Abstract
The abdominal wall is a composite of muscles that are important for the mechanical stability of the spine and pelvis. Tremendous clinical attention is given to these muscles, yet little is known about how they function in isolation or how they interact with one another. Given the morphological, vascular, and innervation complexities associated with these muscles and their proximity to the internal organs, an appropriate animal model is important for understanding their physiological and mechanical significance during function. To determine the extent to which the rat abdominal wall resembles that of human, 10 adult male Sprague-Dawley rats were killed and formalin-fixed for architectural and morphological analyses of the four abdominal wall muscles (rectus abdominis, external oblique, internal oblique, and transversus abdominis). Physiological cross-sectional areas and optimal fascicle lengths demonstrated a pattern that was similar to human abdominal wall muscles. In addition, sarcomere lengths measured in the neutral spine posture were similar to human in their relation to optimal sarcomere length. These data indicate that the force-generating and length change capabilities of these muscles, relative to one another, are similar in rat and human. Finally, the fiber lines of action of each abdominal muscle were similar to human over most of the abdominal wall. The main exception was in the lower abdominal region (inferior to the pelvic crest), where the external oblique becomes aponeurotic in human but continues as muscle fibers into its pelvic insertion in the rat. We conclude that, based on the morphology and architecture of the abdominal wall muscles, the adult male Sprague-Dawley rat is a good candidate for a model representation of human, particularly in the middle and upper abdominal wall regions.
Keywords: abdominal muscles, abdominal wall, animal model, comparative morphology, muscle architecture, spine
Introduction
The four abdominal wall muscles [rectus abdominis (RA), external oblique (EO), internal oblique (IO), and transversus abdominis (TrA)] play a variety of essential roles in human function. They create the torques necessary to flex, twist and laterally bend the spine (McGill, 1991; Arjmand et al., 2008), stiffen the abdominal cavity and lumbar spine during simple tasks such as standing, sitting, and locomotion (Callaghan et al., 1999; Masani et al., 2009) as well as during demanding tasks such as dynamic loading and heavy lifting (Cholewicki & McGill, 1996; El Ouaaid et al., 2009), and finally, assist the expiration of air in challenged breathing (Campbell & Green, 1953). The breadth of these roles has garnered these muscles a great deal of clinical attention in recent years. Despite this, relatively little is understood about the specific physiology and mechanics of these muscles, acting individually and together as a composite, multifunctional structure. Invasive physiological testing of these muscles is required to develop an understanding of the unique passive and active force–length, force transmission, and synergistic properties that these muscles may have. Because such invasive testing is impossible in human volunteers, an appropriate animal model must be developed.
The integrated morphology of the four abdominal wall muscles in quite unique. The RA is a long muscle that runs in a superior–inferior orientation and is symmetrical about the anterior midline of the trunk, separated into right and left muscles by the linea alba. The EO, IO and TrA are broad sheet-like muscles that are tightly bound to one another through networks of connective tissues. Each of these muscles has coplanar fiber orientations that run oblique to the fibers in the adjacent muscle layers, creating what is likened to a composite laminate structure (Rizk, 1980; Hukins, 1984). Fibers of the EO, IO and TrA terminate into the rib cage and pelvis, as well as into aponeuroses forming the rectus sheath at the anterior of the trunk and thoraco-lumbar fascia at the posterior of the trunk. This structural arrangement, it has been suggested, strengthens and stiffens the abdominal wall against multidirectional forces, enabling force to be transmitted around the torso while pressurizing the abdominal cavity and stiffening the spine.
To answer specific questions pertaining to the mechanical output of these muscles, both individually and as an integrated whole, an animal model needs to be validated. Previously, mammals such as the dog, rabbit and hamster have been used to test isolated active (dog, Farkas & Rochester, 1988; hamster, Arnold et al., 1987) and composite passive (dog, Hwang et al., 2005; rabbit, Nilsson, 1982a; rabbit, Nilsson, 1982b) properties of abdominal wall muscles. More recently, the male Sprague-Dawley rat has been used to study the transmission of force between abdominal muscle layers (Brown & McGill, 2009), and to study the formation, repair and atrophic effects of abdominal hernias (DuBay et al., 2005, 2007). However, none of these studies has directly compared the abdominal wall of the animal of choice with that of human. It is possible, based on differences in posture, methods of locomotion, different use of forelimbs, and size, that abdominal wall muscles could show significant specialization across species. This would render comparisons with humans problematic. As the adult male Sprague-Dawley rat is a relatively inexpensive, accessible and widely-used animal model, the purpose of this study was to examine the architectural and morphological properties of rat abdominal wall muscles, and to compare them with human.
