure to the opiate. It is noteworthy that such release was seen in astrocytes or mixed neuronal/glial cultures but not in microglial or pure neuronal cultures. Moreover, the release of CCL5 was not seen at physiological conditions but occurred after at least 6 hr of exposure to morphine. Therefore, astrocytes must be activated by morphine before promoting the release of CCL5. The increased release of CCL5 is accompanied by an increase in CCL5 mRNA, suggesting that morphine activates both synthesis and release of this chemokine. Hence, we propose that the neuroprotective activity of morphine against gp120BaL may rely on its ability to promote a paracrine loop in which activation of μ receptor on astrocytes enhances the production and release of CCL5 from these cells. CCL5, in turn, may either activate a neuroprotective pathway involving the protein kinase Akt (Kaul et al. 2007) or compete with M-tropic gp120 for binding to neuronal CCR5 receptors, or both; therefore, CCL5 reduces the sensitivity of neurons to the toxic effect of this viral glycoprotein. Nevertheless, this suggestion must be tested in vivo. Indeed, morphine may behave differently in the presence of HIV and other opportunistic infections. Moreover, our studies reflect a “subchronic” situation as CCL5 expression was measured only up to 72 hr. Indeed, studies in rhesus monkeys infected with simian immunodeficiency virus have revealed that CCL5 expression rises acutely, but declines during the chronic phase of the infection (Roberts et al. 2006). Thus, more studies are needed to establish the persistence of CCL5 expression in vivo as well as the relative amount produced by morphine because, although it is generally believed that CCL5 has a neuroprotective property, abnormally chronically elevated levels of CCL5 could be detrimental for neuronal survival.
The neurotoxic activity of both strains of gp120s can be blocked by the neurotrophin brain-derived neurotrophic factor (BDNF). In fact, BDNF prevents gp120-mediated neuronal apoptosis in vitro (Bachis et al. 2003; Bachis and Mocchetti 2005) and in vivo (Nosheny et al. 2007). Morphine has been shown to increase BDNF in microglia (Takayama and Ueda 2005). Thus, BDNF could be a potential candidate to explain morphine neuroprotective activity. However, we can discard this hypothesis because morphine did not block gp120IIIB toxicity, which our previous data have shown to be preventable by BDNF in CGC (Bachis et al. 2003). Most importantly, the neuroprotective effect of morphine was inhibited by preincubating neurons with a CCL5 blocking antibody. Lastly, independent studies have shown that morphine and other μ-receptor agonists increase CCR5 and CXCR4 expression in cultured human astrocytes, monocytes and lymphocytes (Burbassi et al. 2008; Happel et al. 2008; Miyagi et al. 2000). Instead, BDNF has been shown to reduce CXCR4 and CCR5 expression (Ahmed et al. 2008; Nosheny et al. 2007). Thus, our data support a role of CCL5 rather than BDNF as a molecular mechanism to explain the neuroprotective effect of morphine observed here.
Evidence has shown that opiates can alter different aspects of the responses of the brain to HIV. Therefore, the need to characterize the complex interaction among opiates, glia and HIV proteins is great and timely. Our findings indicate that morphine prevents gp120BaL neurotoxicity though the release of CCL5 from astrocytes. The majority of viruses that have been isolated from postmortem human brains prefer CCR5, whereas CXCR4-preferring and dual tropic variants have been found in only 40% of patients. Nevertheless, it is important to recall that M-tropic strains of HIV that infect the CNS are not sufficient to cause dementia or encephalitis (Power et al. 1994). Moreover, HIV isolates, such as YU2 and JR-FL which use CCR5, do not always cause neuronal apoptosis despite the fact that they replicate efficiently in brain cultures (He et al. 1997; Ohagen et al. 1999; Zheng et al. 1999). Thus, our data, although preliminary, may shed some light into why only a subset of HIV drug abusers develops ADC.
Acknowledgements
This work was supported by HHS DA026174 and T32 DA007291. Special thank to NIH AIDS Research Reference Reagent Program for gp120BaL and NIH NIDA, Division of Neuroscience & Behavioral Research for morphine and naloxone and to Drs. Timothy Mhyre (Georgetown University) and Andrey Gortchakov (Harvard Medical School) for advice on qPCR.
