Abstract
Background
Defects in lymphoproliferative responses to mitogen/antigens in women >45 years old ith a persistent type-specific HPV infection have been reported.
Methods
To determine whether these defects were associated with altered cytokine profiles, plasma and PBMC culture supernatants from 50 cases (persistent HPV infection and weak lymphoproliferative responses) and 50 uninfected controls were examined for 24 cytokines using multiplexed bead-based immunoassays and enzyme-linked immunosorbent assay (ELISA).
Results
The following plasma cytokines were significantly increased from cases relative to controls: (cases vs. controls (median pg/ml); IL-6: 393.1 vs. 14.5, IL-8: 1128.5 vs. 43.9, TNF-α: 164.1 vs. 9.2, MIP-1α: 1368.9 vs. 25.5, GM-CSF: 13.8 vs. 7.3, IL-1β: 8.3 vs. 1.6, all p<0.0001, and IL-1α: 218.2 vs. 169.5, p=0.02). We focused our analysis on the following cytokines: IL-6, IL-8, TNF-α, and MIP-1α due to high fold change (>10) and highly statistically significant difference between cases and controls. Moreover, length of persistence or type of infection (high risk and low risk) did not affect these differences. IL-6, TNF-α, MIP-1α levels were increased in unstimulated PBMC culture supernatants from cases compared to controls (p <0.05), except for IL-8 (p=0.09). However, the cytokine levels from PHA-stimulated PBMC culture supernatants were significantly lower in the cases (p<0.0001).
Conclusions
Persistent HPV infection in older women with evidence of immune deficit is associated with an increase in systemic inflammatory cytokines.
Impact
Future studies are needed to determine whether the inflammatory profile is age dependent and to examine the role inflammatory cytokines play in HPV-induced progression from infection to cervical cancer.
Keywords: Human papillomavirus, inflammation, cytokines, persistent infection
Introduction
Cervical cancer and its precursor lesions have been extensively shown to be caused by human papillomaviruses (HPV) (1–2). The prevalence of HPV infections in women peaks shortly after sexual debut (20– 25 years old) and declines thereafter. In many regions of the world, a second wave of increased HPV prevalence has been described amongst women older than 55 (3) and postulated to be the result of factors such as age-induced immune suppression or cervical changes that occur with menopause.
We have previously observed a decrease in mitogen and recall antigen lymphoproliferation among women persistently infected with HPV (4), suggesting that HPV persistence in the cervix is associated with a decrease in peripheral immune fitness.
Several factors can lead to a decrease in peripheral immune responsiveness. Cytokines and chemokines are essential for a multitude of immune-related activities such as recruiting phagocytes to areas of insult, stimulating the expansion of T cells and B cells upon antigen recognition, and regulating the activation state of the immune system (5–7). Previous studies have noted associations between cytokine alterations and various infection-related states.
To determine biomarkers of immune suppression in older women who are persistently infected with HPV, we evaluated cytokine profiles in a subpopulation of older women with evidence of persistent infection with HPV and similarly aged women without HPV infection.
Materials and Methods
Participants in this study were selected from a population of women who participated in a 10,049 woman population-based natural history cohort study of HPV and cervical neoplasia in the province of Guanacaste, Costa Rica. Details on the design and methods of the main cohort and the nested case-control study evaluating lymphoproliferative responses among HPV positive and negative older women have been published (4, 8–9). Briefly, we previously conducted a nested case-control study to examine lymphoproliferative responses within the Costa Rican natural history cohort where a group of women (n=284) older than 45 who were infected with HPV were compared with a similarly sized control group of HPV negative women (n=291) of the same age distribution. Testing was performed on peripheral blood mononuclear cells (PBMC) collected at the final visit of natural history cohort study (7–9 years after enrollment). We selected a subpopulation of these women based on the following criteria: cases (n=50), women with type-specific persistent HPV infection as denoted from PCR results obtained at enrollment, follow up (5–6 years after enrollment), and final visit (~9th year after enrollment) and weak lymphoproliferative responses to PHA or HPV16 L1 VLP (within the lowest tertile); controls (n=50), women with no evidence of HPV infection at follow up (5–6 years after enrollment) and final visit (7–9 years after enrollment) and strong lymphoproliferative responses to PHA and HPV16 L1 VLP (within the highest tertile of response). Characteristics of the selected cases and controls are described in Supplementary Table 1. All participants provided informed consent, and this study was approved by the United States National Cancer Institute (NCI) and Costa Rica INCIENSA ethical committees.
