Abstract
The heat shock (HS) response is an important cytoprotective response comprising expression of heat shock proteins (HSP) and orchestrated by the heat/stress-induced transcription factor, heat shock factor-1 (HSF-1). Previous studies suggest that the activation threshold and magnitude of the HS response may be modified by treatment with arachidonic acid (AA). We analyzed the effect of exogenous AA and its metabolites, PGE2, LTD4, and 15-HETE on HSF-1-dependent gene expression in A549 human respiratory epithelial-like cells. When added at 1 μM, PGE2 much more than LTD4, but not 15-HETE increased activity of a synthetic HSF-1-dependent reporter after HS exposure (42°C for 2h), but had no effect in the absence of HS. Exposing A549 cells to HS stimulated release of PGE2 and treatment with the cyclooxygenase inhibitor, ibuprofen, reduced HS-induced HSF-1-dependent transcription. PGE2 increased HS-induced HSP72 mRNA and protein expression but EMSA and Western blot analysis failed to show an effect on HSF-1 DNA binding activity or post-translational modification. In summary, we showed that HS stimulates generation of PGE2, which augments generation of HSPs. The clinical consequences of this pathway have yet to be determined.
Keywords: Heat shock, HSP-72, Prostaglandin E2, Arachidonic Acid
Introduction
The heat shock (HS) response is an evolutionary conserved response to stress, including high temperatures, which is characterized by the generation of a family of heat shock proteins (HSPs), including HSP72 (HSPa1a, b) 1. In vertebrates, HSP genes are regulated by the heat/stress activated transcription factor, heat shock factor-1 (HSF-1) 2, which is required for HS-induced HSP expression 3. When exposed to heat or other stresses such as ischemia and aging, HSF-1 undergoes a stepwise activation including trimerization, nuclear translocation, and independent acquisition of DNA binding and transactivating capacities. While HSF-1 trimerization is sufficient for it to bind to heat shock response elements (HSE) in the promoter regions of HSPs and other genes, transcriptional activation requires further modifications, including phosphorylation and sumoylation, that are detectable as a decrease in electrophoretic mobility on SDS-PAGE 4, 5.
Our group has shown that the activation of HS gene expression is both temperature- and time-dependent 6. We found that exposing A549 human lung epithelial-like cells to an incubation temperature as low as 38.5°C was sufficient to activate HSP72 expression, the magnitude of which increased progressively as incubation temperatures were incrementally increased from 38.5°C to 41°C. In contrast to the proportional response of HSP72 gene expression to increasing temperature, the DNA-binding activity of HSF-1 was activated to similar levels in cells exposed to 38.5°, 39.5°, and 41°C. However, the electrophoretic mobility of HSF-1 on Western blots progressively decreased with increasing incubation temperature between 38.5°C and 41°C and the activity of an HSF-1-dependent reporter plasmid increased, suggesting that the extent of HSF-1 post-translational modification and its trans-activating potential are proportional to temperature in this range.
These results demonstrate that the thermal threshold for activation of the HS response is variable and the magnitude of HSP gene expression is proportional to the magnitude of stress. Previous studies suggest that the thermal threshold for activation of the HS response and the magnitude of HSP expression may also be modified by exposure to certain inflammatory mediators, including arachidonic acid (AA) 5, 7 and type I interferons 5, 8. Jurivich et al. 5, 7 showed that treating HeLa cells with 10 μM AA prior to a 30 min thermal stress reduced the threshold for HSP gene expression from 41°C to 39°C. Treatment with higher levels of AA (20μM) was sufficient to activate HSP expression at 37°C in these cells. The capacity of immunologic mediators such as AA to reduce the threshold for activation of the HS response may explain the lower thermal threshold required to activate the HS response in vivo especially during inflammation 9, 10
Free AA is formed by release from the sn-2 position of phospholipids by A2 phospholipases and is the precursor for the generation of important lipid second messengers including prostaglandins (PGs), leukotrienes (LTs), and hydroxyeicosatetraenoicacids (HETEs) 5, 11. Cytoplasmic phospholipase A2-α (cPLA2-α)-mediated AA release is the rate-limiting step in the generation of downstream mediators in most systems 5, 12 and is responsible for the stimulated release of AA in vivo. cPLA2-α knockout mice lack the ability to generate LTs, PGs, or PAF in a variety of cell types utilizing a wide range of stimuli 13.
The fate of AA once released depends on the presence and activity of downstream enzymes responsible for the formation of specific lipid mediators. The three best-described pathways for processing AA are cyclooxygenase metabolism resulting in PG formation, 5-lipoxygenase activity forming LTs, and 15-lipoxygenase reactions yielding 15-HETEs 5, 11. Of these families of AA metabolites, only 15-deoxy-Delta 12,14-prostaglandin J2, the ultimate metabolite of cyclooxygenase-derived PGD2, has been shown capable of activating HSF-1 and HSP gene expression 14.
