Abstract
Background
Carpal tunnel release and conservative interventions are widely used in clinical therapies of carpal tunnel syndrome. The efficacy of these treatment and interventions mainly lies in the exploitation of the mechanical properties of carpal tunnel. This study investigated the structural mechanics of the transverse carpal arch using cadaveric hands.
Methods
Paired force was applied to the insertion sites of the transverse carpal ligament at both the distal (hamate -trapezium) and proximal (pisiform - scaphoid) level of the carpal tunnel. The two pairs of forces were simultaneously applied in an inward or outward direction when the transverse carpal ligament was intact and transected. Transverse carpal arch and carpal tunnel compliance in response to the forces were analyzed. Three-way repeated measures ANOVA were used to examine the effect of the transverse carpal ligament status (intact/transected), the level of the carpal tunnel (distal/proximal) and the force application direction (inward/outward) on the biomechanics of the transverse carpal arch.
Findings
Transverse carpal ligament plays a stabilizing role in resisting outward deformation of the carpal tunnel. The carpal tunnel at the proximal level is more flexible than the carpal tunnel at the distal level. The carpal tunnel is more compliant under the inward force application than under the outward force application.
Interpretation
The understanding of carpal tunnel mechanics potentially helps to improve the existing strategies and to develop alternatives for the treatment of carpal tunnel syndrome.
Keywords: Carpal tunnel, Transverse carpal ligament, Carpal arch, Compliance, Carpal tunnel syndrome
Introduction
The carpal tunnel is formed by multiple carpal bones and the transverse carpal ligament (TCL). The carpal bones are connected by the intercarpal ligaments to form the dorsal, medial and lateral aspects of the tunnel. The TCL constitutes the palmar roof of the carpal tunnel by attaching to the pisiform, scaphoid, hamate and trapezium (Cobb et al., 1993). The carpal tunnel is rather inflexible and tends to cause compression on the median nerve, which results in carpal tunnel syndrome.
To relieve the compression on the median nerve, carpal tunnel release by transecting the TCL is the standard surgical treatment for patients with carpal tunnel syndrome (Agee et al., 1992, Palmer et al., 1993). Some conservative interventions have been suggested to improve the treatment of carpal tunnel syndrome by mobilizing the carpal tunnel via myofascial manipulation (Sucher, 1993, Moraska et al., 2008), carpal traction (Porrata et al., 2007), and splinting (Kruger et al., 1991, Manente et al., 2001). These treatment and intervention strategies mainly rely on the understanding of the mechanical properties of carpal tunnel. Few studies have been designed to address the basic biomechanics of the carpal tunnel (Garcia-Elias et al., 1989a, Garcia-Elias et al., 1989b, Fuss and Wagner, 1996). The understanding of carpal tunnel mechanics helps to improve the existing strategies and to develop alternatives for the treatment of carpal tunnel syndrome.
The purpose of this study was to investigate the biomechanical properties of the carpal tunnel using cadaveric specimens. We hypothesized that changes in carpal arch width would be (1) affected by TCL status (intact/transected), (2) dependent on the levels of the carpal tunnel (distal/proximal), and (3) dependent on the direction of the force application (inward/outward).
Materials and Methods
Specimens and preparation
Eight fresh frozen cadaveric hands, seven males and one female, were selected by reviewing the medical records to exclude those with musculoskeletal injuries to the hand and wrist. Fluoroscopic examinations were performed on each specimen to confirm the absence of traumatic injuries or arthritic changes in the hand and wrist. The mean age of the specimens was 63 (SD 18) years. Prior to the experiment, each specimen was thawed overnight at room temperature. The specimen was then dissected to expose the TCL by removing its skin tissues and volar fascia. The TCL was recognized by its transverse fibers and insertions at the hook of hamate, trapezium, pisiform and tuberosity of the scaphoid. The carpal tunnel was evacuated by removing the median nerve and all flexor tendons. Individual cortex screws (Synthes Inc, West Chester, PA), 2.0 mm in diameter, were drilled into each carpal bone where the TCL inserts. A thin wire was connected to each screw head to apply the force. The cortex screws were drilled till the screw head with the wire were being flush with the bony surface.