HPV DNA testing was evaluated at enrollment, during follow up (5–6 years after enrollment), and at the final study visit (~9th year after enrollment) as reported (4), PCR testing was performed with the MY09/11 consensus primers and AmpliTaq Gold polymerase and dot blot hybridization was used for genotyping (10). The control group (HPV DNA negative women) is defined as not having a type-specific persistent infection at two essential time points within the natural history cohort study of HPV and cervical neoplasia [follow up (5–6 years after enrollment) and final visit (7–9 years after enrollment)]. Although the controls were HPV DNA negative at these time points, some (n=6) did have transitory HPV infections as detected during additional clinic visits between enrollment and final visit. The cases were defined into two groups (long-term persistors; n=21; median persistence=108 months and short-term persistors; n=29; median persistence=19 months). The long-term persistors have a type-specific HPV DNA positive result at enrollment, follow up (5–6 years after enrollment), and final visit (7–9 years after enrollment), and the short-term persistors have a type-specific HPV DNA positive result at follow up (5–6 years after enrollment) and final visit (7–9 years after enrollment).Furthermore, we classified women as being infected with a high-risk (HR) HPV type if they were positive for HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, or 68 and as being infected with a low-risk (LR) HPV type if they were positive for HPV types 6, 11, 26, 32, 34, 40, 42, 53, 54, 55, 57, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 74, 81, 82, 83, 84, 85, or 89. Four women were excluded from the HR and LR analysis because they had a HR and LR persistent infection.
All heparinized plasma specimens were collected at the final visit of the natural history cohort study of HPV and cervical neoplasia (approximately 9 years following enrollment). The plasma was tested blindly for twenty-two cytokines and chemokines (IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17, IL-1α, IFN-γ, GM-CSF, TNF-α, MCP-1, MIP-1α, IP-10, RANTES, EOTAXIN, G-CSF, IL-12, IL-15, IL-7, and IL-1β) using the Linco-plex assay (Linco Research, Millipore, St. Charles, MO). TGF-β1 was measured by enzyme-linked immunosorbent assay (ELISA) (Biosource, Camarillo, CA). Interferon (IFN)-α was measured as a single analyte in a bead array (Biosource, Camarillo, CA). Cell supernatants from PBMC incubated with AIM V media or PHA for 48 hours were also assayed with the aforementioned cytokine assays. Specimens that were below the minimum detectable limit for any cytokine were assigned a value of ½ the lowest detectable limit.
The cytokine data was not normally distributed. The Wilcoxon rank-sum non-parametric test was used for all analyses comparing cases and controls. P values <0.05 were considered significant.
Results
The mean, median, and range of responses for each of the 24 different cytokines tested are shown in Supplementary Table 2. The following plasma cytokines were significantly increased from cases relative to controls (median among cases, median among controls, p value for IL-6 =393.1, 14.5, p<0.0001, for IL-8 =1128.5, 43.9, p<0.0001, for TNF-α =164.1, 9.2, p<0.0001, for MIP-1α =1368.9, 25.5, p<0.0001, for GM-CSF =13.8, 7.3, p<0.0001, for IL-1β =8.3, 1.6, p<0.0001, and for IL-1α =218.2, 169.5, p=0.02) (Table 1). Next, we focused our analysis on the following cytokines: IL-6 IL-8, TNF-α, and MIP-1α due to the high fold change (>10) between controls and cases and having a highly statistically significant difference between controls and cases. The length of persistence or type of infection (high versus low risk, Figure 1a–d.) did not affect these differences. The predominance of inflammatory cytokines in the plasma led us to examine the transient secretion of cytokines from 48-hour cultured PBMC to determine whether levels of these cytokines produced by PBMCs paralleled those observed in plasma. The same inflammatory cytokines were also increased in unstimulated PBMC culture supernatant fluid from cases compared to controls (median among cases, median among controls, p value for IL-6 =471.4, 13.8, p<0.0001; for TNF-α =52.3, 11.8, p=0.01; for MIP-1α =503.3, 8, p<0.0001) except for IL-8 (6360.9, 4663.2, p=0.09) as shown in Figure 2a. Finally, we measured the concentration of cytokines in supernatants from PHA-stimulated PBMC cultures. In contrast to the results from the supernatants from the 48-hour culture of PBMC with media alone, the levels IL-6, IL-8, MIP-1α, and TNF-α measured in supernatants collected from PHA-stimulated PBMC cultures were significantly lower in the cases (median among cases, median among controls, for IL-6 =1287.2, 3122.8; for IL-8 14059.8, 45160.3; for TNF-α =993.8, 3312.1; for MIP-1 α =3372.3, 9449.3; p value for all analytes, p<0.0001) (Figure 2b) compared to controls.