Materials and methods
Ten adult male Sprague-Dawley rats (mass, mean ± SD, 455 ± 26 g) were killed by intracardiac injection of sodium pentobarbital (mixed as 390 mg/ml solution and given at a dose of 1 ml per 10 pounds body weight), immediately skinned and immersion fixed in 10% formalin for 72 h. Rats were under no external load while immersed, and therefore maintained a posture of neutral spine elastic equilibrium. Animals were then removed from the formalin and immersed in phosphate-buffered saline for 24 h to wash out residual fixative.
The abdominal wall muscles (RA, EO, IO, and TrA) from one side of the body were sharply isolated from the skeleton and separated. To enhance the architectural analysis, muscles were then divided regionally as follows: the RA was divided into three serial sections (divided at tendinous intersections where visible; where not visible, the RA was divided into similar length sections as those in which the intersections were visible); and the EO, IO and TrA were each divided into three regions, determined to maintain approximately homogeneous fascicle lengths within each region (Fig. 1).
Fig. 1.
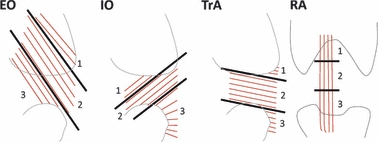
Schematic side view of the external oblique, internal oblique and transversus abdominis, and anterior view of the rectus abdominis, to illustrate the regional division of muscles for architectural analysis. Thick black lines represent regional divisions (shown as 1–3) and thin red lines represent approximate muscle fiber lines of action.
All external connective and adipose tissues were removed, and each muscle section was weighed (resolution 0.01 g) and the length of a representative fascicle was measured with a digital caliper (resolution 0.01 mm). A minimum of three representative fascicles were removed from each muscle section, placed on a slide and measured for sarcomere length by laser diffraction (Lieber et al., 1990). Normalized fascicle lengths [LfN (cm)] were calculated as follows:
| (1) |
where Lfm is measured fascicle length (cm), Lsm is measured sarcomere length (μm), and Lso is estimated optimal sarcomere length for rat muscle (2.40 μm, Burkholder & Lieber, 2001). This normalization procedure had the effect of minimizing differences in torso and pelvis positions among specimens.
The physiological cross-sectional area [PCSA (cm2)] of each muscle section was calculated as (Sacks & Roy, 1982):
| (2) |
where M is muscle mass (g), LfN is normalized fascicle length (cm), θ is pennation angle (0° for all abdominal muscles), and ρ is muscle density (1.112 g cm−3; Ward & Lieber, 2005).
The whole muscle architectural properties were determined as follows for each rat based on the morphological relationship among regions sampled: RA (where regions act in series) PCSA was calculated as the largest measured regional PCSA; RA optimal fascicle length was summed for all regions; RA sarcomere length was averaged across all regions; EO, IO and TrA (where regions act in parallel) PCSA was summed across all regions; and EO, IO and TrA optimal fascicle length and sarcomere length were calculated as weighted averages (weighted to regional PCSAs) across all regions.
The muscle fiber orientations of the EO, IO and TrA were measured for each muscle section as the angle between a representative fascicle and a line cutting through the transverse plane of the abdomen. This was done by tracing lines on each muscle representing the transverse plane and fiber lines of action, then removing the muscles and measuring relative angles with a goniometer. Positive angles indicate an infero-medial fiber direction and negative angles indicate an infero-lateral fiber direction. To facilitate the comparison of rat fiber orientations with those previously measured for human, a second regionalization of EO, IO and TrA was utilized. Urquhart et al. (2005) measured the human anterior muscle fiber orientations from the EO, IO and TrA in the following three regions: (i) upper: superior to base of rib-cage; (ii) middle: between base of rib-cage and iliac crest; and (iii) lower: inferior to iliac crest. Our measurements of fiber orientation were adapted to these three regions and presented additionally as such in the Results section to facilitate comparison with human data.