The purpose of our study was to determine whether other AA metabolites activate or modify the HS response. We analyzed the effect of exogenous AA metabolites on HSF-1-dependent reporter plasmid expression, HSF-1 activation, and HSP72 mRNA and protein generation in the human A549 respiratory epithelial-like adenocarcinoma cell line. PGE2 to a much greater extent than LTD4, but not 15-HETE, increased HSF-1-dependent gene expression.
Materials and Methods
Cell culture and Heating protocol
The human alveolar type II epithelial-like cell line, A549 cells (American Type Cell Collection; Manassas, VA), were maintained in RPMI 1640 containing 10% defined fetal bovine serum (FBS; Hyclone, Logan, UT) at 37°C in 5% CO2-enriched air. Cells were adjusted to 1 × 105 cells/ml and plated in a 24-well plate and incubated at 37°C for 24 h, prior to exposure to HS (42°C for 2 h). Some cells were exposed to PGE2, 15-HETE, AA or LTD4 added simultaneously with HS. In other experiments, cells were pretreated with 5 μM ibuprofen added 30 min prior to HS. Stock solutions of 0.1 mg/ml 15-HETE and LTD4 and 100 mg/ml AA in ethanol were purchased from Cayman Chemical (Ann Arbor, MI). PGE2 was purchased from Cayman Chemical and dissolved in ethanol to produce a 10 mg/ml stock solution. Stock solutions were diluted further in culture medium to achieve the final concentrations reported. To control for the effects of ethanol, control cells were treated with 0.4% ethanol. Ibuprofen sodium was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in PBS to produce a 10 mM stock solution.
Western blot
A549 cells were incubated with or without AA or one of its metabolites and with or without a 2 h HS at 42°C and 4 h recovery at 37°C for a total of 6 h. Following treatment, cells were lysed in RIPA buffer (Teknova; Hollister, CA) containing protease inhibitors (Roche, Palo Alto, CA) and phosphatase inhibitors (Sigma, St. Louis, MO), resolved on 10% SDS-polyacrylamide gels, and transferred to PVDF membrane (Millipore, Billerica, MA). The membranes were blocked for 1 h at room temperature in blocking buffer (TBS-T (10 mM Tris-HCl, pH7.5, 136 mM NaCl, 2 mM KCl, 0.1% Tween 20) containing 5% nonfat dry milk). Following blocking, the membranes were washed with TBS-T, and incubated with rabbit antibodies against HSP72 (Stressgen; Ann Arbor, MI), β-tubulin (Milipore), or rat anti-HSF-1 (Santa Cruz; Santa Cruz, CA) in blocking buffer for 2 h. After primary antibody reactions, the membranes were washed with TBS-T, incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (Santa Cruz) and developed with a chemiluminescence detection system (Renaissance™; New England Nuclear; Boston, MA), quantified by direct imaging (Fuji gel documentation system and ImageGauge software).
HSE reporter analysis
For stable transfections, A549 cells were co-transfected with a luciferase reporter construct driven by a synthetic promoter composed of multiple HSEs and the TATA-like promoter region from the Herpes simplex virus thymidine kinase gene (pHSE-Luc) along with the blasticidin resistance plasmid, pcDNA6/TR, using Fugene-6 transfection agent. Transfectants were selected using 4 μg/ml blasticidin, and maintained in media containing 2 μg/ml blasticidin and cloned by limiting dilution. Single colonies were isolated, analyzed for HS-induced activation of the HSE-containing promoter and a single clone was used for further studies. For reporter assays, 1 × 105 cells/ml were plated in blasticidin-free medium for 24 h, then were incubated with or without AA or one of its metabolites and with or without a 2 h HS and 4 h recovery at 37°C for a total of 6 h. Controls were treated with the AA/PGE2 vehicle, EtOH at 1%, the highest concentration present in the AA/PGE2 dose response. The cells were then lysed in 1× Cell culture lysis buffer (Promega; Madison, WI) and luciferase activity was analyzed using a commercial luciferase assay kit (Promega).
Quantitative Reverse Transcription/Polymerase Chain Reaction Assay (qRT-PCR)
Total RNA from 2 × 106 A549 cells was isolated using RNeasy (Qiagen; Valencia, CA) and contaminating DNA was eliminated using DNase I digestion (Qiagen). RNA was reverse-transcribed using 1 μg total RNA and oligo-dT primer using a cDNA synthesis kit according to the manufacturer’s protocol (Promega). Duplicate 25 μl real–time PCR reactions were performed in 96 well plates using SYBR-Green reaction mix (Biorad; Herculus, CA) and Biorad iCycler according to the supplier’s protocol with the following forward and reverse primers: glyceraldehydes-3 phosphate dehydrogenase (GAPDH), 5′-agcctcgtcccgtagacaaaat and 5′-tggcaacaatctccactttgc; and HSP72, 5′-accaagcagacgcagatcttc and 5′-agcctcaagatcatcagcaatg. Data were quantified using the Gene Expression Ct Difference method described by Schefe et al. 15 and standardized to levels of the housekeeping gene, GAPDH, using Ct values automatically determined by the thermocycler. Briefly, the efficiency of amplification for each primer pair was calculated using the equation:
where slope is measured from the linear portion of the log fluorescence vs. cycle number curve. The expression of the gene of interest (GOI) in each sample relative to a reference, untreated 37°C cells, was calculated using the equation:
where DCt (GOI) and DCt (GAPDH) is the difference in threshold cycle between sample and reference for the gene of interest and GAPDH, respectively.