Apparatus
After the dissection preparation, the hand specimen was attached to a custom designed experimental apparatus for biomechanical testing. An aluminum base plate was used as a sturdy platform to mount the hand specimen and fixation accessories. A plywood block wedge-shaped at 20 degrees was secured to the platform. The wrist of the hand specimen was configured to its 20 degrees of extension by aligning the dorsal aspect of the specimen with the surface of the wedged wooden block. The specimen was then stabilized by drilling a screw through the middle shaft of the third metacarpal into the plywood. The forearm was fastened to the wooden block with Velcro strips (Figure 1).
Figure 1.
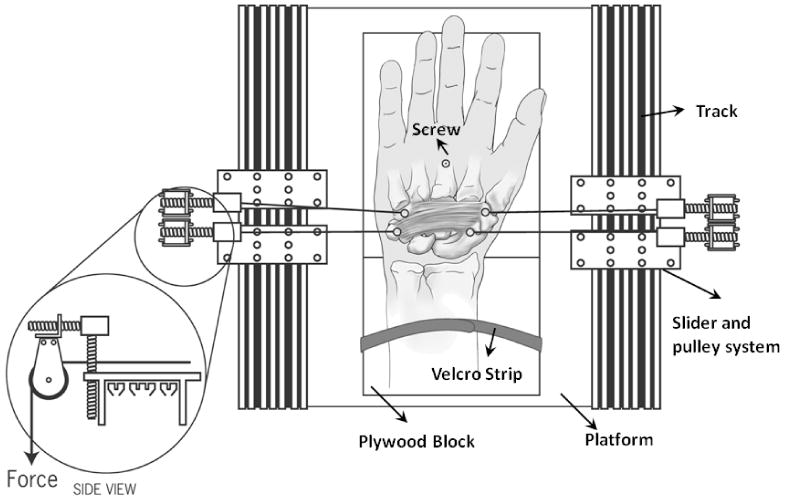
Two pairs of forces simultaneously applied to the carpal bones at the proximal and distal levels. Note that shown in the figure is the outward force application, and the inward force application was achieved by swapping the connection wires.
Four pulley systems were established on the platform for the purpose of force application to the carpal bones. Two T-slotted tracks were fixed to the platform at the parallel edges of the platform, which were along the proximal-distal direction of the hand specimen. Two pulley systems were attached to each track through sliders whose locations were adjustable along the track. In addition, each pulley system also had a movable slider for the alignment of the pulley in the vertical (palmar-dorsal) direction. With the pulley systems, forces were applied to the carpal bones by hanging weights (Figure 1).
Experimental procedure
Two pairs of forces were applied. One pair aligned along the connection line between the hamate and trapezium at the distal level, and the other along the connection line between the pisiform and scaphoid at the proximal level (Figure 1). The two pairs of forces were applied simultaneously in an inward or outward direction to compress or outstretch the carpal tunnel, respectively. The inward/outward force application sequence was randomized, and the five force magnitudes were applied in a sequential order of 2, 4, 6, 8, 10 N.
While the carpal bones were under loading, the coordinates at the center of each screw head surface were digitized using a 3D digitizer (Microscribe, San Jose, CA). The specimen was unloaded at the completion of each digitization. There was a lapse of one-minute unloading period between consecutive trials. The data collection was firstly performed with the TCL intact, and then repeated after the TCL was transected.
Data analysis
The distance (i.e. carpal arch width) between the hamate and trapezium at the distal level of the carpal tunnel, and the distance between the pisiform and scaphoid at the proximal level of the carpal tunnel were calculated based on the digitized coordinates. Two dependent variables were considered. The first was the arch width change at the 10 N force relative to the original arch width without force application, and the second was the compliance of the carpal tunnel. The compliance was defined as the slope of the linear regression of the arch width change as a function of forces. The independent variables were TCL status (intact/transected), tunnel level (distal/proximal) and force direction (inward/outward). Three-way (2×2×2) repeated measures ANOVA were performed to examine the effects of these independent variables on the arch width and compliance of the carpal tunnel (α = 0.05).
Results
The original carpal arch widths at the distal and proximal levels were 26.3 (SD 2.4) mm and 30.3 (SD 2.3) mm, respectively. Statistical analyses of the arch width change of the carpal tunnel at the 10 N force application revealed that the TCL status (P < 0.001), the carpal tunnel level (P < 0.001) and force direction (P < 0.001) had significant effects on the arch width change.