The whole muscle PCSA, optimal fascicle length and sarcomere length were compared among muscles by repeated-measures one-way anova. Tukey's HSD post-hoc test was utilized when significant effects were revealed by anova. Statistical significance was set at P < 0.05.
Results
The relationship between the PCSA and normalized fascicle length expresses both the force-producing and excursion capability of a muscle (Lieber & Fridén, 2000) and is displayed for each of the rat abdominal wall muscles (Fig. 2). The IO and EO have the largest PCSA, significantly greater than the TrA, which is significantly greater than the RA (P < 0.0001). An opposite pattern is seen in terms of normalized fascicle length, with the RA being the longest muscle and the IO the shortest (all significantly different from one another, P < 0.0001). To facilitate the comparison between rat and human abdominal wall muscles by accounting for differences in absolute size, the PCSA and normalized fascicle length for each muscle were normalized to that of the RA (Fig. 3). A very similar pattern between the PCSA and normalized fascicle length is observed between rat and human. Architectural data are also shown for all muscle regions in Table 1.
Fig. 2.
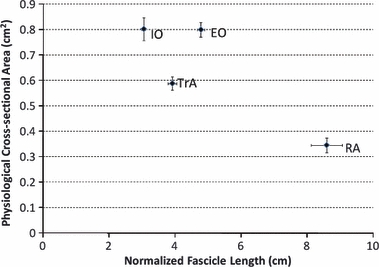
Scatterplot of physiological cross-sectional area (PCSA) vs. normalized fascicle length for abdominal wall muscles of the rat. A large PCSA indicates large isometric force-generating ability, and a long normalized fascicle length indicates the ability to generate force across a wide range of lengths. Data are plotted as mean ± SE. RA, rectus abdominis; EO, external oblique; IO, internal oblique; TrA, transversus abdominis.
Fig. 3.
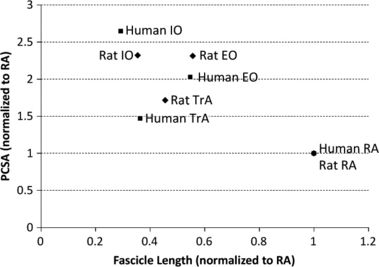
Scatterplot of physiological cross-sectional area (PCSA) vs. optimal fascicle length for abdominal wall muscles of rat and human. For comparison between the two, the mean PCSA and optimal fascicle length are normalized to the means of the rectus abdominis (RA) within each species. Human data taken from Brown et al. (in press) (n = 11). EO, external oblique; IO, internal oblique; TrA, transversus abdominis.
Table 1.
Architectural properties of rat abdominal wall muscles.
| Muscle (region) | PCSA (cm2) | Sarcomere length (μm) | Normalized fascicle length (cm) |
|---|---|---|---|
| RA1 | 0.28 ± 0.04 | 2.84 ± 0.03 | 2.4 ± 0.2 |
| RA2 | 0.33 ± 0.02 | 2.78 ± 0.04 | 1.5 ± 0.1 |
| RA3 | 0.28 ± 0.02 | 2.88 ± 0.03 | 4.7 ± 0.2 |
| RA (whole) | 0.35 ± 0.03 | 2.83 ± 0.03 | 8.6 ± 0.5 |
| EO1 | 0.40 ± 0.02 | 2.70 ± 0.03 | 3.2 ± 0.1 |
| EO2 | 0.25 ± 0.01 | 2.77 ± 0.04 | 7.8 ± 0.2 |
| EO3 | 0.14 ± 0.00 | 2.57 ± 0.04 | 3.9 ± 0.2 |
| EO (whole) | 0.80 ± 0.03 | 2.70 ± 0.02 | 4.8 ± 0.1 |
| IO1 | 0.19 ± 0.01 | 2.43 ± 0.04 | 2.7 ± 0.1 |
| IO2 | 0.29 ± 0.01 | 2.53 ± 0.02 | 4.6 ± 0.2 |
| IO3 | 0.32 ± 0.04 | 2.39 ± 0.04 | 1.9 ± 0.1 |
| IO (whole) | 0.80 ± 0.04 | 2.45 ± 0.02 | 3.0 ± 0.1 |
| TrA1 | 0.22 ± 0.01 | 2.32 ± 0.05 | 2.3 ± 0.1 |
| TrA2 | 0.27 ± 0.01 | 2.38 ± 0.06 | 5.6 ± 0.1 |
| TrA3 | 0.09 ± 0.01 | 2.54 ± 0.05 | 3.1 ± 0.2 |
| TrA (whole) | 0.59 ± 0.03 | 2.39 ± 0.05 | 3.9 ± 0.1 |
See text for details regarding the calculation of whole muscle parameters [note, for example, that the whole rectus abdominis (RA) mean physiological cross-sectional area (PCSA) is higher than any of the individual region means because the whole RA PCSA for each rat was calculated as the highest PCSA within any of its regions]. Data represented as mean ± SEM (n = 10).
