Abstract
Purpose
In preclinical models, non-cytotoxic suramin (concentrations <50 μM) potentiates the activity of multiple chemotherapeutic agents. The present study evaluated the safety and tolerability of suramin in combination with docetaxel or gemcitabine in previously chemotherapy-treated patients with advanced non-small cell lung cancer.
Methods
Patients received suramin intravenously in combination with either docetaxel on day 1 or gemcitabine on days 1 and 8, of each 21-day treatment cycle. After 3 cycles, patients with partial response (PR) or better continued on the same combination, whereas patients with stable disease (SD) or worse crossed-over to the other combination. Pharmacokinetic analyses were performed before and after each treatment.
Results
Eighteen patients received a total of 79 courses (37 suramin plus docetaxel, 42 suramin plus gemcitabine). The dose-limiting toxicity (DLT) was febrile neutropenia, observed in three of six patients treated with suramin and docetaxel 75 mg/m2. No DLTs were observed with suramin plus docetaxel 56 mg/m2 or suramin plus gemcitabine 1,250 mg/m2. Common adverse events included neutropenia, thrombocytopenia, anemia, fatigue, nausea, vomiting, skin rash, hyperglycemia, and electrolyte abnormalities. The target plasma suramin concentration range of 10–50 μM was achieved in 90% of treatments. Discernable antitumor activity was noted in 11 patients (2 PR, 9 SD).
Conclusions
Non-cytotoxic suramin, in combination with docetaxel 56 mg/m2 or gemcitabine 1,250 mg/m2, was reasonably well-tolerated with a manageable toxicity profile. Target plasma concentrations were correctly predicted by our previously described dosing nomogram. The observed preliminary evidence of antitumor activity encourages evaluation of this strategy in efficacy trials.
Keywords: Suramin, Docetaxel, Gemcitabine, Chemosensitizer, Modulator, Non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States, with an estimated 219,000 new cases and roughly 159,000 deaths in 2009 [1]. The majority of patients diagnosed with non-small cell lung cancer (NSCLC) have an advanced stage of disease and poor long-term survival [2]. Standard platinum-based combinations used in the first-line setting yield a median survival of only 8 to 12 months [3–6].
Gemcitabine is a nucleoside analog that is phosphorylated intracellularly to its active diphosphate and triphosphate forms. Gemcitabine diphosphate and gemcitabine triphosphate, respectively, inhibit ribonucleotide reductase and DNA polymerase, interfering with DNA synthesis and function. Incorporation of gemcitabine into RNA also results in alterations in RNA processing and translation. Gemcitabine is approved in combination with cisplatin for first-line therapy in locally advanced and metastatic NSCLC based on positive phase III trials [6–8]. Although not formally approved for second- or third-line treatment of metastatic NSCLC, gemcitabine has been studied in phase II trials with activity at doses of 1,000 mg/m2 weekly for 3 weeks every 28 days or 1,250 mg/m2 weekly for 2 weeks every 21 days [9, 10].
Docetaxel is a taxane derivative that promotes the assembly of microtubules from tubulin dimers and inhibits the depolymerization of tubulin which stabilizes microtubules in the cell, leading to inhibition of DNA, RNA, and protein synthesis. Docetaxel is approved for use as a single agent in the second-line setting and in combination with cisplatin for first-line metastatic NSCLC. In a phase III trial of docetaxel compared with vinorelbine or ifosfamide in the second-line setting, docetaxel yielded better response rate (RR) (6.7–10.8 vs. 0.8%), longer time to progression (TTP), and statistically significant greater 1-year survival (32 vs. 19%) [11]. In a separate study, docetaxel was superior to best supportive care for relapsed or refractory NSCLC [12, 13]. Since agents such as gemcitabine and docetaxel can provide modest activity for refractory or relapsed NSCLC, effective therapeutic strategies which enhance their antitumor effects may provide tangible clinical benefit for such patients.