When the 10 N inward force was applied, the arch width for distal and proximal levels of the carpal tunnel decreased by 10.8% (SD 4.0%) and 37.5% (SD 10.5%), respectively; after the TCL was transected, the arch width for distal and proximal levels of the carpal tunnel decreased by 10.6% (SD 4.7 %) and 37.9% (SD 11.5%), respectively (Figure 2a).
Figure 2.
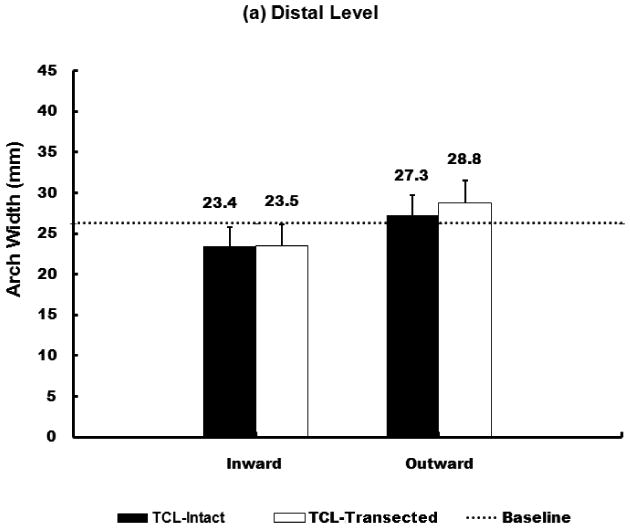
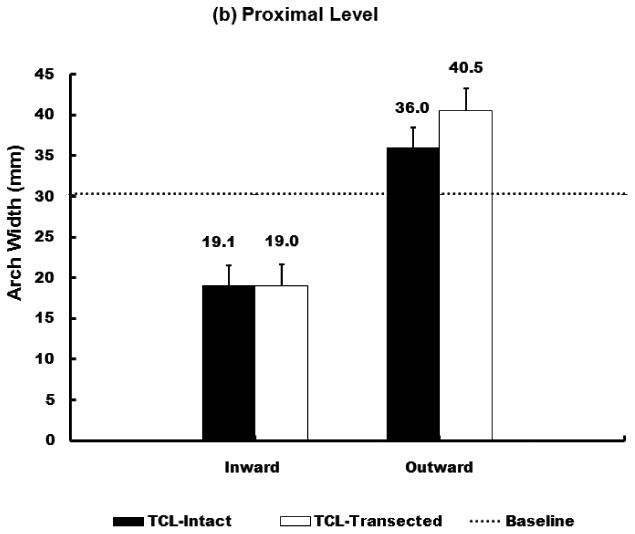
Arch widths of the carpal tunnel under 10 N force application at distal (a) and proximal (b) level.
When the 10 N outward force was applied, the arch width for distal and proximal levels of the carpal tunnel increased by 3.7% (SD 1.5%) and 18.8% (SD 5.2 %), respectively; after the TCL was transected, the arch width for distal and proximal levels of the carpal tunnel increased by 9.6% (SD 4.1%) and 33.9% (SD 10.2%), respectively (Figure 2b).
Under the 10 N force application, the arch width change at the proximal level was significantly greater than that at the distal level regardless of force direction and TCL status. Similarly, the distance change during the inward loading was significantly greater than that during outward loading regardless of the level of the carpal tunnel and TCL status. Further two-way repeated measures ANOVA showed that the removal of the TCL did not significantly affect the arch width changes in the inward direction (P = 0.086). However, TCL removal significantly increased the width changes in the outward direction (P < 0.001).
The arch width change in response to the individual applied forces was investigated (Figure 3). The carpal tunnel compliance was calculated in the 2 – 10 N force range and the regression analyses showed strong linear relationship in this this range (R2 = 0.951-0.988).
Figure 3.