EO, external oblique; IO, internal oblique; TrA, transversus abdominis.
In the neutral spine posture, the RA and EO had sarcomere lengths well above optimal and significantly greater than both the IO and TrA (P < 0.0001), which acted near optimal sarcomere length (Fig. 4). Human sarcomere lengths (Brown et al., in press) showed the same pattern for each of these muscles in human (Fig. 4).
Fig. 4.
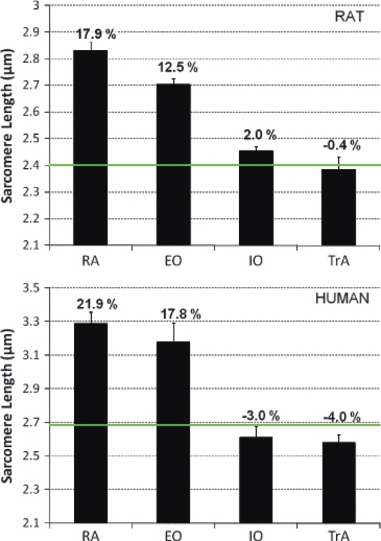
Mean (± SE) sarcomere lengths for the abdominal wall muscles of rat and human. Bold horizontal green line represents the optimal sarcomere length for each species. Numerical values corresponding to each bar represent the percent difference from optimal length. Human data taken from Brown et al. (in press) (n = 11). EO, external oblique; IO, internal oblique; RA, rectus abdominis; TrA, transversus abdominis.
The fiber orientations for each region of the EO, IO and TrA are reported in Table 2. Table 3 also displays the fiber orientations regionalized to facilitate comparison to human data (as per Urquhart et al., 2005). Figure 5 displays a representative anterior view diagram of the fiber orientations documented in the EO, IO and TrA in the current study.
Table 2.
Mean ± SE for the angles (°) of the external oblique (EO), internal oblique (IO) and transversus abdominis (TrA) muscle fibers measured relative to the axis cutting through the transverse plane of the abdomen.
| Regionalization 1 | Regionalization 2 (for comparison to human) | |||||
|---|---|---|---|---|---|---|
| Muscle | 1 | 2 | 3 | Upper | Middle | Lower |
| EO | 49 ± 2 | 71 ± 1 | 64 ± 2 | 49 ± 2 | 71 ± 1 | 71 ± 1 |
| IO | −40 ± 3 | −46 ± 2 | −15 ± 2 | −46 ± 2 | −46 ± 2 | −15 ± 2 |
| TrA | 5 ± 2 | 14 ± 1 | 28 ± 1 | 5 ± 2 | 14 ± 1 | 28 ± 1 |
Regionalization 1 divides the muscles into three regions corresponding to Fig. 1. Regionalization 2 divides the same muscles into three regions chosen to facilitate comparison to human, corresponding to the data provided in Table 2 of Urquhart et al. (2005) (see text for details). Positive angles indicate an infero-medial fiber direction; negative angles indicate an infero-lateral fiber direction.
Table 3.
Angles (°) calculated between the average measured muscle fiber orientations in adjacent muscle layers.
| Region | Layers | Rat (°) | Human (Urquhart et al., 2005) (°) |
|---|---|---|---|
| Upper | EO–IO | 85 | 83 |
| IO–TrA | 51 | 51 | |
| Middle | EO–IO | 63 | 86 |
| IO–TrA | 60 | 48 | |
| Lower | EO–IO | 85 | n/a |
| IO–TrA | 42 | 21 |
Rat data from current study; human data from Urquhart et al. (2005). Angle between the external oblique (EO) and internal oblique (IO) in human is not applicable (n/a) because the EO muscle does not traverse inferior to the anterior superior iliac spine, where the lower region is defined.