Table 1.
Elevated plasma cytokine levels from women with persistent HPV infection.
| HPV−* | HPV+† | ||
|---|---|---|---|
| IL-6 | N | 50 | 50 |
| Responders | 50% | 96% | |
| Mean | 206.1 | 715.5 | |
| Median | 14.5 | 393.1 | |
| Range | 8.0 – 3202.5 | 8.0 – 3338.0 | |
| p-value‡ | <.0001 | ||
| IL-8 | N | 50 | 50 |
| Responders | 96% | 100% | |
| Mean | 392.5 | 1545.7 | |
| Median | 43.9 | 1128.5 | |
| Range | 1.6 – 3615.8 | 17.8 – 7535.2 | |
| p-value | <.0001 | ||
| IL-1α | N | 50 | 50 |
| Responders | 100% | 100% | |
| Mean | 244.8 | 328.5 | |
| Median | 169.5 | 218.2 | |
| Range | 52.4 – 2714.8 | 57.6 – 2113.9 | |
| p-value | 0.02 | ||
| GM-CSF | N | 50 | 50 |
| Responders | 100% | 100% | |
| Mean | 14.5 | 33.6 | |
| Median | 7.3 | 13.8 | |
| Range | 2.1 – 293.5 | 4.4 – 886.4 | |
| p-value | <.0001 | ||
| TNF-α | N | 50 | 50 |
| Responders | 96% | 100% | |
| Mean | 61.4 | 189.1 | |
| Median | 9.2 | 164.1 | |
| Range | 0.8 – 833.6 | 12.3 – 608.1 | |
| p-value | <.0001 | ||
| MIP-1α | N | 50 | 50 |
| Responders | 54% | 100% | |
| Mean | 576.0 | 2164.9 | |
| Median | 25.5 | 1368.9 | |
| Range | 8.0 – 10097.2 | 17.9 – 10097.2 | |
| p-value | <.0001 | ||
| IL-1β | N | 50 | 50 |
| Responders | 48% | 84% | |
| Mean | 6.6 | 23.6 | |
| Median | 1.6 | 8.3 | |
| Range | 1.6 – 73.9 | 1.6 – 536.5 | |
| p-value | <.0001 |
HPV- women (controls) were negative for any HPV type at follow up (5–6 years after enrollment) and final visit (9 years after enrollment).
HPV+ women (cases) tested positive on at least the follow up visit (5–6 years after enrollment) and final visit (9 years after enrollment) for a type specific HPV infection.
The p-value was calculated using the Wilcoxon rank sum test to compare cytokine values between cases and controls.
Figure 1.