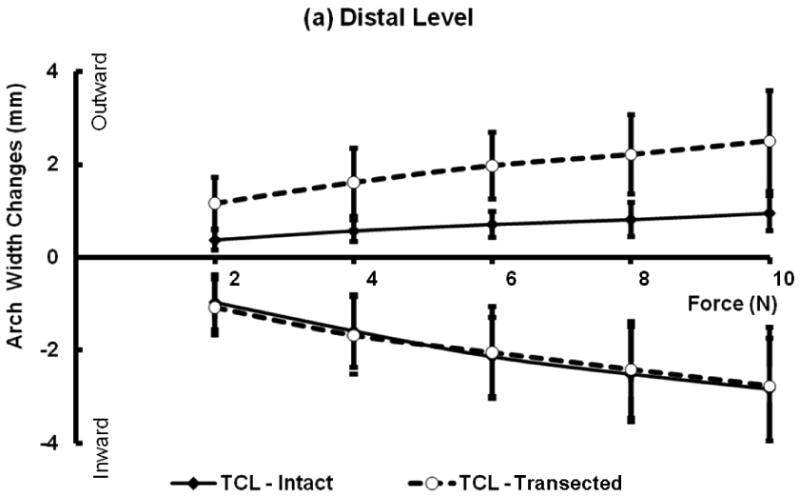
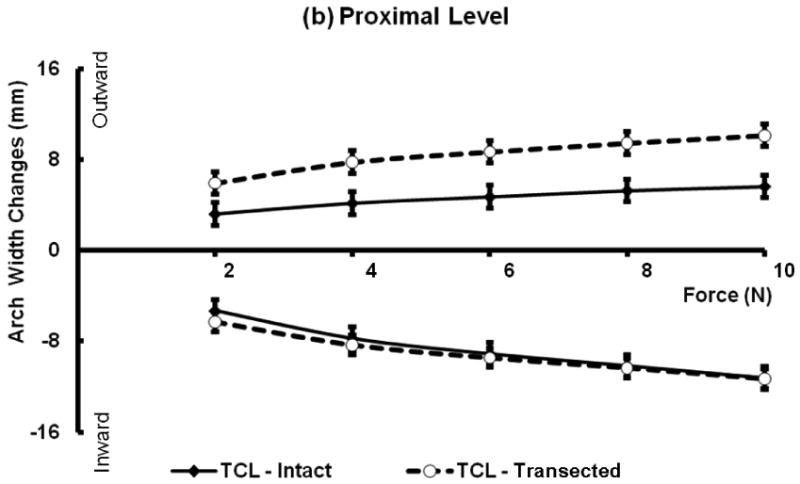
Force and arch width changes with and without TCL at the distal (a) and proximal (b) levels. Positive values correspond to increased distances under outward forces, and negative values represent distance decreases under inward forces.
Statistical results indicated significant main effects of tunnel level (P < 0.001) and force direction (P < 0.001) on the compliance. The compliance at the distal tunnel is significantly smaller than that at the proximal tunnel regardless of force direction and TCL status (Figure 4). The compliance at inward force was significantly greater than that at the outward force regardless of the tunnel level and TCL status.
Figure 4.
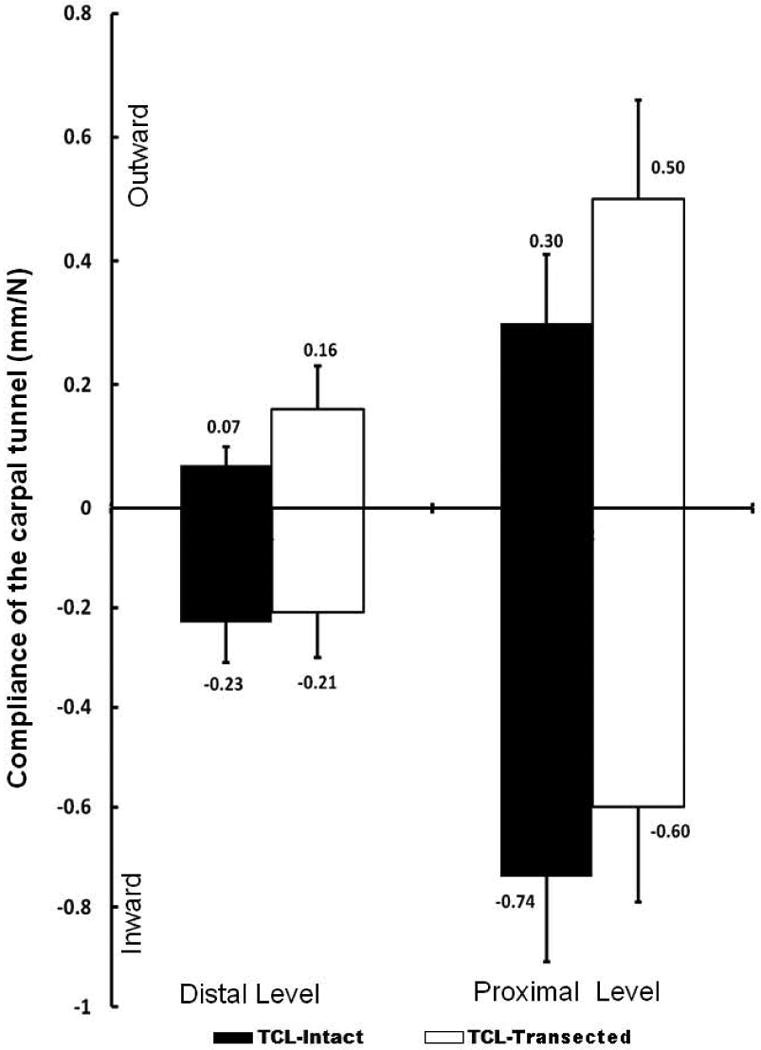
Compliance at the distal and proximal level of the carpal tunnel in the inward and outward directions.
When inward forces were applied, the compliance of the carpal tunnel decreased about 8.7% at the distal level and 18.9% at the proximal level after the transection of the TCL. Due to the non-compressibility of the TCL under inward forces, the compliance change after TCL transection was relatively small (Figure 4).
When outward forces were applied, the compliance of the carpal tunnel increased about 128.6% at the distal level and 66.7% at the proximal level after the transection of the TCL.The TCL transection led to a significant increase in carpal tunnel compliance at both distal and proximal levels (P < 0.001) (Figure 4).
Discussion
In this study, the change of the carpal arch width was investigated in response to the forces applied to carpal bones. We concluded that the carpal arch width will be affected by the TCL status (intact/transected), the levels of the carpal tunnel (distal /proximal) and the direction of the force application (inward/outward).