TrA, transversus abdominis.
Fig. 5.
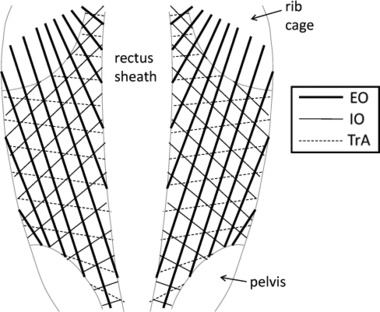
Diagram of the anterior view of the rat abdominal wall representing the approximate fiber lines of action of the external oblique (EO), internal oblique (IO) and transversus abdominis (TrA). Note the composite laminate-like structure formed by the overlying of these three muscle layers.
To evaluate the relative muscle sizes between rat and human, muscle masses were normalized to body mass and compared. Masses were relatively larger for each of the muscles in rat compared with human (Fig. 6). Caution should be taken, however, in interpreting these relative masses, as the rats were young adults and the humans were elderly individuals.
Fig. 6.
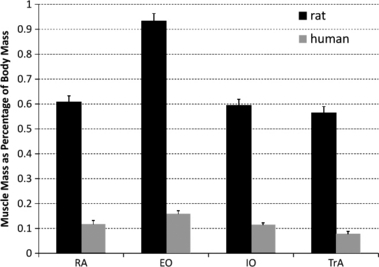
Mean (± SE) abdominal wall muscle masses normalized to body mass for both rat and human. Human data taken from Brown et al. (in press). EO, external oblique; IO, internal oblique; RA, rectus abdominis; TrA, transversus abdominis.
Discussion
To acquire insights into the physiological and mechanical function of the human abdominal wall muscles, an appropriate animal model is required for physiological experimentation. The purpose of this study was to define the morphology and architecture of the abdominal wall muscles of the adult male Sprague-Dawley rat, and to compare them with human. Architectural characteristics dictating the force-generating and excursion capabilities (PCSA and normalized fascicle length, respectively), sarcomere lengths in the neutral spine posture, and muscle fiber orientations were measured and shown to be quite similar, in terms of relative patterns, to those previously reported for human (Urquhart et al., 2005; Brown et al., in press).
The muscle PCSA is defined as the summated cross-section of all muscle fibers, relative to the axis of force generation (Lieber & Fridén, 2000). Thus, the PCSA determines a muscle's ability to generate isometric force (Powell et al., 1984); the greater the PCSA the more force the muscle can produce. The PCSAs of the rat abdominal muscles demonstrate the same pattern from largest to smallest (IO, EO, TrA, and RA), as has been reported for human (Brown et al., in press) (Fig. 3). The only relative difference in PCSAs between human and rat was that the human IO was significantly larger than the EO (Brown et al., in press), whereas in the current study they were almost identical. However, in contrast to the study from Brown et al. (in press), who measured PCSAs from elderly cadavers, computed tomography and magnetic resonance imaging studies of young healthy humans have estimated the EO and IO PCSAs to be more similar (McGill, 1996; Marras et al., 2001), thereby more closely approximating the young healthy rat. Measuring the PCSA of these muscles via imaging is very difficult owing to limitations in assessing fibers that are out of, or acting at an oblique angle to, the scan plane. Therefore, it is not clear if the close matching of the EO and IO PCSA is unique to the rat, or whether this relationship is skewed towards younger populations in both rat and human.
The normalized fascicle length of a muscle determines its ability to generate active force across a range of lengths (Bodine et al., 1982). A longer fascicle length indicates more sarcomeres acting in series and therefore an ability to produce forces over a wider range of length changes. The current study demonstrated an identical pattern of optimal fascicle lengths in the rat abdominal wall muscles (from longest to shortest: RA, EO, TrA, and IO) as has been shown for human (Brown et al., in press).