Prostaglandin E Assay
Media from cell cultures was assayed in triplicate for PGE2 using an EIA kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol.
Electrophoretic Mobility Shift Assays (EMSA)
Nuclear extracts from A549 cells were prepared according to the method of Schreiber et al. 16 as described earlier 17 and total protein concentration was measured using a commercial reagent (Biorad) against a bovine serum albumin standard curve. Double-stranded oligonucleotide containing the HSE corresponding to -107/-83 of the human Hspa1a promoter 18 (5′-GATCTCGGCTGGAATATTCCCGACCTGGCAGCCGA-3′) was generated by annealing complementary strands and radiolabeling using T4 polynucleotide kinase (Promega) and [γ-32P]ATP according to the manufacturer’s protocol. The DNA-protein complexes were then electrophoretically resolved on 4% nondenaturing polyacrylamide gels. The dried gels were analyzed by phosphorimaging (Molecular Dynamics, Sunnyvale, CA) and exposed to X-ray film. Where indicated, 30-fold excess unlabeled competitor (Hspa1a promoter) or nonspecific oligonucleotide or 1:20 dilution of anti-HSF-1 antibody (Santa Cruz) were incubated with the nuclear extracts for 30 min at room temperature before the addition of the radiolabeled probe.
Statistical Analysis
Data are presented as mean±SE. Differences among groups were analyzed by applying a Tukey Honestly Significant Difference test to a one-way ANOVA. Differences with p < 0.05 were considered significant.
Results
Effect of AA and metabolites on HSF-1-dependent gene expression
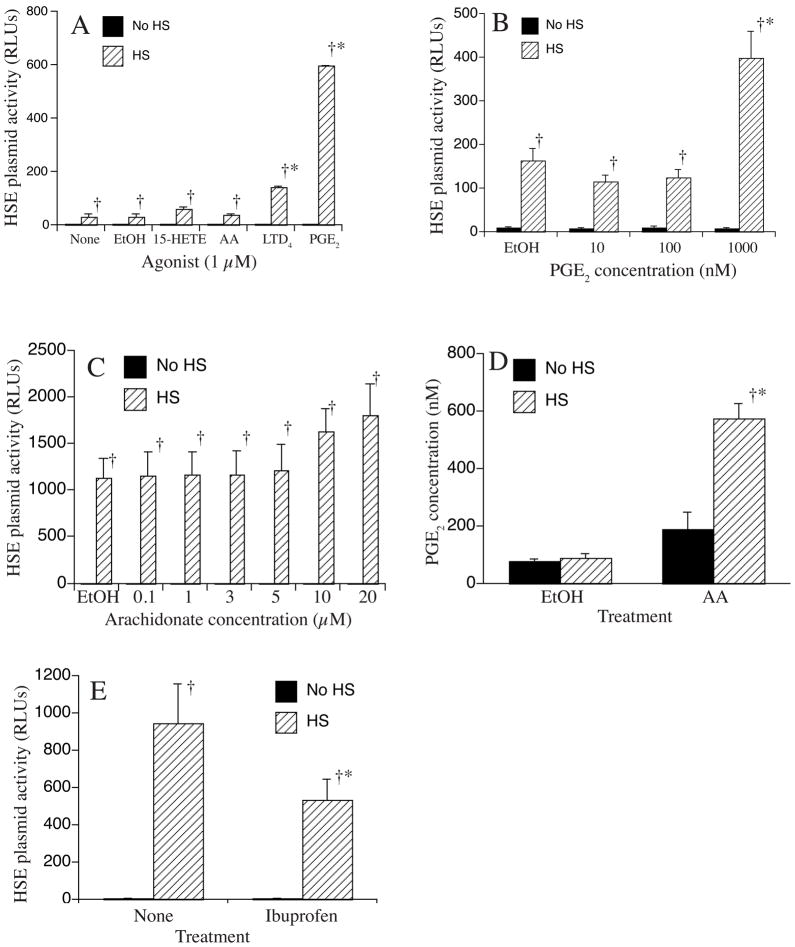
Adding exogenous AA has been reported to augment activation of HSF-1-dependent gene expression. Since AA is predominantly metabolized to three families of potent biochemical mediators, we performed an initial experiment to identify the AA metabolite family that augments HSF-1-dependent gene expression using A549 cells stably expressing a HSF-1-responsive promoter as we have previously described 6 (Fig. 1A). Cells were treated with 1 μM concentrations of AA, 15-HETE, LTD4, or PGE2. Controls were incubated in medium alone or with 0.4% ethanol, the diluent used for AA and its metabolites. Treated cells were either exposed to HS for 2 h at 42°C followed by 4 h recovery at 37°C or were incubated for 6 h at 37°C without HS, and luciferase levels were measured. Ethanol-treated controls exhibited luciferase activity virtually identical with untreated cells. PGE2 and LTD4 augmented HS-induced luciferase expression compared with ethanol-treated controls; however, the effect was much greater with PGE2. Neither PGE2 nor any of the other lipid mediators tested activated luciferase expression in the absence of HS. Analysis of the PGE2 dose response indicated that PGE2 concentrations below 1 μM are not sufficient to modify HSF-1-dependent transcription (Fig. 1B). Because Jurivich et al. 7 demonstrated that 20 μM AA was sufficient to activate HSP72 expression at 37°C and 10 μM AA synergized with heat to activate HSP72 transcription in HeLa cells, we analyzed the effect of AA concentrations between 1 and 20 μM on activation of an HSF-1-responsive reporter plasmid (1C). AA at concentrations of 10 and 20 μM tended to increase HS-induced reporter plasmid activation, but the difference did not reach statistical significance. AA at concentrations up to 20 μM had no effect on reporter activity in the absence of HS. Since AA is a precursor for AA, we analyzed the effect of HS on PGE2 synthesis without and with exogenous AA (1D).
