Abstract
Background
Children undergoing stem cell transplant (SCT) experience high levels of somatic distress and mood disturbance. This trial evaluated the efficacy of complementary therapies (massage, humor therapy, relaxation/imagery) for reducing distress associated with pediatric SCT.
Methods
Across 4 sites, 178 pediatric patients scheduled to undergo SCT were randomized to a child-targeted intervention involving massage and humor therapy (HPI-C), the identical child intervention plus a parent intervention involving massage and relaxation/imagery (HPI-CP) or standard care (SC). Randomization was stratified by site, age, and type of transplant. The interventions began at admission and continued through SCT week +3. Primary outcomes included patient and parent reports of somatic distress and mood disturbance obtained weekly from admission through week +6 using the BASES scales. Secondary outcomes included length of hospitalization, time to engraftment, and usage of narcotic analgesic and antiemetic medications.
Results
A mixed model approach was used to assess longitudinal trends of patient and parent-report outcomes and test differences between groups on these measures. Significant changes across time were observed on all patient and parent-report outcomes. However, no significant differences between treatment arms were found on the primary outcomes. Similarly, no signficant between group differences were noted on any of the medical variables as secondary outcomes.
Conclusions
Results of this multi-site trial failed to document significant benefits of complementary interventions in the pediatric SCT setting.
Keywords: stem cell transplant, children, complementary therapy, massage, symptoms
The use of hematopoietic stem cell transplantation (SCT) for the treatment of childhood malignancies and other serious pediatric disorders poses unique challenges for patients and their families.1,2 Despite advances in supportive care, SCT remains a high risk medical procedure involving a prolonged and physically demanding treatment regimen that can create high levels of distress, and may lead to later adjustment difficulties for survivors.3-8 Our prior studies of the natural history of patient adjustment during the acute phase of SCT demonstrated that patients continue to experience a high level of somatic distress and associated mood disturbance during the acute phase of SCT.9,10 Further, the parental caregivers also experience a high level of emotional distress during the same timeframe.11,12 The distress of patients and parents appears to be relatively transient, peaking in the early weeks following transplant and returning to near baseline levels within 4-6 months post-SCT.9-12 Nevertheless, such unacceptably high levels of distress, which persist despite aggressive, state-of-the-art supportive care, point to the need for the development of novel interventions for distress reduction.
Several intervention trials in adult SCT settings have involved complementary therapies. Mind-body approaches, including trials of hypnosis, and relaxation/imagery have demonstrated benefit in reducing pain, nausea and use of analgesics.13,14 A massage therapy intervention with adults undergoing autologous SCT showed significant immediate reductions in fatigue, nausea, and anxiety relative to controls, but the effects were not maintained.15 In another randomized trial in adults undergoing SCT, patients receiving massage and therapeutic touch reported greater comfort and overall benefit than a comparison group that received a ‘friendly visit’, but there were no differences between groups in time to engraftment or toxicity scores.16 Another complementary approach that has been tested in adult SCT settings is music therapy.17 Adults undergoing SCT who music therapy during transplant hospitalization reported lower levels of mood disturbance relative to controls. Similar findings were found in a trial of music therapy with children with cancer during inpatient hospitalization.18
We conducted pilot studies demonstrating the feasibility of complementary interventions in the SCT setting, exposing patients to multiple stress reduction techniques including:1) relaxation training, with use of imagery; 2) massage therapy, which was taught to, and administered by parents; 3) humor therapy; and 4) emotional expression therapy.19 Feasibility was demonstrated, and the intervention as a whole was rated as very helpful or better by 85% of patients and parents. Moreover, a clear pattern was observed regarding the individual components, indicating that massage and humor therapy were rated more highly than the others. A subsequent pilot study compared the benefits of massage administered by massage professionals vs. parents.20 No significant differences were found between either massage group and controls on measures of somatic distress and mood disturbance, although sample size was small, and trends were in the predicted direction. Patients receiving massage however, did show evidence of faster engraftment and earlier hospital discharge.20
This paper presents findings from a multi-site study of complementary interventions designed to reduce distress and promote well-being in children undergoing SCT. The primary focus was on a child-targeted intervention that included massage therapy and humor therapy. A parent-targeted intervention involving massage therapy and relaxation/imagery was included to assess whether this would provide incremental benefits beyond the child-targeted intervention.
The massage intervention was designed to produce relaxation and reduce distress, while increasing positive affect. Massage therapy has been used for reduction of anxiety, pain, and distress in a variety of medical settings, particularly oncology, and across a wide age range, including pediatric patients.21,24 The evidence thus far has been encouraging, but not unequivocal. The immediate effects of massage in reducing anxiety and pain complaints have been demonstrated more consistently, whereas the evidence for sustained effects over time has been less compelling.21-24 The humor intervention was also designed to increase positive affect with anticipated positive physiological changes. Empiric data on the effects of humor are limited, but there is some evidence suggesting that humor interventions may reduce pain, or increase pain tolerance and improve immune function.25-28 Moreover, humor is viewed as a cognitive-behavioral coping strategy that allows for increased tolerance of stress.25
High levels of parental anxiety and distress in the SCT setting have been well documented.11,12 Parental distress is a concern not only for the parents themselves, but for the patients, since parental stress levels have been associated with coping responses and adjustment of children with cancer.29 Consequently, parents are frequently an intervention target in studies designed to reduce distress in children with cancer.30-33 In addition to massage, the parent intervention included relaxation with imagery, an approach that has been widely studied in oncology settings.34
The primary objectives of this 3-group randomized trial were: 1) To evaluate the efficacy of a child-targeted health-promotion intervention (HPI-C) in improving patient well-being during the acute phase of BMT; 2) To evaluate the incremental improvement in child and parent well-being obtained by combining parent-targeted and child-targeted health promotion interventions (HPI-CP); and 3) To assess the impact of the interventions on short-term medical outcomes. We hypothesized that patients in both intervention groups would demonstrate less transplant-related somatic distress, and greater well-being than those randomized to standard care, and that greater benefits would be demonstrated in the HPI-CP group than in those receiving HPI-C alone. This paper focuses on child outcomes.