Knowledge of instantaneous sarcomere length is required to predict the ability of a muscle to generate active force (Gordon et al., 1966). Both the current study and Brown et al. (in press) measured sarcomere lengths of the abdominal wall muscles with the muscles fixed at a neutral spine posture. Again, in comparison to human, a very similar pattern of relative sarcomere lengths was discovered (Fig. 4), with the RA having the longest lengths followed by the EO (17.9 ± 1.3 and 12.5 ± 1.4% above optimal length, respectively), and the IO and TrA both acting near optimal length (2.0 ± 1.1% above and 0.4 ± 3.0% below optimal length, respectively). This approximates the trend measured for human: RA and EO, 21.9 ± 2.4 and 17.8 ± 4.1% above optimal length, respectively, and IO and TrA, 3.0 ± 2.3 and 4.0 ± 1.8% below optimal length, respectively. These similarities would seem to indicate similar relative ranges of sarcomere lengths over which these muscles generate force in vivo, and similar relative interactions between the muscles with regards to where they lie on the force–length relationship as the spine moves through its ranges of motion.
One of the most interesting morphological features of the abdominal muscles is their composite laminate-like morphology. The EO, IO and TrA are broad sheet-like muscles that overlie one another and are composed of fibers that act highly obliquely with respect to each adjacent layer. This structural make-up may be hypothesized to give these muscles a specialized spine-stiffening role (Nilsson, 1982a; Hwang et al., 2005; Brown & McGill, 2009). Thus, for the rat to be regarded as a valid animal model of human, the relative fiber orientations between the muscle layers must be representative of human. To facilitate this comparison, the muscles are regionalized (Regionalization 2 in Table 2) according to that previously reported for human (Urquhart et al., 2005). Based on this regionalization, Table 3 reports a general pattern of similar angles between fiber lines of action relative to adjacent layers for both rat and human. In the upper region, muscle fibers have relative orientations almost identical to those in human. In the middle region, the angle between the EO–IO is less in the rat, whereas the IO–TrA angle is larger. The greatest difference between rat and human exists in the lower muscle region, where in human the EO muscle becomes aponeurotic (fibers terminate above the anterior superior iliac spine level), whereas in rat the EO fibers extend to the muscle's pelvic insertion. Thus, in human there is no overlap between fibers of the the EO and IO in this lower region, whereas in the rat the fibers act at a relative angle of 85°. Despite this difference, the overall orientation of fibers in the three muscle layers is similar between human and rat, suggesting that the mechanical interaction between muscle layers should be similar between the two species, especially in the upper two regions. The more striking difference in the lower abdominal region may be a requirement of the longer lumbar spine of the rat, which increases the relative distance between the rib cage and pelvis, and may necessitate longer traversing EO fibers and more cranially/caudally oriented IO fibers.
Whereas the current study described the architectural and morphologic parameters of the rat abdominal wall muscles in comparison with human, the connective tissue networks between, and adjacent to, the muscles were not described nor compared. The gross morphology of the terminal aponeuroses of the EO, IO and TrA has been previously described in detail as being generally similar between rat and human (Rizk, 1980). However, neither the more detailed (protein-level) morphology nor the mechanical characteristics of these connective tissues have been examined or compared. These characteristics will influence the mechanical interaction between the muscle layers in terms of force transmission (Yucesoy et al., 2006) and torso stiffening. Future studies will be designed to examine these characteristics.
The functional requirements of the abdominal wall muscles were not assessed in the current study; however, some of these will be briefly discussed. First, the abdominal wall muscles are activated to assist with the expiration phase of ventilation in both human (Goldman et al., 1987; De Troyer et al., 1990) and rat (Reilly et al., 2009), especially as ventilatory demand increases. Next, the muscles play active roles in human and rat locomotion, albeit demonstrating subtly different patterns. In human locomotion the abdominal wall muscles demonstrate phasic activation patterns, overlying some tonic activity in the TrA and IO (Saunders et al., 2004; Anders et al., 2007), whereas in rat locomotion the EO and IO are activated phasically together corresponding to contralateral hindlimb stance, with the TrA and RA maintaining an entrainment to the expiration phase of respiration (Reilly et al., 2009). Finally, in recent years, the importance of abdominal wall activation in stiffening and stabilizing the human lumbar spine has been well documented. This was recently demonstrated as being essential when the human adopts a quadrupedal posture (push-up stance), in which the external destabilizing gravitational load acts more in a shear than a compressive mode on the lumbar spine (Freeman et al., 2006; Howarth et al., 2008). A recent study of a quadruped (dog) (Fife et al., 2001) demonstrated strategic activation of the EO and IO muscles (RA and TrA were not monitored) to support a similar destabilizing mode. In fact, the relative size of the abdominal muscles, when normalized to body mass, is much larger in rat than in human (Fig. 6), suggestive of a greater stabilizing role of these muscles in the rat. Thus, despite the obvious bipedal vs. quadrupedal difference between human and rat, the requirements imposed on the abdominal muscles by the central nervous system appear to be similar, providing evolutionary selection pressure for the similar architecture and morphology.