Figure 1. Effect of PGE2 and other stimuli on HS-induced activation of a HS-responsive reporter construct.
A549 cells stably transfected with a HS-responsive luciferase reporter plasmid were incubated for 6 h with or without a 2 h 42°C HS and coincident treatment with medium alone (none), vehicle (0.4% EtOH), 1 μM of AA or indicated AA metabolite (A), the indicated concentration of PGE2 (B), the indicated concentration of AA (C), 20 μM AA (D) or 30 min pretreatment with 5 μM ibuprofen (E). The cells were then lysed and luciferase measured (A-C, E) and expressed as fold-change compared with untreated controls without HS or PGE2 measured by EIA (D). Mean±SE, n = 4 (A–C, E) or 3 (D); * and † denotes p<0.05 vs. untreated, HS-exposed controls and cells receiving the same treatment but without HS, respectively.
While HS did not induce a detectable increase from basal PGE2 levels in ethanol-treated control cells, combined treatment with 20 μM AA and exposure to 42°C increased PGE2 levels by 6.6-fold compared with ethanol-treated cells incubated at 37°C and by 2.8-fold vs. cells treated with AA-treated cells at 37°C. Finally, to determine whether generation of endogenous PGE2 contributed to HS-induced activation of HSF-1-dependent gene expression, we analyzed the effect of pretreatment with the cyclooxygenase inhibitor, ibuprofen, on HS-induced reporter plasmid activity (1E). Ibuprofen (5 μM) added 30 min prior to HS reduced the HS-induced reporter plasmid activity by 44% compared with heat-shocked cells incubated without ibuprofen, but had no effect on activity in 37°C cells.
PGE2 Enhances HS-induced HSP72 expression
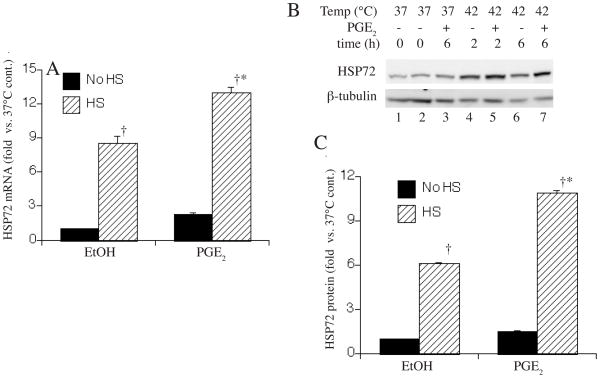
To determine whether PGE2 has similar effects on HS-induced expression of endogenous HSF-1-responsive genes, we analyzed the effect of adding 1 μM PGE2 with or without HS on levels of HSP72 mRNA and protein. Non-transfected A549 cells were incubated with 1 μM PGE2 or 0.4% ethanol with or without 2 h HS at 42°C followed by recovery at 37°C. HSP72 mRNA levels were analyzed by quantitative RT-PCR after 2 h (Fig. 2A) and HSP72 protein levels were analyzed by Western blotting after the 2 h HS and 4 h recovery (Fig. 2B, C). PGE2 exerted effects on HSP72 gene expression that were similar to its effects on activation of the HSF-1-responsive reporter plasmid. PGE2 increased HS-induced HSP72 mRNA levels by 42% and HSP72 protein levels by 77%, but in the absence of HS, PGE2 had no detectable effect on HSP72 expression.
Figure 2. Effect of PGE2 on HS-induced activation of HSP72 expression.