Methods
Patients and Settings
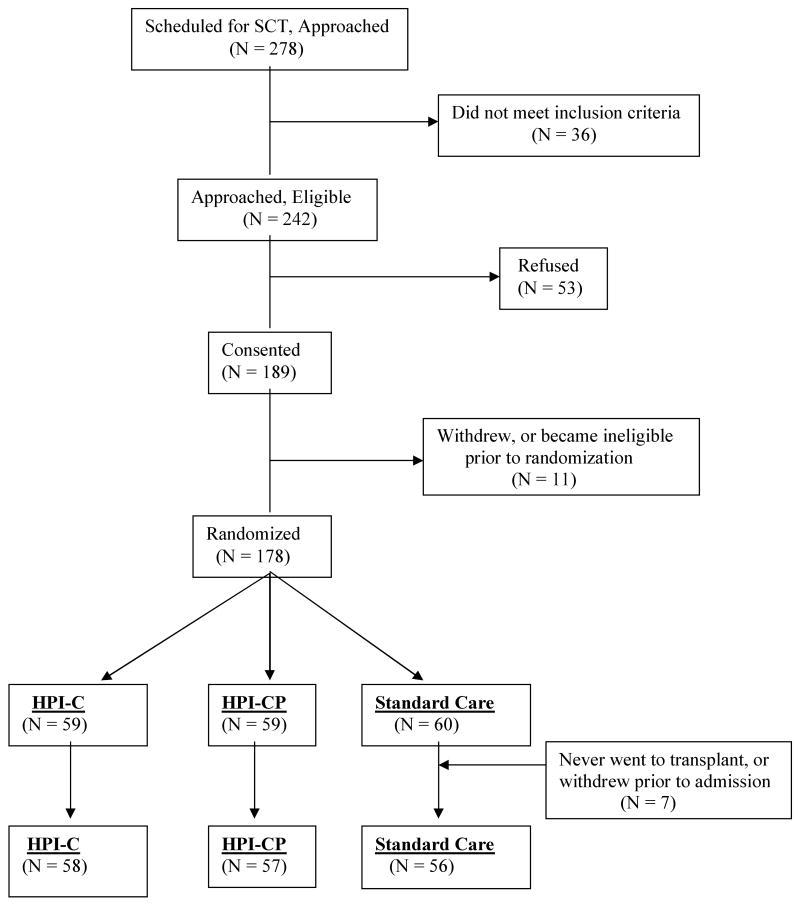
Children were recruited from 4 pediatric transplant centers: St Jude Children's Research Hospital, Memphis (SJCRH); The Hospital for Sick Children, Toronto (HSCT); Children's Hospital of Philadelphia (CHOP); and Nationwide Children's Hospital, Columbus (NCH). Eligibility criteria included: 1.) Patient undergoing allogeneic or autologous transplant with bone marrow or peripheral blood stem cells; 2.) Anticipated inpatient hospital stay of at least 3 weeks; 3.) Age 6-18 years; 4.) English speaking; and 5.) approval by the patient's SCT attending physician. For each patient, a resident parent was also enrolled. Eligibility criteria for parents included: 1) English speaking; 2) primary caregiver for the child during SCT; and 3) parent planned to be available for the duration of the transplant hospitalization. The study flow diagram is illustrated in Figure 1. Across all sites, a total of 278 patients/families were approached; 36 of these were subseqently found to be ineligible. Of the 242 eligible families, 189 (78.1%) consented to participate in the study. Following consent, 17 families withdrew, or became ineligible prior to randomization. Ineligibility following consent occurred due to failure to complete baseline measures in a timely fashion (7), or relapse/medical complication prohibiting transplant (4). Of randomized patients, an additional 4 never proceeded to transplant and 3 withdrew prior to initiation of the intervention. The final sample of 171 is comprised of families who completed baseline measures, were randomized, and admitted for transplant. The demographic and medical background of the participants is summarized in Table 1.
Figure 1.
CONSORT diagram for trial accrual.
Table 1.