The biomechanical functions of the TCL include preventing the bowstring of the flexor tendons (Manske and Lesker, 1983), anchoring the thenar and hypothenar muscles (Rotman and Manske, 1993), and maintaining the stability of the carpal bones (Seradge and Seradge, 1989, Garcia-Elias et al., 1989b). The stabilization role of the TCL was confirmed in the current study. For example, TCL transection resulted in increases in carpal tunnel outward compliance by 128% and 67% at the distal and proximal levels, respectively. Under the 10 N outward force, the carpal arch width at the distal level increased by 1.5 mm when the TCL was transected, agreeing well with a previous study which reported a 1.6 mm increase under comparable experimental conditions (Fuss and Wagner, 1996). In a study to understand the stretchability of the carpal tunnel and the TCL, Sucher et al. (2005) applied a 10 N static force to the carpal tunnels and reported that the TCL elongation (by measuring the transverse carpal arch) at the distal level was between 5% and 7% (1.6 mm and 2.3 mm). In the current study, the carpal arch width increased about 1.0 mm with intact TCL under the 10 N outward forces. The greater distance change reported by Sucher et al. may be explained by the prolonged (up to 2 hours) loading.
Garcia-Ellias et al. studied the contribution of the TCL to the stability of the carpal tunnel by applying the compression forces in the dorsopalmar direction (Garcia-Elias et al., 1989a). The authors reported that the stiffness of the carpal tunnel decreased by 7.5% after transection of the TCL, suggesting that the TCL plays a relatively minor role in carpal tunnel stability. In our study, however, the results indicated a much greater role of the TCL in stabilizing the carpal tunnel. The discrepancy could be explained by the difference in the direction of force application. Under the dorspalmar compression, the carpal tunnel was likely deformed in the dorsopalmar direction with little changes of the carpal arch width and the TCL could be isometrically preserved. In our study, the force was applied outwardly to expand the carpal tunnel arch and the TCL was directly against the outward deformation. After the TCL transection, the restriction from the TCL to the carpal tunnel was lost and therefore the carpal tunnel became more flexible.