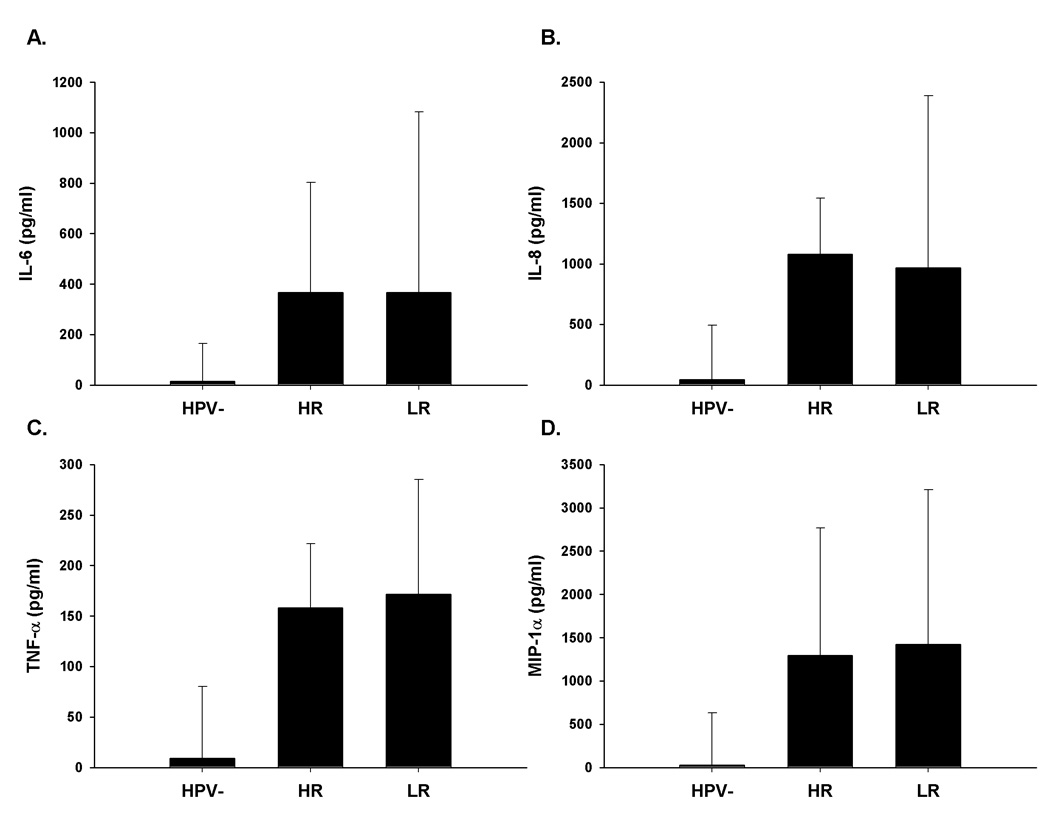
Comparisons of inflammatory cytokine levels in plasma from women with 'High Risk' (HR) or 'Low Risk' (LR) HPV type-specific persistent infection. HR and LR were each compared to the HPV- control group. There was not a significant difference between the HR and LR groups for the cytokines shown. All comparisons between HPV- and HR or LR were highly significant (p<0.0001) using the Wilcoxon rank sum test. Vertical bars represent 75th percentile.
Figure 2.
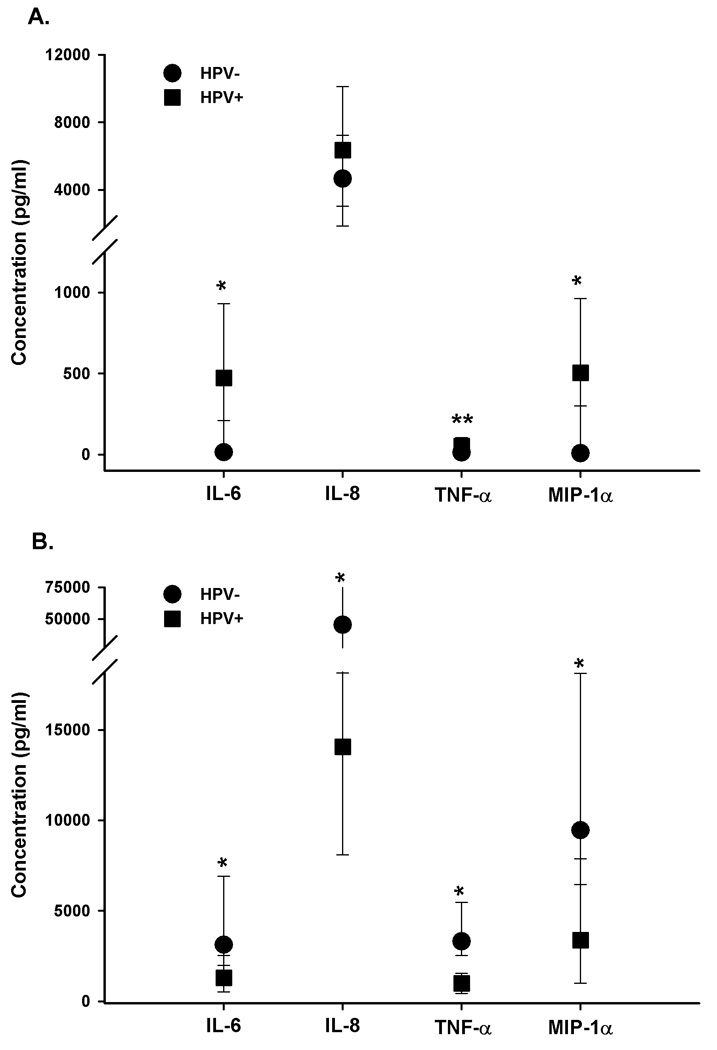
a–b. (A)Cytokine levels in supernatants collected from 48 hour unstimulated PBMC. Pooled supernatants from 2×105 PBMC cultured in AIM V media for 48 hours were collected and frozen at −80°C. (B) Cytokine levels in supernatants collected from 48 hour PHA-stimulated PBMC. Pooled supernatants from 2×105 PBMC stimulated with PHA (1 mg/ml) for 48 hours were collected and frozen at −80°C. Samples were tested with Linco-plex bead assay to examine the cytokine concentrations in the supernatants. Bars represent interquartile range. Wilcoxon rank sum used to determine statistical significance in differences between HPV- (controls) and HPV+ (cases) groups for each cytokine (*= p<0.0001; **= p<0.01).
Discussion
We have previously observed that older women who were persistently infected with HPV had a marked decrease in lymphoproliferation in response to PHA, FLU, and HPV16 L1 VLP (4). To investigate possible underlying mechanisms for this phenomenon, we evaluated a subset of participants from our previous study and explored whether alterations in their cytokine profile might account for the increased risk of HPV persistence observed among women with low proliferative response. Our results suggest that the cases have markedly increased levels of inflammatory cytokines as indicated by the significant increase in IL-6, IL-8, TNF-α, and MIP-1α levels as compared to controls. These were the only peripheral markers we have noted to be altered in this group of persistently infected women with evidence of immune dysfunction in vitro.