Demographic and Medical Background
| Age (M = 12.8; SD = 3.9) | ||
| 6 – 12 years | 84 | 49.1 |
| > 12 years | 87 | 50.9 |
| Gender | ||
| Male | 101 | 59.1 |
| Race/Ethnicity | ||
| White | 121 | 70.7 |
| Black | 26 | 15.2 |
| Hispanic | 8 | 4.7 |
| Asian | 7 | 4.1 |
| Other/Unkown | 9 | 5.3 |
| Socioeconomic Status | ||
| I | 28 | 16.4 |
| II | 60 | 35.1 |
| III | 35 | 20.5 |
| IV & V | 27 | 15.8 |
| Unknown | 21 | 12.3 |
| Resident Parent | ||
| Mother | 141 | 82.4 |
| Father | 20 | 11.7 |
| Other | 10 | 5.8 |
| Site | ||
| St. Jude | 71 | 41.5 |
| HSC-Toronto | 41 | 23.9 |
| CHOP | 35 | 20.5 |
| NCH-Columbus | 24 | 14.0 |
| Type of Transplant | ||
| Autologous | 31 | 18.1 |
| Allo-matched sib | 44 | 25.7 |
| Allo-other | 96 | 56.1 |
| Diagnostic Group | ||
| ALL | 46 | 26.9 |
| AML | 42 | 24.6 |
| Other Leukemia | 23 | 13.5 |
| HD/NHL | 18 | 10.6 |
| Solid Tumor | 21 | 12.3 |
| Non-malignancy | 19 | 11.1 |
Design and Procedure
Patients were recruited prior to admission for SCT, and informed consent and child assent was obtained from all participants. Patients and parents completed a battery of baseline measures prior to randomization. Randomization was stratified by site, age (6-12; >12) and type of transplant (autologous; allogeneic-matched sibling; allogeneic-other). The intervention began at the time of admission, and continued through SCT week +3. Primary outcomes were self-report from patient and parent obtained weekly for 8 observations from admission (week -1) though week + 6. Secondary outcomes included medical variables obtained from chart review, and longer term self-report outcomes obtained at 12 and 24 weeks.
Measures
Behavioral, Affective and Somatic Experiences Scales (BASES)35,36
The BASES scales provide a rating of acute aspects of health related quality of life (HRQL) for pediatric patients undergoing aggressive treatments. These instruments were designed for repeated use and sensitivity to change and provide scores on 5 subscales, labeled, ‘Somatic Distress’, ‘Mood Disturbance’, ‘Compliance’, ‘Quality of Interactions’, and ‘Activity’.35 The scales initially contained 38 items, but were subsequently abbreviated to 22 items, with no loss of reliability.21 Parent, child, and nurse report versions are available that contain essentially identical item content. BASES data were obtained from parents and patients on a weekly basis from admission for transplant to 6 weeks following SCT.
Positive and Negative Affect Schedule, for Children (PANAS-C)37
Because the BASES scales focus solely on distress, the PANAS-C was added to provide a measure of positive affect. We used a modified version of the PANAS-C scale described by Crook et al.37, using only the positive affect items, to which 4 items were added, ‘calm’, ‘relaxed’, ‘comfortable’, and ‘peaceful’ reflecting the intended effect of our interventions. These scales were obtained on the same schedule as the BASES.
Medical Variables
Medical variables included: days in hospital, time to engraftment, medication usage, and specific toxicities. Days in hospital was measured using the number of days from transplant to first hospital discharge, and also assessing the number of days hospitalized through month +3 (day 90). Time to engraftment was assessed by recording the number of days until the patient obtained an ANC > 500 for three consecutive days. Two measures of medication usage were obtained re: narcotic analgesic and antiemetic usage. All narcotics dispensed through day +21 were recorded, and total dose of each agent was converted to morphine equivalents. Doses of all agents were combined and divided by the patients' weight at admission to provide a single index in mg/kg of morphine equivalent. All antiemetics were recorded from admission through day +21. Because of the large number of agents used, and differences in standard procedures for prophylactic treatment across sites, we recorded the number of days that any unscheduled antiemetics were dispensed. Thus a single score was obtained, indicating the number of days that any unscheduled antiemetics were dispensed through day +21. To assess toxicity, we used NCI toxicity ratings obtained routinely at all sites, and applied the summary scale developed by Bearman and colleagues.38 Toxicity scores were calculated at week +3.
Intervention
Standardization of the intervention was facilitated with the use of written manuals and electronic media. The massage routine was manualized, and a DVD demonstrating the massage routines was developed and circulated to massage therapists at all sites. All massage therapists were licensed in their state or province. A CD of the relaxation and imagery procedures was developed specifically for the study, and distributed to all participating parents. Both the relaxation and humor interventions were conducted by research assistants (RA) following a 2-day centralized training.
Child Intervention
Children randomized to receive the intervention were informed about the benefits of massage and humor. Age appropriate handouts reinforcing the benefits of taking massage and humor breaks were provided. Patients and parents met with a licensed massage therapist at the time of admission to introduce them to the rationale of the massage intervention before providing the initial massage session. Massage sessions of ½ hour were scheduled 3 times per week from admission through week +3. For the humor intervention, each site developed a humor cart stocked with humorous video shorts, books, gags, etc. The intervention focused on 3 procedures: 1) education re: the benefits of laughter and regular laugh breaks; 2) easy access to humor materials via the humor cart; and 3) continued encouragement in the use of the humor materials by the RA therapist who met with the patient on a weekly basis.
Parent Intervention
The parent intervention began with educating the parent about the benefits to their child that result from improvement in their own well-being. The parent massage intervention was identical to the child's, with sessions 3× weekly, scheduled together so that the parent massage occurred either immediately following or prior to the child massage. For the relaxation intervention, a session was held near the time of admission with the RA therapist, who described the benefits of a regular practice of relaxation. Parents were taught a relaxation induction, using breath awareness and muscle release, followed by guided imagery, including end state imagery of competence in parenting to promote a sense of confidence in handling stressful aspects of their child's treatment. A relaxation CD was provided and parents were encouraged to set aside 20 minutes each day for this purpose. In addition the RA met with them for booster sessions weekly through week +3.