A549 cells were incubated for 6 h with or without a 2 h, 42°C HS and with 1 μM PGE2 or 0.4% ethanol and HSP72 mRNA was quantified by real-time RT-PCR standardized to GAPDH (A) and protein was quantified by Western blotting with β-tubulin as a control (representative of 4 similar gels in B). The bands were quantified by direct imaging of the chemilluminescent bands, expressed as a ratio to β-tubulin, and as fold change vs untreated cells without HS (C). Data are expressed as mean±SE, n = 4; * and † denotes p<0.05 vs. untreated, HS-exposed controls and cells receiving the same treatment but without HS, respectively.
Effect of PGE2 on HSF-1 activation
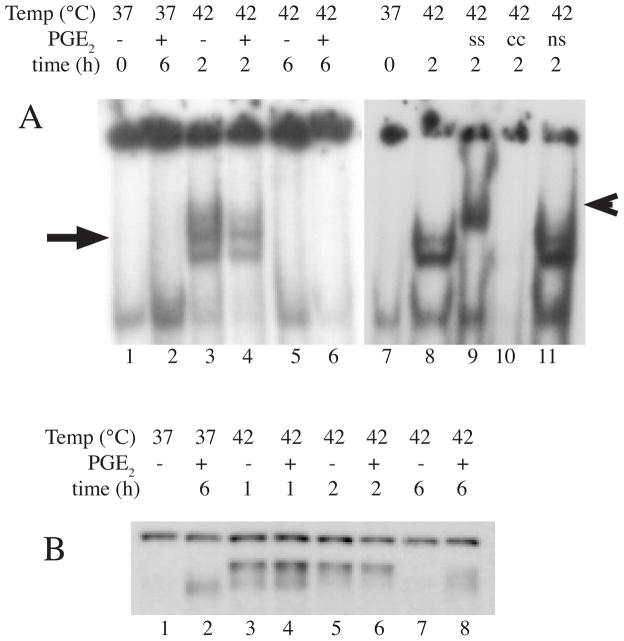
HSF-1 exists as a monomer and homotrimerizes in response to stress 5, 19. Mutational analyses of HSF-1 suggests that homotrimerization is sufficient for HSF-1 to attain capacity for specific, high affinity DNA binding to three contiguous inverted nGAAn dyad repeats 20–22. However, the additional gain of transcriptional activating activity requires further posttranslational modification of the HSF-1 trimers, including phosphorylation23–26, SUMOylation 27 and possibly oxidation 5, 28. To determine whether PGE2 exerted its effects on HSF-1 trimerization/DNA binding or covalent modification, we analyzed nuclear extracts from A549 cells incubated with 1 μM PGE2 or 0.4% ethanol with or without 1 or 2 h HS at 42°C for HSF-1 DNA binding activity by EMSA (Fig. 3A) and covalent modification by analyzing the elecrophoretic mobility of HSF-1 on an SDS-PAGE/Western blot (Fig. 3C). As expected, exposure to HS increased both DNA-binding activity and apparent molecular weight of HSF-1, but PGE2 did not cause further increases in these measures of HSF-1.
Figure 3. Effect of PGE2 on HSF-1 activation.
Nuclear extracts were collected from A549 cells treated with 1 μM PGE2 or 0.4% ethanol with or without HS at 42°C and analyzed for HSF-1 DNA binding activity by EMSA (representative of 4 similar autoradiographs in A; HSF-1-shifted complex indicated by arrow). HSF-1 binding was confirmed by supershifting with anti-HSF-1 antibody (ss, lane 9; indicated by arrowhead) and competition with 30X excess of unlabeled hspa1a oligonucleotide (cc, lane 10). Competition with a nonspecific (ns) oligonucleotide is shown as a negative control in lane 11. B. Covalent modification of HSF-1 was analyzed by measuring the elecrophoretic mobility of HSF-1 in nuclear extracts by Western blotting; a representative of 3 similar blots is shown.
Discussion
Jurivich et al. showed that treatment with exogenous AA synergizes with heat to activate HSF-1 and induce HSP gene expression, but only at high AA concentrations 5, 7. 29–31 In our current study, we extend these observations by demonstrating that the AA metabolites PGE2 to a much greater extent than LTD4 but not 15-HETE, augmented HS-induced HSF-1-dependent gene expression.