Suramin (8,8′-carbonyl-bis [imino-3,1-phenylenecarbonylimino (4-methyl-3,1-phenylene) carbonylimino] bis-1,3,5-naphthalene-trisulfonic acid) is a polysulfonated naphthylurea that has been used for more than 60 years in the treatment of certain African parasitic infections such as Rhodesian and Gambian trypanosomiasis [14]. Suramin inhibits the binding of several polypeptide growth factors (including platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, and insulin-like growth factor) to their respective receptors [15–17]. Suramin has concentration-dependent antiproliferative activity against a variety of human tumor cell lines [18, 19]. Suramin has been evaluated in numerous clinical trials as a cytotoxic agent in a variety of solid malignancies; however, its complex pharmacology and broad spectrum of toxicity have limited further investigation as an anticancer agent [20–24]. At cytotoxic concentrations above 275 μM, sustained over several weeks, suramin has been associated with severe neurological toxicity, renal toxicity, adrenal insufficiency, immune- and anticoagulant- mediated blood dyscrasias, and dermatological toxicity including toxic epidermal necrolysis [25–29]. However, our group has shown that at much lower and non-cytotoxic concentrations of 10–20 μM, suramin can augment the activity of other chemotherapeutic agents [30]. At these non-cytotoxic concentrations, suramin was shown to enhance the antitumor activity of 5-fluorouracil, doxorubicin, paclitaxel, docetaxel, and gemcitabine in tumor-bearing animals, including animals bearing human NSCLC A549 xenografts pretreated with chemotherapy [31–34]. Our earlier studies showed that these suramin effects are in part due to its inhibition of acidic and basic FGF [15–17]. However, additional FGF-independent mechanisms are likely. Other investigators have also shown that bFGF can induce chemoresistance in small cell lung cancer cells and that bFGF is frequently over-expressed in NSCLC tissues [35, 36].
In previous phase I and II clinical trials at our institution, we have investigated the use of non-cytotoxic doses of suramin in combination with paclitaxel and carboplatin in chemotherapy-naïve and chemo-refractory patients [37, 38]. In these studies, suramin was dosed using a previously validated dosing nomogram which correctly predicted the target suramin concentration range of 10–50 μM over 48 h in 93% of treatment cycles [39]. Given the overexpression of bFGF and FGF receptors in lung cancer, the potentiation of chemotherapeutic agents by non-cytotoxic doses of suramin in preclinical studies, and the moderate activity of the docetaxel and gemcitabine in platinum-refractory NSCLC, we conducted this phase I study to evaluate the safety and tolerability of non-cytotoxic suramin in combination with docetaxel or gemcitabine in patients with previously treated advanced NSCLC.
Patients and methods
Eligibility
Patients with histologically confirmed advanced NSCLC (stage IIIB with malignant pleural effusion or stage IV) who had progressed on a platinum-containing regimen were eligible for this study. Up to two prior chemotherapy regimens were allowed, with a washout period of 28 days. No prior docetaxel or gemcitabine was allowed. Prior suramin, epidermal growth factor receptor (EGFR)-targeted therapy, and radiation therapy were permitted. Additional eligibility criteria included age ≥18 years; Eastern Cooperative Oncology Group performance status of 0–2; a life expectancy ≥12 weeks; and adequate hematopoietic, hepatic, and renal function. Patients were excluded from participation if they had untreated brain metastases or leptomeningeal involvement; a history of myocardial infarction within the previous six months, congestive heart failure requiring therapy, or unstable angina; any known active serious infectious process or current treatment for human immunodeficiency virus type 1 infection; uncontrolled diabetes mellitus; grade ≥2 neuropathy; any history of hypersensitivity to suramin; or if they were pregnant or lactating. The treatment protocol was approved by the Cancer Therapy Evaluation Program at the National Cancer Institute and the Institutional Review Board at The Ohio State University. Written, informed consent was obtained from all patients prior to study entry, according to federal and institutional guidelines.