Treatment Integrity Procedures
Procedures were monitored to ensure compliance with treatment protocols and consistency across sites. Massage therapists completed a checklist of their activities after each session, rating the participants' attitude towards massage, the length of the session, and reasons for any deviations in routine. The RA therapist also completed a checklist following each relaxation session. All relaxation sessions were audiotaped and sessions were chosen at random for review, with feedback provided to the sites. Likewise the RAs completed a checklist after each weekly humor session, which included a review of the patients'humor activities over the past week and identification of obstacles to the use of humor. Parents also completed compliance checklists as part of their weekly questionnaires, assessing their and their child's use of the intervention techniques during the previous week.
Statistical Analysis
The primary outcomes comprised the BASES-R scales by patient and parent report, and the PANAS-C. Given the complexity of comparing groups across repeated measures, two inferential approaches were utilized. First, we compared differential area under the curve from week-1 to week +3 after subtracting the week -1 value. This allows a single estimate of overall treatment effects during the intervention period. Secondly, to better appreciate longitudinal trends, a mixed model approach was used to assess change over time on these measures. These trends (curves) were estimated using a polynomial function, in which week is the number of weeks from SCT (i.e., the linear trend), week2 for the quadratic effect, week3 for the cubic effect, etc, using SAS Proc Mixed.39 Initially, we examined between-group differences for all the BASES subscales of somatic distress, mood disturbance, compliance, quality of interaction, activity, and sleep. Because these subscales were highly interrcorrelated, we examined the BASES-R data as single scale combining all subscales, to reduce experiment-wise error and to make findings more easily interpreteable. This reduced BASES outcomes to two variables; a composite BASES-R score by patient report and by parent report. For secondary outcomes of length of hospital stay, time to engraftment, and medication usage, group differences were examined using a Kruskal-Wallace test.
Results
Intervention Compliance
The number of massage sessions provided was used as the indicator of study compliance. With an expected ‘dosage’ of 12 massage sessions (3/Week × 4 weeks), the mean number of massages per patients was 8.8 (S.D. = 3.1; Median = 10), while for parents it was slightly lower, with a mean of 7.6 (S.D. = 3.2, Median = 8). Thus, approximately 75% of the planned interventions were provided. Missed sessions were generally passively refused or missed for logistical reasons, with a small number actively refused. Another indicator of study compliance is the number of self-report observations obtained. The number of successful observations in each arm over the course of the study are presented in Table 2. Overall, 73% of all possible observations were obtained from admission through week + 6, and the rate remained reasonably consistent over the course of the study period. This rate is comparable to that obtained in prior natural history studies.12
Table 2.
Number of observations per study arm.
| Week | HPI-C | HPI-CP | Standard Care | Total |
|---|---|---|---|---|
| Week -1 | 45 | 40 | 30 | 115 |
| Week 0 | 48 | 46 | 42 | 136 |
| Week +1 | 46 | 53 | 39 | 138 |
| Week +2 | 43 | 42 | 34 | 119 |
| Week +3 | 46 | 45 | 39 | 130 |
| Week +4 | 39 | 47 | 38 | 124 |
| Week +5 | 40 | 40 | 37 | 117 |
| Week +6 | 37 | 42 | 37 | 116 |
| Total Week -1 - +6 | 344 | 355 | 296 | 995 |
| Week +12 | 38 | 44 | 34 | 116 |
| Week +24 | 33 | 40 | 25 | 98 |
| Total | 415 | 439 | 355 | 1209 |
Primary Outcomes
Differential area under the curve for week -1 to week +3 for the Total BASES score by child report revealed no significant differences across treatment arms [F (2, 109) = 0.1, p = .95]. All 3 groups showed a slight increase in total distress across this time frame, and all pairwise contrasts were non-significant. Similarly for Total BASES score by parent report, there were no between group differences [F (2, 109) = 1.1, p = .35], and all pairwise contrasts were non-significant. Finally, the effects of the intervention on positive affect were examined using the PANAS-C. Again, no group differences were found [F (2, 117) = 1.0, p = .36], and there were no significant pairwise comparisons.