Transcription of heat shock protein genes is predominantly regulated by the heat-activated transcription factor, HSF-1, but other transcriptional regulators, including STAT-1, NF-IL-6 and TFIID, have also been shown to bind to HSF-1 with variable effects on HSF-1 function. For example, STAT-1 and HSF-1 synergistically activated transcription of HSP72 and HSP90 5, 32, whereas HSF-1 and NF-IL-6 are mutually antagonistic 33, 34. Similarly, Yuan and Gurley 35 reported that HSF-1 could bind both TATA-binding protein (TBP) and the TFIIB transcription factor complex, and implied that these interactions determine whether the complex is dysfunctional or a transcriptionally competent pre-initiation complex. We initially analyzed the effect of AA and its metabolites on activity of a luciferase reporter driven by an artificial HSF-1-responsive promoter stably transfected into A549 cells. The promoter, which contains multiple HSEs and the TATA-like promoter region from the Herpes simplex virus thymidine kinase gene, exhibited an 84-fold increase in activity following exposure to HS. Utilizing this system, we showed that HS-induced transcriptional activation was further enhanced by 1 μM PGE2. Since this promoter lacks binding sequences for transcriptional activators other than HSF-1, our results suggest that PGE2 and LTD4 increase HSF-1-dependent transcription. Jurivich et. al. 5, 7 demonstrated in HeLa cells that 10 μM AA reduced the thermal threshold for heat-induced HSF-1 activation and HSP72 transcription and 20 μM AA was sufficient to induce sub-maximal activation of HSF-1 and HSP72 transcription at 37°C. Our results suggested a similar interaction between AA and heat in activating HSF-1-dependent gene expression, but the trend in increased HS-induced HSF-1-responsive reporter plasmid activity in cells treated with 10 and 20 μM AA did not reach statistical significance. The AA concentrations analyzed in these studies appears to be achievable in vivo, especially in the presence of inflammation. For example, concentrations of AA have been reported to be ~0.5–1 μM in resting leukocytes 36, 15 μM in isolated islets of Langerhans 37, and 100 μM in the inflamed skin tissue of psoriasis 38. However, whether exogenous AA exerted its effects directly or through effects of one or more of its metabolites was not addressed in these studies.
In this study, we demonstrated that exposing A549 cells to HS tended to increase secretion of PGE2, but the effect of HS on PGE2 generation was much greater in the presence of exogenous AA, suggesting that HS may exert its effects predominantly distal to AA release from membrane lipids. Furthermore, we found that treating A549 cells with the cyclooxygenase inhibitor, ibuprofen, attenuated the HS-induced activation of HSF-1-dependent gene expression. Taken with our demonstration that exogenous PGE2 increases HS-induced activity of an HSF-1-responsive reporter plasmid, these results suggest that HS induces generation of PGE2, which is required for maximal HSF-1-dependent gene transcription.
Surprisingly, we found that PGE2 levels ≤100 nM had no effect on HSF-1-dependent gene expression. Most studies show that PGE2 at nanomolar levels activates a family of membrane-associated G-protein coupled receptors, EP1 through EP4, all of which are expressed in epithelial cell culture lines 39. Yano et al 40 showed in A549 cells that 10 nM PGE2 increased proliferation and activation of extracellular signal-regulated kinase activation via EP3 and 4 engagement, raising questions about whether 1 μM PGE2 modifies HSF-1-dependent gene expression through alternative mechanisms. The PGD2 metabolite and potent HSF-1 activator, 15-deoxy-Delta12,14-prostaglandin J2, activates the nuclear transcription factor PPARγ 41, 42. Several studies show that non-lipid PPARγ agonists also activate HSF-1 and HSP gene expression. However, to our knowledge, there are no reports of PGE2 exhibiting PPARγ agonist activity. Of note, Yamaki et al. 43 found that 100 μM but not 1 μM PGE2 activated src protein tyrosine kinsase in A549 cells; however, they did not identify the mechanisms through which PGE2 stimulated this cell response.
Other groups have shown that cyclooxygenase metabolites, including PGE2, enhance activation of the HS response in other cell systems and tissues while inhibition of cyclooxygenase reduces this activation. Togo et al. 44 showed that treating rats with PGE1 enhanced HSP70 generation in residual liver and reduced acute liver failure after massive hepatectomy. Hwang et al. 45 reported that the cyclopentenone prostaglandin, 15-deoxy-Delta(12,14)-prostaglandin J(2) (15d-PGJ(2)), induced HSP70 expression in endothelial cells. Xu et al. 46 showed that PGA1 pretreatment increased HSP70 and glucose-regulated-protein 78 (GRP78) levels and reduced brain infarct size after middle cerebral artery occlusion in rats. Warzecha et al. 47 demonstrated that the increase in pancreatic HSP70 levels induced by ischemic preconditioning in a cerulein-induced pancreatitis model was reduced by pretreatment with COX-2 inhibition. Matsuo et al. 48 demonstrated that treating rats with PGE1 increased hepatic expression of several HSP genes after ischemia reperfusion. Ethridge et al. 49 found that overexpression of COX-2 reduced HSP70 mRNA and protein after HS in rat intestinal epithelial cells, suggesting that some COX-2 metabolites may reduce HSP expression in some cell types. In contrast with these studies largely demonstrating positive regulation of the HS response by prostaglandins, other investigators have shown that cyclooxygenase inhibitors, including salicylate 29, 30 and indomethacin 31, activated HSF-1 to its DNA binding form in HeLa cells. However these effects were only found when the drugs were used at toxic levels and neither drug activated expression of HSF-1-dependent genes. Housby et al. 50 found that the cyclooygenase inhibitor, sulindac, activated HSF-1 DNA binding activity and HSP70B transcription; however, like the other studies, these effects were only seen at toxic levels of the drug. Collectively, these data suggest that endogenous PGE2 generation may contribute to heat shock gene expression through activation of HSF-1 to a transcriptionally active form.