The finding of elevated systemic levels of inflammatory cytokines in women with persistent cervical HPV infections and marked decrease in immune function is novel and somewhat unexpected because there is little evidence for an HPV-induced systemic inflammatory reaction. Also, HPV infections described here are believed to remain localized to the cervix. Studies have shown that persistent HPV infection may lead to local immune tolerance (11), which suggests that local inflammation at the cervix would be minimal as well as markers of peripheral inflammation. In addition, several studies have described HPV-induced anti-inflammatory mechanisms such as HPV16 E6 protein inhibiting the expression and signaling of interferons (12)and IL-18 (13). Secondly, HPV does not have a lytic life cycle within differentiating keratinocytes which would induce an inflammatory response. Interestingly, the measured inflammatory response in the periphery is not restricted to individuals infected with ‘High Risk’ HPV types such as HPV16, HPV18, HPV31, and HPV45. We observed that women infected with ‘Low Risk’ HPV types also had a marked increase in IL-6, IL-8, TNF-α, and MIP-1α when compared to controls. This finding suggests that the peripheral inflammatory response is not influenced by the oncogenicity of the HPV type involved. Although increased levels of inflammatory cytokines have been previously noted in a study of cervical cancer (14), women in our current study were healthy and had normal cervical cytology at each visit.
Our study focused on comparing cytokine measures from age-matched cases and controls, suggesting that the presence of inflammatory cytokines in the periphery is associated with HPV persistence and weak lymphoproliferative responses, but not necessarily age. Because women aged 20–30 years old have the highest prevalence of HPV infection (15–18) and also are most likely to progress to CIN3 (19), it would be important to conduct a longitudinal study in younger women (20–30 years old) to define the associations between lymphoproliferative responses, inflammatory cytokine levels, and HPV persistence in order to track these events early on.