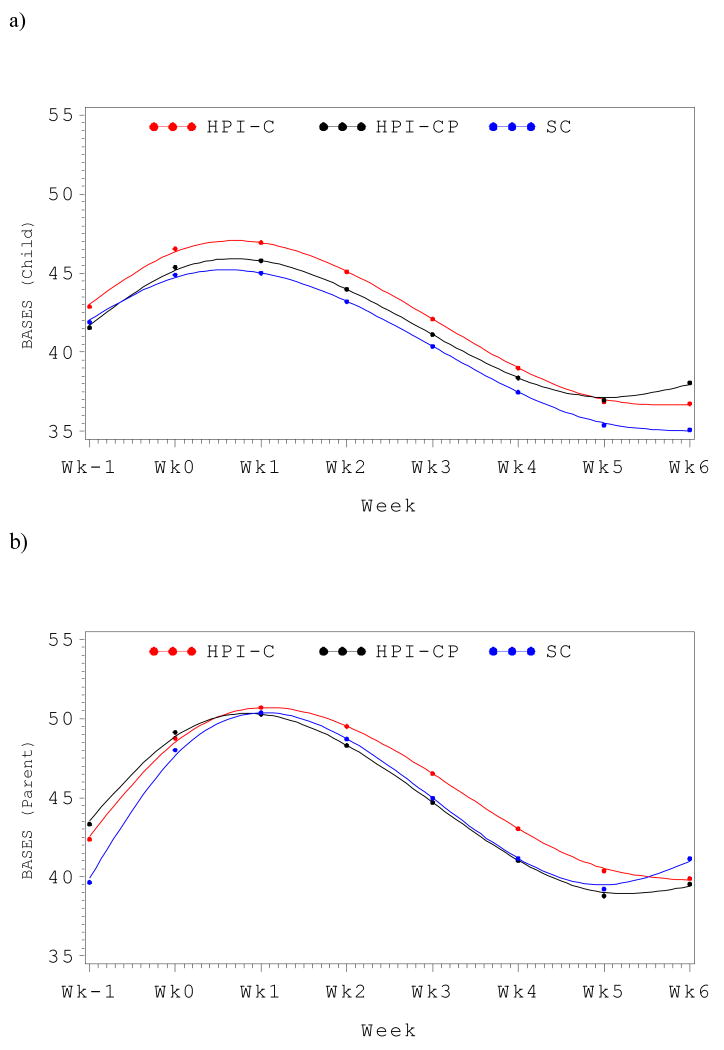
For examination of the longitudinal trends on the BASES scales, a model was fitted for each of the 3 intervention arms (HPI-C, HPI-CP, and SC). The longitudinal trends included the linear effects as well as polynomial functions of time, including the quadratic (time2) and cubic (time3) terms. Across the entire cohort, clear longitudinal trends were observed for the Total BASES score by child report, with significant linear (p <.01), quadratic (p <.001) and cubic (p <.01) effects. However, mirroring the area under the curve comparisons, no significant between-group differences were observed. Examination of the main effects for treatment group, revealed no difference (Type III p-value > .8). Using SC as the reference group, neither the HPI-C (t = 0.36, p > .7) nor HPI-C (t = -0.21, p > .8) differed from the control group based on patient report. Likewise, there were no between group differences in change over time, including linear, quadratic or cubic trends (all Type-III p-values > .5). This is perhaps best appreciated graphically, as depicted in Figure 2a. The longitudinal trends of the 3 groups were remarkably similar. Likewise, by parent report, significant linear, quadratic and cubic trends were seen (all p's <.001), but again there were no significant overall group differences (Type III p-value >.4). Neither the HPI-C group (t = 0.97, p > .3) nor the HPI-CP group (t = 1.3, p = .18) differed significantly from the SC group. Examination of longitudinal effects also revealed no group differences for linear, quadratic or cubic trends (all p's > .4). This is depicted graphically in Figure 2b.
Figure 2.
Distress trajectories from admission through SCT week +6 by intervention arm. A) Total BASES distress score by child report. B) Total Bases distress score by parent report.
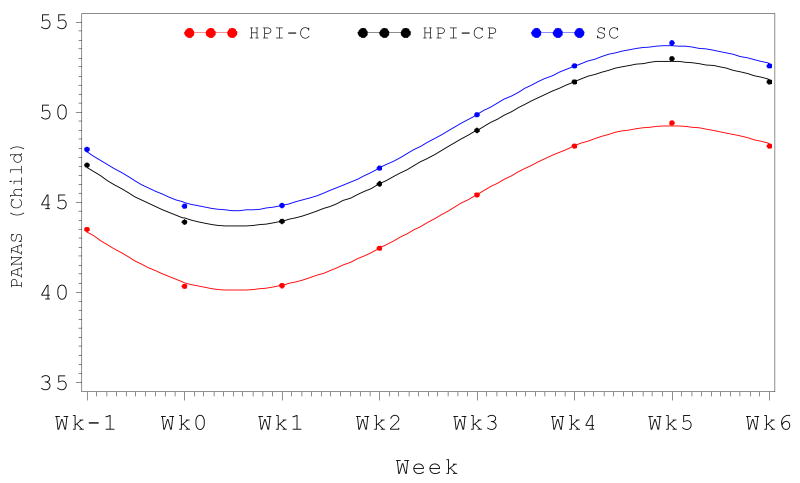
On the PANAS-C, significant longitudinal trends across the entire cohort were observed, with linear, quadratic and cubic effects (all p's <.001). A significant group difference was found for the overall main effect (Type III p-value < .05). With SC as the reference group, there was no difference between the SC and HPI-CP groups (t = -.99, p > .3), but a significant difference was found between HPI-C and SC groups (t = -2.9, p <.01), indicating lower positive affect overall in the HPI-C group. This contrasts with the findings using area under the curve, but the discrepancy is explained enirely by baseline differences as there were no between group differences in linear, quadratic or cubic longitudinal trends (all p's > .5). Again, this is best appreciated graphically (Figure 3).
Figure 3.
Positive affect trajectory (PANAS-C total score) from admission through SCT week+6 by intervention arm.
Medical outcomes
Results for length of hospital stay and time to engraftment are presented in Table 3. Given the skewed nature of this data, group differences were assessed with a one-way analysis of variance using the Kruskal-Wallis test. No differences between groups were observed for days in hospital (p > .4) or time to engraftment (p > .3). Likewise, there were no significant between group differences for use of narcotic analgesics (p > .8) or anti-emetics (p > .5).