References
- Ahmed F, Tessarollo L, Thiele C, Mocchetti I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008;1227:1–11. doi: 10.1016/j.brainres.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo N, Bayer B. uOpioids, immunology, and host defenses of intravenous drug abusers. Infect Dis Cli N Am. 2002;16:553–569. doi: 10.1016/s0891-5520(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Anthony I, Arango J, Stephens B, Simmonds P, Bell J. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- Arango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? Aids. 2004;18 Suppl 1:S69–S74. [PubMed] [Google Scholar]
- Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009;4(1):150–160. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21(9):3104–3112. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23(13):5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann NY Acad Sci. 2005;1053(1):247–257. doi: 10.1196/annals.1344.022. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz SM. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16(9):1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56(13):1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3(1):26–34. doi: 10.1007/s11481-007-9083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483(2–3):175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88(1):36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, Dickson DW, Chern Y. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci. 2008;28(13):3277–3290. doi: 10.1523/JNEUROSCI.0116-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter MA. Cerebral cortex in tissue culture. In: Barker JL, editor. Current Methods in Cellular Neurobiology. New York: Whiley; 1983. pp. 81–106. [Google Scholar]
- Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, Simmonds P. HIV-1 tropism and co-receptor use. Nature. 1997;385(6616):495–496. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53(2):132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gray L, Roche M, Churchill MJ, Sterjovski J, Ellett A, Poumbourios P, Sherieff S, Wang B, Saksena N, Purcell DF, et al. Tissue-specific sequence alterations in the human immunodeficiency virus type 1 envelope favoring CCR5 usage contribute to persistence of dual-tropic virus in the brain. J Virol. 2009;83(11):5430–5441. doi: 10.1128/JVI.02648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998;188(2):317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102(3):555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-{beta}1. J Leukoc Biol. 2008 doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100(3):567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385(6617):645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8(10):595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis. 2005;191(6):886–889. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Wu J, Karl N, Leshchyns'ka I, Sytnyk V, Chen J, Irintchev A, Schachner M. Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J Neurosci. 2007;27(27):7222–7233. doi: 10.1523/JNEUROSCI.0739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96(14):8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14(2):296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MPN. Morphine Regulates Gene Expression of {alpha}- and {beta}-Chemokines and Their Receptors on Astroglial Cells Via the Opioid {micro} Receptor. J Immunol. 2002;169(7):3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95(24):14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEM×174 lymphocytes. J Biol Chem. 2000;275(40):31305–31310. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31 Suppl 2:S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Amhed F, Yakovlev AG, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, et al. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol. 1999;73(2):897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74(5):691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Doms RW, Pohlmann S. Prospects of HIV-1 entry inhibitors as novel therapeutics. Rev Med Virol. 2004;14(4):255–270. doi: 10.1002/rmv.435. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273(36):23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Power C, McArthur JC, Johnson RT, Griffin DE, Glass JD, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68(7):4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ES, Huitron-Resendiz S, Taffe MA, Marcondes MC, Flynn CT, Lanigan CM, Hammond JA, Head SR, Henriksen SJ, Fox HS. Host response and dysfunction in the CNS during chronic simian immunodeficiency virus infection. J Neurosci. 2006;26(17):4577–4585. doi: 10.1523/JNEUROSCI.4504-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, Rubin JB, Meucci O. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29(8):2534–2544. doi: 10.1523/JNEUROSCI.5865-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Wilkinson D, Reeves JD, Dittmar MT, Beddows S, Weber J, Carnegie G, Desselberger U, Gray PW, Weiss RA, et al. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70(12):8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280(2):192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99(16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J Neurosci. 2005;25(2):430–435. doi: 10.1523/JNEUROSCI.3170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367(6459):188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotox Res. 2005;8(1–2):149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D, Rabin RL, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber JM, Fauci AS. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389(6654):981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Alvarez X, deBakker C, Aye P, Wilson ML, Williams KC, Lackner AA. Developmental expression patterns of CCR5 and CXCR4 in the rhesus macaque brain. J Neuroimmunol. 2002;122(1–2):146–158. doi: 10.1016/s0165-5728(01)00457-x. [DOI] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Eisenstein TK, Adler MW, Henderson EE, Rogers TJ. Mu-opioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-gamma-inducible protein-10 expression in human peripheral blood mononuclear cells. J Immunol. 2000;165(11):6519–6524. doi: 10.4049/jimmunol.165.11.6519. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ghorpade A, Niemann D, Cotter RL, Thylin MR, Epstein L, Swartz JM, Shepard RB, Liu X, Nukuna A, et al. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 1999;73(10):8256–8267. doi: 10.1128/jvi.73.10.8256-8267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]