One of the limitations of our study is that the samples were all collected at the final visit. Future longitudinal studies will help define whether the increases in inflammatory cytokine levels observed are constitutive or induced following a persistent HPV infection. Furthermore, we do not know whether the inflammatory cytokines in the present study place women at risk for progression to CIN3 or if they are beneficial to these women.
There are several possible hypotheses as to why cases have increased levels of systemic inflammatory cytokines, such as the presence of simultaneous infections with other microbial agents, which deserve attention in future studies. For example, chronic viral infections such as hepatitis B and C have been shown to induce inflammatory cytokines such as IL-8, TNF-α, and IL-6 in the periphery (20–21). Furthermore, high body mass index (BMI) has been associated with an increase in peripheral inflammation in some studies (22–23). Future studies are needed to define the potential role of concomitant infections and obesity in the inflammation and immune deficit seen in this subset of older women with persistent HPV infection.
Another hypothesis as to why proliferation was significantly lower in the cases is that their PBMC may be exhausted or more susceptible to cell death due to the peripheral cytokine milieu. Support for this hypothesis comes from our cytokine results which showed significantly lower concentrations for all of the cytokines tested in the supernatants from PHA-stimulated PBMC. In order to find a dramatic decrease in nearly all of the cytokine values, we would have to suspect a global effect on the cells such as an increased susceptibility to cell death or anergy. Due to the limited number of cells isolated from our study participants, follow up analysis of cell exhaustion or cell death mechanisms will require future studies to examine this hypothesis thoroughly.
In conclusion, we have described an increase in inflammatory cytokine levels among women persistently infected with HPV, with evidence of marked decrease in immune function. Future studies are needed to determine role of this inflammatory cytokine profile in progression to pre-cancer and cancer and whether this inflammatory profile is present in persistent infections in women from this cohort with preserved immune function and in younger women.
Supplementary Material
Footnotes
Grant Support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflict of Interest: None of the authors have any potential financial conflict of interest related to this manuscript.
Bibliography
- 1.Munoz N, Bosch FX, de Sanjose S, et al. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int J Cancer. 1992;52:743–749. doi: 10.1002/ijc.2910520513. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 3.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Pineres AJ, Hildesheim A, Herrero R, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070–11076. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 7.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 8.Bratti MC, Rodriguez AC, Schiffman M, et al. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica. 2004;15:75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 9.Herrero R, Schiffman MH, Bratti C, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salud Publica. 1997;1:362–375. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 10.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–423. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 11.Doan T, Herd K, Street M, et al. Human papillomavirus type 16 E7 oncoprotein expressed in peripheral epithelium tolerizes E7-directed cytotoxic T-lymphocyte precursors restricted through human (and mouse) major histocompatibility complex class I alleles. J Virol. 1999;73:6166–6170. doi: 10.1128/jvi.73.7.6166-6170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koromilas AE, Li S, Matlashewski G. Control of interferon signaling in human papillomavirus infection. Cytokine Growth Factor Rev. 2001;12:157–170. doi: 10.1016/s1359-6101(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 13.Cho YS, Kang JW, Cho M, et al. Down modulation of IL-18 expression by human papillomavirus type 16 E6 oncogene via binding to IL-18. FEBS Lett. 2001;501:139–145. doi: 10.1016/s0014-5793(01)02652-7. [DOI] [PubMed] [Google Scholar]
- 14.Chopra V, Dinh TV, Hannigan EV. Circulating serum levels of cytokines and angiogenic factors in patients with cervical cancer. Cancer Invest. 1998;16:152–159. doi: 10.3109/07357909809050029. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–2684. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 16.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 17.Lazcano-Ponce E, Herrero R, Munoz N, et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412–420. doi: 10.1002/1097-0215(20010201)91:3<412::aid-ijc1071>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Molano M, Posso H, Weiderpass E, et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87:324–333. doi: 10.1038/sj.bjc.6600442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SS, Zuna RE, Wentzensen N, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev. 2009;18:113–120. doi: 10.1158/1055-9965.EPI-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polyak SJ, Khabar KS, Rezeiq M, Gretch DR. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falasca K, Ucciferri C, Dalessandro M, et al. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36:144–150. [PubMed] [Google Scholar]
- 22.Festa A, D'Agostino R, Jr, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25:1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 23.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