Table 3.
| Hospital Stay (days) |
Time to Engraftment (days) |
|||||
|---|---|---|---|---|---|---|
| Mean |
Median |
Range |
Mean |
Median |
Range |
|
| HPI-C | 31.1 | 26.0 | 12 – 131 | 18.5 | 16.0 | 11 – 40 |
| HPI-CP | 34.7 | 26.0 | 14 – 180 | 20.0 | 19.5 | 10 – 38 |
| SC | 30.6 | 23.0 | 11 – 130 | 19.8 | 19.0 | 10 – 92 |
Discussion
The current study examined a multi-site trial of complementary therapies designed to reduce distress and improve well-being in pediatric patients undergoing SCT. Contrary to hypothesis, the trial produced largely null findings, and no clear evidence of benefit for either the child-targeted intervention alone, or in combination with a parent-targeted intervention. On the primary outcomes of patient and parent reported somatic distress, mood disturbance, and activity levels as captured on the BASES scales, there were no differences in mean distress relative to baseline (area under the curve) and the longitudinal trajectories of the three groups were remarkably similar. The only marginally significant effect was seen for positive affect, where the HPI-C intervention group reported lower levels of overall positive affect across the study period compared to the SC or HPI-CP groups. However, this was due primarily to differences at baseline, as there were no group differences in longitudinal trends. Regarding medical outcomes, there were no significant intervention effects observed on length of hospitalization, time to engraftment, or use of analgesic or antiemetic medications.
This study was designed to detect a difference of 0.45SD between the SC group and intervention groups (HPI-C or HPI-CP) in the area under the curve mean scores on the BASES scales from week -1 to week +3, at α = .05 with 80% power. Because of a somewhat smaller sample at study entry and further reduction in N from missing data, power was reduced such that there was 80% power to detect an effect size of .55SD, generally considered a moderate effect. Thus it is possible that the null findings reflect lack of sufficient power to detect smaller group differences. Descriptively however, the observed effects were quite small, with effects sizes between the SC and HPI_C groups of .05 on BASES child report; .07 on BASES parent report, and -.22 on the PANAS-C. Similarly, effect size for comparisons between SC and HPI-CP were .04 for BASES child report, .24 for BASES parent report, and -.15 on the PANAS-C. These effects are of a magnitude that we would not consider clinically relevant.
The absence of significant intervention effects is disappointing and somewhat surprising, in light of prior studies of complementary therapies in adult transplant settings, as well as our own pilot studies.16-21 This compels an examination of factors that might have contributed to this lack of intervention effects. One issue is whether the most appropriate outcome measures have been assessed. It was the anecdotal impression of study staff, based on observation and patient/parent report, that those receiving the intervention were experiencing benefits. However, when objective, repeated measures were obtained, no evidence of benefits was seen. Certainly, it is possible that there are relevant outcomes that were not measured, but the BASES scales were developed specifically for the purpose of measuring transplant-related distress, and were shown to be sensitive to change over time in this study. Thus the null findings cannot be attributed to measurement insensitivity. Moreover, the absence of group differences on the medical outcomes of days in hospital, time to engraftment, and medication usage, is consistent with our null findings on self-report outcomes, and point to the conclusion that the interventions did not produce their intended effects.
The timing of our measurements is another potential issue. In many prior studies of complementary therapies, particularly massage, measures have been obtained pre- and immediately post-intervention.16-19, 22-25 The immediate effects of massage in reducing reports of anxiety and pain are well established.22,25 However, these effects appear to be relatively short-lived, and may not reflect the sustained effects of intervention, nor the cumulative effect of repeated interventions over the course of transplant hospitalization, which was our primary focus. The current findings do not demonstrate significant sustained effects of the interventions. Patient and parent perception of benefit may not reflect actual positive change, a trend which has been found previously with massage in the transplant setting.40
Another factor that may have contributed to the null findings is the relatively low levels of distress in the sample overall. Although there were clear longitudinal trends observed, with a peak in distress around week +1 as reported in our prior natural history studies9,10, the absolute levels of distress were surprisingly low, and the amplitude of change from admission to peak levels was smaller relative to prior reports. Perhaps improvements in standard supportive care have led to continued decreases in distress such that it is more difficult to demonstrate effects. This is not to say that transplant-related distress has been eliminated, but that it may be difficult to reduce distress further beyond that of current aggressive standard care.
The current interventions were designed to reduce acute distress during the early and most intense phase of transplant. Given recent concerns regarding posttraumatic stress and other potential psychosocial late effects, perhaps the impact of the intervention may become more measurable over time despite the apparent absence of effects during the acute phase. The current design included follow-up through 6-months post-transplant. The effects of the intervention on more global quality of life and adjustment outcomes at 6-months are currently being examined.
There are some study limitations which deserve mention. The age range of 6-18 years is rather broad, and required some developmental tailoring which reduced standardization, particularly for the humor intervention. There were also potential regional differences that required flexibility and thus less standardization in regard to the humor materials. Differences in practice, and in availability of patients prior to admission for transplant led to variation in timing of baseline assessements both across and within sites. The study design required the baseline assessment to be completed prior to randomization in all cases. However, this may have occurred on the day of admission for some patients and one to two weeks prior to admission for others. As in most repeated measures studies in clinical settings, we also experienced missing data. Across the course of the study, 27% of possible observations were missed. Our statistical models assume data were missing at random, an assumption that is rarely met. However, our rate of missing data is similar to that of other studies with comparable designs, including our prior studies in the same setting, and more importantly, the rate of missing data did not differ across treatment arms.