In summary, we have shown that exogenous PGE2, added at levels achieved in vivo, increase the magnitude of HS-induced gene expression. The mechanism underlying this effect and its clinical consequences are not yet known.
Acknowledgments
This work was supported by NIH grants GM069431 (ISS) and GM066855, HL69057 and HL085256 (JDH), and by VA Merit Review grants (JDH and ISS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–82. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 2.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress [published errata appear in Mol Cell Biol 1993 May;13(5):3122–3 and 1993 Jun;13(6):3838–9] Mol Cell Biol. 1993;13(3):1392–407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273(13):7523–8. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 4.Hietakangas V, Anckar J, Blomster HA, et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A. 2006;103(1):45–50. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CY, Mayo MW, Baldwin ASJscx, et al. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 6.Tulapurkar ME, Asiegbu BE, Singh IS, Hasday JD. Hyperthermia in the febrile range induces HSP72 expression proportional to exposure temperature but not to HSF-1 DNA-binding activity in human lung epithelial A549 cells. Cell Stress & Chaperones. 2008;14:499–508. doi: 10.1007/s12192-009-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurivich DA, Sistonen L, Sarge KD, Morimoto RI. Arachidonate is a potent modulator of human heat shock gene transcription. Proc Natl Acad Sci U S A. 1994;91(6):2280–4. doi: 10.1073/pnas.91.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morange M, Dubois MF, Bensaude O, Lebon P. Interferon pretreatment lowers the threshold for maximal heat-shock response in mouse cells. J Cell Physiol. 1986;127(3):417–22. doi: 10.1002/jcp.1041270310. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Q, DeTolla L, Kalvakolanu I, Fitzgerald B, Hasday JD. Fever upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653–R60. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- 10.Rice P, Martin E, He JR, et al. Febrile-range Hyperthermia Augments Neutrophil Accumulation and Enhances Lung Injury in Experimental Gram-negative Bacterial Pneumonia. J Immunol. 2005;174:3676–85. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 11.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 12.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A(2) Biochim Biophys Acta. 2000;1488(1–2):124–38. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- 13.Bonventre JV, Huang Z, Taheri MR, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390(6660):622–5. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi T, Negishi M, Ichikawa A. Activation of heat shock transcription factors by delta 12-prostaglandin J2 and its inhibition by intracellular glutathione. Biochem Pharmacol. 1993;45(12):2457–64. doi: 10.1016/0006-2952(93)90227-n. [DOI] [PubMed] [Google Scholar]
- 15.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med. 2006;84(11):901–10. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding protein with mini extracts prepared from a small number of cells. Nucl Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh IS, Calderwood S, Kalvakolanu I, Viscardi R, Hasday JD. Inhibition of tumor necrosis factor-α transcription in macrophages exposed to febrile range temperature: A possible role for heat shock factor-1. J Biol Chem. 2000;275:9841–8. doi: 10.1074/jbc.275.13.9841. [DOI] [PubMed] [Google Scholar]
- 18.Mosser DD, Theodorakis NG, Morimoto RI. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988;8:4736–44. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9(2):122–33. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo J, Baler R, Dahl G, Voellmy R. Activation of the DNA-binding ability of human heat shock transcription factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled-coil structure. Mol Cell Biol. 1994;14(11):7557–68. doi: 10.1128/mcb.14.11.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo J, Rungger D, Voellmy R. Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol. 1995;15(8):4319–30. doi: 10.1128/mcb.15.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farkas T, Kutskova YA, Zimarino V. Intramolecular repression of mouse heat shock factor 1. Mol Cell Biol. 1998;18(2):906–18. doi: 10.1128/mcb.18.2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmberg CI, Hietakangas V, Mikhailov A, et al. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. Embo J. 2001;20(14):3800–10. doi: 10.1093/emboj/20.14.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soncin F, Zhang X, Chu B, et al. Transcriptional activity and DNA binding of heat shock factor-1 involve phosphorylation on threonine 142 by CK2. Biochem Biophys Res Commun. 2003;303(2):700–6. doi: 10.1016/s0006-291x(03)00398-x. [DOI] [PubMed] [Google Scholar]
- 25.Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung JJ, Cheng TJ, Lai YK, Chang MD. Differential activation of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinases confers cadmium-induced HSP70 expression in 9L rat brain tumor cells. J Biol Chem. 1998;273(48):31924–31. doi: 10.1074/jbc.273.48.31924. [DOI] [PubMed] [Google Scholar]