We conclude that, based on the morphology and architecture of the abdominal wall muscles, the adult male Sprague-Dawley rat is a valid model of the human abdominal wall musculature. The relative force-generating and length-excursion capabilities, neutral posture sarcomere lengths, and muscle fiber orientations are similar in rat and human. Thus, employing a rat model to test various hypotheses regarding the active and passive interactions between these muscles should provide significant insight into their function in humans.
Acknowledgments
This work was supported by the Department of Veterans Affairs Rehabilitation Research and Development, NIH grants HD048501 and HD050837. S.H.M.B. is supported by a post-doctoral Fellowship from NSERC Canada.
References
- Anders C, Wagner H, Puta C, et al. Trunk muscle activation patterns during walking at different speeds. J Electromyogr Kinesiol. 2007;17:245–252. doi: 10.1016/j.jelekin.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Arjmand N, Shirazi-Adl A, Parnianpour M. Trunk biomechanics during maximum isometric axial torque exertions in upright standing. Clin Biomech. 2008;23:969–978. doi: 10.1016/j.clinbiomech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Arnold JS, Thomas AJ, Kelsen SG. Length-tension relationship of abdominal expiratory muscles: effect of emphysema. J Appl Physiol. 1987;62:739–745. doi: 10.1152/jappl.1987.62.2.739. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Meadows DA, et al. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982;48:192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Brown SHM, McGill SM. Transmission of muscularly generated force and stiffness between layers of the rat abdominal wall. Spine. 2009;34:E70–E75. doi: 10.1097/BRS.0b013e31818bd6b1. [DOI] [PubMed] [Google Scholar]
- Brown SHM, Ward SR, Cook MS, et al. Architectural analysis of human abdominal wall muscles: implications for mechanical function. Spine. doi: 10.1097/BRS.0b013e3181d12ed7. (in press) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol. 2001;204:1529–1536. doi: 10.1242/jeb.204.9.1529. [DOI] [PubMed] [Google Scholar]
- Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech. 1999;14:203–216. doi: 10.1016/s0268-0033(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Campbell EJM, Green JH. The variations in intra-abdominal pressure and the activity of the abdominal muscles during breathing: a study in man. J Physiol. 1953;122:282–290. doi: 10.1113/jphysiol.1953.sp004999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Estenne M, Ninane V, et al. Transversus abdominis muscle function in humans. J Appl Physiol. 1990;68:1010–1016. doi: 10.1152/jappl.1990.68.3.1010. [DOI] [PubMed] [Google Scholar]
- DuBay DA, Wang X, Adamson B, et al. Progressive fascial wound failure impairs subsequent abdominal wall repairs: a new animal model of incisional hernia formation. Surgery. 2005;137:463–471. doi: 10.1016/j.surg.2004.12.016. [DOI] [PubMed] [Google Scholar]
- DuBay DA, Choi W, Urbanchek MG, et al. Incisional herniation induces decreased abdominal wall compliance via oblique muscle atrophy and fibrosis. Ann Surg. 2007;245:140–146. doi: 10.1097/01.sla.0000251267.11012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ouaaid Z, Arjmand N, Shirazi-Adl A, et al. A novel approach to evaluate abdominal coactivities for optimal spinal stability and compression force in lifting. Comput Methods Biomech Biomed Engin. 2009 doi: 10.1080/10255840902896018. DOI: 10.1080/10255840902896018. [DOI] [PubMed] [Google Scholar]
- Farkas GA, Rochester DF. Characteristics and functional significance of canine abdominal muscles. J Appl Physiol. 1988;65:2427–2433. doi: 10.1152/jappl.1988.65.6.2427. [DOI] [PubMed] [Google Scholar]
- Fife MM, Bailey CL, Lee DV, et al. Function of the oblique hypaxial muscles in trotting dogs. J Exp Biol. 2001;204:2371–2381. doi: 10.1242/jeb.204.13.2371. [DOI] [PubMed] [Google Scholar]