Certainly, there may be other design elements and limitations that could have hindered our ability to detect intervention effects. However, overall study compliance was good, and treatment integrity procedures suggest that most participants received the majority of the intended interventions, and in an adequate/appropriate manner. The null findings reported here are not sufficient to yield a conclusion that massage and humor therapy are ineffective in the pediatric transplant setting. This is a single trial and other trials are necessary before any such generalization should be made. As mentioned previously, our study was not adequately powered to detect small effects. We are aware of other trials that are currently underway, and remain hopeful that benefits may be more clearly demonstrated. In the meantime, complementary therapies such as massage are becoming more widely available and offered as supportive care in many major cancer centers.43 Should oncologists and transplant physicians be recommending these approaches? Such therapies are relatively inexpensive to provide, are perceived positively by patients, and while not completely without risk, when provided by trained practitioners are generally safe and involve minimal risk of significant harm. In three studies involving massage, we have yet to experience a single adverse event. At the same time, the measurable benefits of such therapies in settings such as SCT have yet to be clearly documented. The current trial does not provide support for the benefits of massage and humor therapy in reducing distress in the pediatric SCT setting, and suggests some caution in the widespread application of these therapies.
Acknowledgments
Support: Supported by grants R01 CA60616 and P30 21765 from the National Institutes of Health, and by the American Lebanese Syrian Associated Charities (ALSAC)
References
- 1.Bollard CM, Krance RA, Heslop HE. Hematopoietic stem cell transplantation in pediatric oncology. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 476–500. [Google Scholar]
- 2.Handretinger R, Turner V, Barfield R. Hematopoietic stem cell transplantation. In: Pui CH, editor. Childhood Leukemias. 2nd. New York: Cambridge University Press; 2006. pp. 599–624. [Google Scholar]
- 3.Phipps S. Psychosocial and behavioral issues in stem cell transplantation. In: Brown RT, editor. Comprehensive Handbook of Childhood Cancer and Sickle Cell Disease. New York: Oxford University Press; 2006. pp. 75–99. [Google Scholar]
- 4.Neitzert CS, Ritvo P, Dancey J, Weiser K, Murray C, Avery J. The psychosocial impact of bone marrow transplantation: a review of the literature. Bone Marrow Transplantation. 1998;22:409–422. doi: 10.1038/sj.bmt.1701358. [DOI] [PubMed] [Google Scholar]
- 5.Vannatta K, Zeller M, Noll RB, Koontz K. Social functioning of children surviving bone marrow transplantation. Journal of Pediatric Psychology. 1998;23:169–178. doi: 10.1093/jpepsy/23.3.169. [DOI] [PubMed] [Google Scholar]
- 6.Barrera M, Pringle LAB, Sumbler K, Saunders F. Quality of life and behavioral adjustment after pediatric bone marrow transplantation. Bone Marrow Transplantation. 2000;26:427–435. doi: 10.1038/sj.bmt.1702527. [DOI] [PubMed] [Google Scholar]
- 7.Barrera M, Atenafu E, Pinto J. Behavioral, social, and educational outcomes after pediatric stem cell transplantation and related factors. Cancer. 2009;115:880–889. doi: 10.1002/cncr.24109. [DOI] [PubMed] [Google Scholar]
- 8.Phipps S. Stem cell transplant. In: Kazak A, Kupst MJ, Pao M, Patenaude A, Wiener L, editors. Quick Reference for Pediatric Oncology Clinicians: The Psychiatric and Psychological Dimensions of Pediatric Cancer Symptom Management. Charlottesville, VA: IPOS Press; 2009. pp. 82–89. [Google Scholar]
- 9.Phipps S, Dunavant M, Garvie P, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: I. Descriptive outcomes. Bone Marrow Transplantation. 2002;29:425–434. doi: 10.1038/sj.bmt.1703377. [DOI] [PubMed] [Google Scholar]
- 10.Phipps S, Dunavant M, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: II. Medical and Demographic Determinants. Bone Marrow Transplantation. 2002;29:435–442. doi: 10.1038/sj.bmt.1703376. [DOI] [PubMed] [Google Scholar]
- 11.Phipps S, Dunavant M, Lensing S, Rai SN. Patterns of distress in parents of children undergoing stem cell tranplantation. Ped Blood & Cancer. 2004;43:267–274. doi: 10.1002/pbc.20101. [DOI] [PubMed] [Google Scholar]
- 12.Phipps S, Dunavant M, Lensing S, Rai SN. Psychosocial predictors of distress in parents of children undergoing stem cell transplantation. J Ped Psychol. 2005;30:139–153. doi: 10.1093/jpepsy/jsi002. [DOI] [PubMed] [Google Scholar]
- 13.Syrjala KL, Cummings C, Donaldson GW. Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: a controlled clinical trial. Pain. 1992;48:137–146. doi: 10.1016/0304-3959(92)90049-H. [DOI] [PubMed] [Google Scholar]
- 14.Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain. 1995;63:189–198. doi: 10.1016/0304-3959(95)00039-U. [DOI] [PubMed] [Google Scholar]
- 15.Ahles TA, Tope DM, Pinkson B, Walch S, Hann D, Whedon M, Dain B, Weiss JE, Mills L, Silberfarb PM. Massage therapy for patients undergoing autologous bone marrow transplant. Journal of Pain and Symptom Management. 1999;18:157–163. doi: 10.1016/s0885-3924(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 16.Smith MC, Reeder F, Daniel L, et al. Outcomes of touch therapies during bone marrow transplant. Alternative Therapies. 2003;9:40–49. [PubMed] [Google Scholar]