Our results showed that the carpal tunnel at the proximal level was significantly more flexible than at the distal level. When TCL was intact, the compliances at the proximal tunnel were about 3.2 (under inward force direction) and 4.3 (under outward force direction) times of the compliance at the distal tunnel. When the TCL was transected, the compliances at the proximal tunnel were about 2.9 (under inward force direction) and 3.1 (under outward force direction) times of the compliances at the distal tunnel. The flexibility of the proximal carpal tunnel is often indicated in clinical observations. Wrist instabilities are often reported at this level, such as the scapholunate instability (Kuo and Wolfe, 2008, Pevny et al., 1995) and pisotriquetral disorders (Pevny et al., 1995, Kuo and Wolfe, 2008). At the distal level, the hamate, capitate, trapezoid and trapezium are tightly intercalated by strong intercarpal ligaments, forming a relatively rigid unit of the hand skeleton (Stuchin, 1992). However, at the proximal level, the scaphoid, lunate, triquetrum and pisiform are inter-articulated by a ligament system, allowing and regulating the mobility of these carpal bones (Landsmeer, 1961). An additional explanation of the proximal flexibility may be related to TCL anatomy. It was shown that the proximal portion of the TCL is thinner than the distal TCL portion (Cobb et al., 1993), and the distal portion of the TCL has a laminar configuration with consistent, strong transverse bundle (Isogai et al., 2002), which helps stabilize the distal carpal arch.
Our results also demonstrated that the carpal tunnel was more compliant along the inward direction than the outward direction. For example, with the intact TCL, the inward compliance at the distal level was about 2.3 times of the outward compliance at the distal carpal tunnel. It indicates that the carpal arch could be better mobilized in the inward direction than in the outward direction for carpal tunnel manipulation. One of our previous studies showed that carpal arch narrowing was effective at causing the TCL to form a palmar arch and therefore increasing the total tunnel cross-sectional area (Li et al., 2009). Our results suggested an alternative therapeutic strategy by inwardly immobilizing the carpal bones for the purpose of carpal tunnel expansion and pressure alleviation for patients with carpal tunnel syndrome.
This study had several limitations. Firstly, the forces were not randomized but applied sequentially from 2 N to 10 N in 2 N increments. Our previous study showed that carpal tunnel deformation did not appear to be affected by the loading history even when the carpal tunnel and TCL underwent much higher load (Li et al., 2009). Secondly, the disruptive nature of TCL transection necessitated the experimental sequence of TCL-intact condition followed by TCL-transected condition. Thirdly, the experiment in this study was based on cadaver specimens; large dissection of the soft tissue around the carpal tunnel area has been made in order to take the measurement, the effects from the soft tissue on the change of the carpal arch width was neglected. Finally, the creeping of the carpal tunnel under the force application was not considered in this study.
In summary, we investigated the changes in carpal arch width in response to forces applied to the carpal tunnel when the TCL was intact and transected. TCL plays a mechanical role in resisting outward deformation of the carpal tunnel. The carpal tunnel is more rigid at the distal, hamate-trapezium level than at the proximal, pisiform-scaphoid level. With an intact TCL, the carpal tunnel is easier to be deformed in the inward direction than in the outward direction.