- 27.Hong Y, Rogers R, Matunis MJ, et al. Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J Biol Chem. 2001;276(43):40263–7. doi: 10.1074/jbc.M104714200. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki M, Deshpande SS, Angkeow P, Suzuki S, Irani K. Rac1 regulates stress-induced, redox-dependent heat shock factor activation. J Biol Chem. 2000;275(45):35377–83. doi: 10.1074/jbc.M005287200. [DOI] [PubMed] [Google Scholar]
- 29.Jurivich DA, Pachetti C, Qiu L, Welk JF. Salicylate triggers heat shock factor differently than heat. J Biol Chem. 1995;270(41):24489–95. doi: 10.1074/jbc.270.41.24489. [DOI] [PubMed] [Google Scholar]
- 30.Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–5. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 31.Lee BS, Chen J, Angelidis C, Jurivich DA, Morimoto RI. Pharmacological modulation of heat shock factor 1 by antiinflammatory drugs results in protection against stress-induced cellular damage. Proc Natl Acad Sci U S A. 1995;92(16):7207–11. doi: 10.1073/pnas.92.16.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephanou A, Latchman DS. Transcriptional regulation of the heat shock protein genes by STAT family transcription factors. Gene Expr. 1999;7(4–6):311–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor-1 (HSF1) represses transcription of the interleukin 1 beta (IL-1 beta) gene through physical interaction with nuclear factor of interleukin-6 (NF-IL6) J Biol Chem. 2002;277:11802–10. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y, Chen C, Stevenson MA, Hume DA, Auron PE, Calderwood SK. NF-IL6 and HSF1 have mutually antagonistic effects on transcription in monocytic cells. Biochem Biophys Res Commun. 2002;291(4):1071–80. doi: 10.1006/bbrc.2002.6562. [DOI] [PubMed] [Google Scholar]
- 35.Yuan CX, Gurley WB. Potential targets for HSF1 within the preinitiation complex. Cell Stress Chaperones. 2000;5(3):229–42. doi: 10.1379/1466-1268(2000)005<0229:ptfhwt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chilton FH, Fonteh AN, Surette ME, Triggiani M, Winkler JD. Control of arachidonate levels within inflammatory cells. Biochim Biophys Acta. 1996;1299(1):1–15. doi: 10.1016/0005-2760(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 37.Ramanadham S, Gross R, Turk J. Arachidonic acid induces an increase in the cytosolic calcium concentration in single pancreatic islet beta cells. Biochem Biophys Res Commun. 1992;184(2):647–53. doi: 10.1016/0006-291x(92)90638-2. [DOI] [PubMed] [Google Scholar]
- 38.Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci U S A. 1975;72(12):5130–4. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavakoli S, Cowan MJ, Benfield T, Logun C, Shelhamer JH. Prostaglandin E(2)-induced interleukin-6 release by a human airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2001;280(1):L127–33. doi: 10.1152/ajplung.2001.280.1.L127. [DOI] [PubMed] [Google Scholar]
- 40.Yano T, Zissel G, Muller-Qernheim J, Jae Shin S, Satoh H, Ichikawa T. Prostaglandin E2 reinforces the activation of Ras signal pathway in lung adenocarcinoma cells via EP3. FEBS Lett. 2002;518(1–3):154–8. doi: 10.1016/s0014-5793(02)02689-3. [DOI] [PubMed] [Google Scholar]
- 41.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 42.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83(5):813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 43.Yamaki T, Endoh K, Miyahara M, et al. Prostaglandin E2 activates Src signaling in lung adenocarcinoma cell via EP3. Cancer Lett. 2004;214(1):115–20. doi: 10.1016/j.canlet.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Togo S, Chen H, Takahashi T, et al. Prostaglandin E1 improves survival rate after 95% hepatectomy in rats. J Surg Res. 2008;146(1):66–72. doi: 10.1016/j.jss.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Hwang J, Lee HI, Chang YS, Lee SJ, Kim KP, Park SI. 15-deoxy-Delta12,14-prostaglandin J2-induced down-regulation of endothelial nitric oxide synthase in association with HSP70 induction. Biochem Biophys Res Commun. 2007;357(1):206–11. doi: 10.1016/j.bbrc.2007.03.127. [DOI] [PubMed] [Google Scholar]
- 46.Xu XH, Zhang HL, Han R, Gu ZL, Qin ZH. Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia. Brain Res. 2006;1102(1):154–62. doi: 10.1016/j.brainres.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 47.Warzecha Z, Dembinski A, Ceranowicz P, et al. Ischemic preconditioning inhibits development of edematous cerulein-induced pancreatitis: involvement of cyclooxygenases and heat shock protein 70. World J Gastroenterol. 2005;11(38):5958–65. doi: 10.3748/wjg.v11.i38.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuo K, Togo S, Sekido H, et al. Pharmacologic preconditioning effects: prostaglandin E1 induces heat-shock proteins immediately after ischemia/reperfusion of the mouse liver. J Gastrointest Surg. 2005;9(6):758–68. doi: 10.1016/j.gassur.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Ethridge RT, Hellmich MR, DuBois RN, Evers BM. Inhibition of heat-shock protein 70 induction in intestinal cells overexpressing cyclooxygenase 2. Gastroenterology. 1998;115(6):1454–63. doi: 10.1016/s0016-5085(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 50.Housby JN, Cahill CM, Chu B, et al. Non-steroidal anti-inflammatory drugs inhibit the expression of cytokines and induce HSP70 in human monocytes. Cytokine. 1999;11(5):347–58. doi: 10.1006/cyto.1998.0437. [DOI] [PubMed] [Google Scholar]