- 17.Cassileth BR, Vickers AJ, Magill LA. Music therapy for mood disturbance during hospitalization for autologous stem cell transplantation. Cancer. 2003;98:2723–2729. doi: 10.1002/cncr.11842. [DOI] [PubMed] [Google Scholar]
- 18.Barrera ME, Rykov MH, Doyle SL. The effects of interactive music therapy on hospitalized children with cancer: a pilot study. Psycho-oncology. 2002;11:379–388. doi: 10.1002/pon.589. [DOI] [PubMed] [Google Scholar]
- 19.Phipps S. Reduction of distress associated with paediatric bone marrow transplant: complementary health promotion interventions. Pediatric Rehabilitation. 2002;5:223–234. doi: 10.1080/1363849021000064553. [DOI] [PubMed] [Google Scholar]
- 20.Phipps S, Dunavant M, Gray E, Rai SN. Massage therapy in children undergoing hematopoietic stem cell transplant: results of a pilot trial. J Cancer Integr Med. 2005;3:62–70. [Google Scholar]
- 21.Ernst E. Massage therapy for cancer palliation and supportive care: a systematic review of randomised clinical trials. Support Care Cancer. 2009;17:333–337. doi: 10.1007/s00520-008-0569-z. [DOI] [PubMed] [Google Scholar]
- 22.Kutner JS, Smith MC, Corbin L, et al. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med. 2008;149:369–379. doi: 10.7326/0003-4819-149-6-200809160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers CD, Walton T, Small BJ. The value of massage therapy in cancer care. Hematol Oncol Clin North Am. 2008;22:649–660. doi: 10.1016/j.hoc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Beider S, Moyer CA. Randomized controlled trials of pediatric massage: a review. Evid Based Complement Alternat Med. 2007;4:23–34. doi: 10.1093/ecam/nel068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin RA. Humor, laughter, and physical health: methodological issues and research findings. Psychol Bull. 2001;127:504–519. doi: 10.1037/0033-2909.127.4.504. [DOI] [PubMed] [Google Scholar]
- 26.Bennett MP, Lengacher C. Humor and laughter influence health: III. Laughter and health outcomes. Evid Based Complement Alternat Med. 2008;5:37–40. doi: 10.1093/ecam/nem041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fry WF. The physiologic effects of humor mirth, and laughter. JAMA. 1992;267:1857–1861. doi: 10.1001/jama.267.13.1857. [DOI] [PubMed] [Google Scholar]
- 28.Stuber M, Hilber SD, Mintzer, et al. Laughter, humor and pain perception in children: a pilot study. Evid Based Complement Alternat Med. 2007 Oct 5; doi: 10.1093/ecam/nem097. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolgin MJ, Phipps S. Reciprocal influences in family adjustment to childhood cancer. In: Baider L, Cooper CL, Kaplan DeNour A, editors. Cancer and the Family. Chichester: John Wiley & Sons; 1996. pp. 73–92. [Google Scholar]
- 30.Streisand R, Rodrigue JR, Houck C, Graham-Pole J, Berlant N. Parents of children undergoing bone marrow transplantation: documenting stress and piloting a psychological intervention program. Journal of Pediatric Psychology. 2000;25:331–338. doi: 10.1093/jpepsy/25.5.331. [DOI] [PubMed] [Google Scholar]
- 31.Hoekstra-Weebers JEHM, Huevel F, Jaspers JPC, et al. An intervention program for parents of pediatric cancer patients: a randomized controlled trial. Journal of Pediatric Psychology. 1998;23:207–214. doi: 10.1093/jpepsy/23.3.207. [DOI] [PubMed] [Google Scholar]
- 32.Kazak AE, Blackall G, Himelstein B, et al. Producing systemic change in pediatric practice: an intervention protocol for reducing distress during pediatric procedures. Family Systems Medicine. 1995;13:173–185. [Google Scholar]
- 33.Sahler OJZ, Fairclough DL, Phipps S, et al. Using Problem-Solving Skills Training to Reduce Negative Affectivity in Mothers of Children with Newly Diagnosed Cancer: Report of a Multi-site Randomized Trial. J Consult Clin Psychol. 2005;73:272–283. doi: 10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- 34.Redd WH, Montgomery GH, DuHamel KN. Behavioral intervention for cancer treatment side effects. J Natl Cancer Inst. 2001;93:810–823. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 35.Phipps S, Hinds PS, Channel S, Bell GL. Measurement of behavioral, affective and somatic responses to pediatric bone marrow transplantation. Development of the BASES scale. J Ped Oncol Nurs. 1994;11:109–117. doi: 10.1177/104345429401100305. [DOI] [PubMed] [Google Scholar]
- 36.Phipps S, Dunavant M, Jayawardene D, Srivastava DK. Assessment of health related quality of life in acute inpatient settings: Use of the BASES scale in children undergoing bone marrow transplantation. Int J Oncol. 1999;12:18–24. doi: 10.1002/(sici)1097-0215(1999)83:12+<18::aid-ijc5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 37.Crook K, Beaver BR, Bell M. Anxiety and depression in children: A preliminary examination of the utility of the PANAS-C. J Psychopathol Behav Assess. 1998;20:333–350. [Google Scholar]
- 38.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. Journal of Clinical Oncology. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 39.SAS Institute Inc. Version 8. Cary, N.C.: SAS Institute Inc.; 1999. [Google Scholar]
- 40.Smith MC, Reeder F, Daniel L, Baramee J, Hagman J. Outcomes of touch therapies during bone marrow transplant. Alternative Therapies. 2003;9:40–49. [PubMed] [Google Scholar]