- Freeman S, Karpowicz A, Gray J, et al. Quantifying muscle patterns and spine load during various forms of the push-up. Med Sci Sports Exerc. 2006;38:570–577. doi: 10.1249/01.mss.0000189317.08635.1b. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Lehr RP, Millar AB, et al. An electromyographic study of the abdominal muscles during postural and respiratory manoeuvres. J Neurol Neurosurg Psychiatry. 1987;50:866–869. doi: 10.1136/jnnp.50.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth SJ, Beach TAC, Callaghan JP. Abdominal muscles dominate contributions to vertebral joint stiffness during the push-up. J Appl Biomech. 2008;24:130–139. doi: 10.1123/jab.24.2.130. [DOI] [PubMed] [Google Scholar]
- Hukins DWL. Collagen orientation. In: Hukins DWL, editor. Connective Tissue Matrix. Weinheim: Verlag Chemie; 1984. pp. 211–240. [Google Scholar]
- Hwang W, Carvalho JC, Tarlovsky I, et al. Passive mechanics of canine internal abdominal muscles. J Appl Physiol. 2005;98:1829–1835. doi: 10.1152/japplphysiol.00910.2003. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. J Hand Surg Am. 1990;15:244–250. doi: 10.1016/0363-5023(90)90103-x. [DOI] [PubMed] [Google Scholar]
- Marras WS, Jorgensen MJ, Granata KP, et al. Female and male trunk geometry: size and prediction of the spine loading trunk muscles derived from MRI. Clin Biomech. 2001;16:38–46. doi: 10.1016/s0268-0033(00)00046-2. [DOI] [PubMed] [Google Scholar]
- Masani K, Sin VW, Vette AH, et al. Postural reactions of the trunk muscles to multi-directional perturbations in sitting. Clin Biomech. 2009;24:176–182. doi: 10.1016/j.clinbiomech.2008.12.001. [DOI] [PubMed] [Google Scholar]
- McGill SM. Kinetic potential of the lumbar trunk musculature about 3 orthogonal orthopedic axes in extreme postures. Spine. 1991;16:809–816. doi: 10.1097/00007632-199107000-00021. [DOI] [PubMed] [Google Scholar]
- McGill SM. A revised anatomical model of the abdominal musculature for torso flexion efforts. J Biomech. 1996;29:973–977. doi: 10.1016/0021-9290(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Nilsson T. Biomechanical studies of rabbit abdominal wall. Part 1. The mechanical properties of specimens from different anatomical positions. J Biomech. 1982a;15:123–129. doi: 10.1016/0021-9290(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Nilsson T. Biomechanical studies of rabbit abdominal wall. Part 2. The mechanical properties of specimens in relation to length, width, and fiber orientation. J Biomech. 1982b;15:131–135. doi: 10.1016/0021-9290(82)90045-8. [DOI] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, et al. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Reilly SM, McElroy EJ, White TD. Abdominal muscle function in ventilation and locomotion in new world opposums and basal eutherians: breathing and running with and without epipubic bones. J Morphol. 2009;270:1014–1028. doi: 10.1002/jmor.10735. [DOI] [PubMed] [Google Scholar]
- Rizk NN. A new description of the anterior abdominal wall in man and mammals. J Anat. 1980;131:373–385. [PMC free article] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Saunders SW, Rath D, Hodges PW. Postural and respiratory activation of the trunk muscles changes with mode and speed of locomotion. Gait Posture. 2004;20:280–290. doi: 10.1016/j.gaitpost.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Urquhart DM, Barker PJ, Hodges PW, et al. Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech. 2005;20:233–241. doi: 10.1016/j.clinbiomech.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38:2317–2320. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Yucesoy CA, Maas H, Koopman BH, et al. Mechanisms causing effects of muscle position on proximo-distal muscle force differences in extra-muscular myofascial force transmission. Med Eng Phys. 2006;28:214–226. doi: 10.1016/j.medengphy.2005.06.004. [DOI] [PubMed] [Google Scholar]