Acknowledgments
This study was supported by the National Institutes Health (NIH R03AR054510) and China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agee JM, Mccarroll HR, Jr, Tortosa RD, Berry DA, Szabo RM, Peimer CA. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17:987–95. doi: 10.1016/s0363-5023(09)91044-9. [DOI] [PubMed] [Google Scholar]
- Cobb TK, Dalley BK, Posteraro RH, Lewis RC. Anatomy of the flexor retinaculum. J Hand Surg [Am] 1993;18:91–9. doi: 10.1016/0363-5023(93)90251-W. [DOI] [PubMed] [Google Scholar]
- Fuss FK, Wagner TF. Biomechanical alterations in the carpal arch and hand muscles after carpal tunnel release: a further approach toward understanding the function of the flexor retinaculum and the cause of postoperative grip weakness. Clin Anat. 1996;9:100–8. doi: 10.1002/(SICI)1098-2353(1996)9:2<100::AID-CA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias M, An KN, Cooney WP, 3rd, Linscheid RL, Chao EY. Stability of the transverse carpal arch: an experimental study. J Hand Surg [Am] 1989a;14:277–82. doi: 10.1016/0363-5023(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias M, An KN, Cooney WP, Linscheid RL, Chao EY. Transverse stability of the carpus. An analytical study. J Orthop Res. 1989b;7:738–43. doi: 10.1002/jor.1100070516. [DOI] [PubMed] [Google Scholar]
- Isogai S, Murakami G, Wada T, Akita K, Yamashita T, Ishii S. Laminar configuration of the transverse carpal ligament. J Orthop Sci. 2002;7:79–83. doi: 10.1007/s776-002-8424-2. [DOI] [PubMed] [Google Scholar]
- Kruger VL, Kraft GH, Deitz JC, Ameis A, Polissar L. Carpal tunnel syndrome: objective measures and splint use. Arch Phys Med Rehabil. 1991;72:517–20. [PubMed] [Google Scholar]
- Kuo CE, Wolfe SW. Scapholunate instability: current concepts in diagnosis and management. J Hand Surg Am. 2008;33:998–1013. doi: 10.1016/j.jhsa.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Landsmeer JM. Studies in the anatomy of articulation. I. The equilibrium of the “intercalated” bone. Acta Morphol Neerl Scand. 1961;3:287–303. [PubMed] [Google Scholar]
- Li ZM, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131:081011. doi: 10.1115/1.3148469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manente G, Torrieri F, Di Blasio F, Staniscia T, Romano F, Uncini A. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;24:1020–5. doi: 10.1002/mus.1105. [DOI] [PubMed] [Google Scholar]
- Manske PR, Lesker PA. Palmar aponeurosis pulley. J Hand Surg Am. 1983;8:259–63. doi: 10.1016/s0363-5023(83)80154-3. [DOI] [PubMed] [Google Scholar]
- Moraska A, Chandler C, Edmiston-Schaetzel A, Franklin G, Calenda EL, Enebo B. Comparison of a targeted and general massage protocol on strength, function, and symptoms associated with carpal tunnel syndrome: a randomized pilot study. J Altern Complement Med. 2008;14:259–67. doi: 10.1089/acm.2007.0647. [DOI] [PubMed] [Google Scholar]
- Palmer DH, Paulson JC, Lane-Larsen CL, Peulen VK, Olson JD. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9:498–508. doi: 10.1016/s0749-8063(05)80396-2. [DOI] [PubMed] [Google Scholar]
- Pevny T, Rayan GM, Egle D. Ligamentous and tendinous support of the pisiform, anatomic and biomechanical study. J Hand Surg Am. 1995;20:299–304. doi: 10.1016/S0363-5023(05)80030-9. [DOI] [PubMed] [Google Scholar]
- Porrata H, Porrata A, Sosner J. New carpal ligament traction device for the treatment of carpal tunnel syndrome unresponsive to conservative therapy. J Hand Ther. 2007;20:20–7. doi: 10.1197/j.jht.2006.10.001. quiz 28. [DOI] [PubMed] [Google Scholar]
- Rotman MB, Manske PR. Anatomic relationships of an endoscopic carpal tunnel device to surrounding structures. J Hand Surg Am. 1993;18:442–50. doi: 10.1016/0363-5023(93)90089-L. [DOI] [PubMed] [Google Scholar]
- Seradge H, Seradge E. Piso-triquetral pain syndrome after carpal tunnel release. J Hand Surg Am. 1989;14:858–62. doi: 10.1016/s0363-5023(89)80090-5. [DOI] [PubMed] [Google Scholar]
- Stuchin SA. Wrist anatomy. Hand Clin. 1992;8:603–9. [PubMed] [Google Scholar]
- Sucher BM. Myofascial release of carpal tunnel syndrome. J Am Osteopath Assoc. 1993;93:92–4. 100–1. [PubMed] [Google Scholar]
- Sucher BM, Hinrichs RN, Welcher RL, Quiroz LD, St Laurent BF, Morrison BJ. Manipulative treatment of carpal tunnel syndrome: biomechanical and osteopathic intervention to increase the length of the transverse carpal ligament: part 2. Effect of sex differences and manipulative “priming”. J Am Osteopath Assoc. 2005;105:135–43. [PubMed] [Google Scholar]