Study design
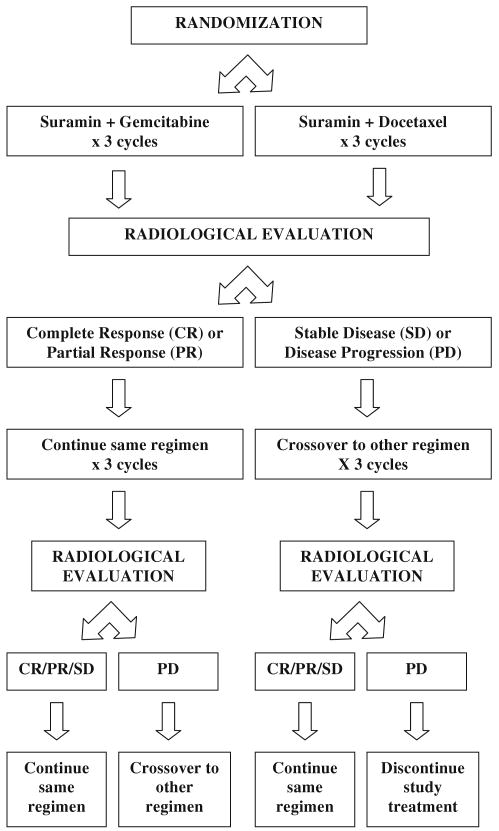
This was a phase I study with two parallel arms in which patients were randomized to receive suramin in combination with either docetaxel or gemcitabine (Fig. 1). Six patients were to be treated in each cohort. In the event of dose-limiting toxicity (DLT) occurring in ≥2 of the 6 patients, the cohort would be expanded to treat an additional six patients at a 25% dose reduction. The starting dose for docetaxel was 75 mg/m2 given intravenously over 1 h on day 1 of each 3-week cycle. The starting dose for gemcitabine was 1,250 mg/m2 given intravenously over 30 min on days 1 and 8 of each 3-week cycle. Suramin was given on day 1 only in the docetaxel arm and on days 1 and 8 in the gemcitabine arm of each 3-week cycle. Suramin was administered as a 30-min infusion, given 2.5 h prior to the initiation of chemotherapy. The dose of suramin was determined using a validated and previously published dosing nomogram, which uses body surface area (BSA) and time elapsed since the previous dose, to calculate the dose that would achieve a target concentration range of 10 to 50 μM from 8 to 48 h [39]. The dose of suramin in milligram was calculated as the product of FACTOR × (BSA)2, where FACTOR is equal to 125 for day 1 of the first cycle and based on the nomogram for all subsequent doses, including the day 8 gemcitabine dose.
Fig. 1.
Study treatment schema
Radiological evaluation was obtained at baseline and after every three cycles, at which point tumor assessments were performed using RECIST criteria to evaluate for objective response [40]. The study treatment schema is illustrated in Fig. 1. After 3 cycles, patients with complete response (CR) or partial response (PR) continued on the same combination, whereas patients with stable disease (SD) or disease progression (PD) crossed-over to the other combination. In patients with SD or better, treatment beyond six cycles was entirely optional.
Histories, physical examinations, and routine laboratory studies were performed at baseline and prior to each cycle of therapy. In addition, complete blood cell counts were also performed weekly. Chest radiographs and electrocardiograms were performed prior to initiating treatment. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 2.0 [41].
Dose-limiting toxicity (DLT) was defined as any of the following: (a) grade 4 neutropenia lasting more than 5 days or accompanied by grade ≥2 fever; (b) grade 4 thrombocytopenia; (c) grade 3 non-hematologic toxicity resulting in treatment interruption for more than 2 weeks; or (d) grade 4 non-hematologic toxicity. Patients who experienced DLT were allowed to be retreated, at a 25% dose reduction of docetaxel or gemcitabine, provided that they had recovery of all prior toxicity to grade ≤ 1.
Pharmacokinetics and correlative studies
Blood samples (10 ml in green-top heparinized tubes) were obtained from a site contralateral to the drug infusion. Pretreatment plasma bFGF levels were determined for all treatments. The sampling times for the determination of plasma suramin levels included pretreatment (1 h before suramin administration), during and at the end of the 30-min suramin infusion, before the start (2.5 h) and at the end of gemcitabine (3 h) or docetaxel (3.5 h) infusion. For the first treatment, samples were taken at pretreatment and at 10, 20, and 30 min and at 1, 1.5, 2, 2.5, 3, 3.5, 5.5, 24, 48, and 72 h after the initiation of suramin infusion. For the remaining treatments, samples were taken 1 h before treatment at the end of the 30-min suramin infusion (0.5 h), the start of the chemotherapy infusion (2.5 h), and at the end of the gemcitabine infusion (3 h) or docetaxel infusion (3.5 h) after initiation of suramin. Suramin concentrations were determined using a previously described high-performance liquid chromatography method [42]. Briefly, a plasma sample was mixed with tetrabutylammonium bromide and extracted with acetonitrile. The organic layer was refrigerated, filtered, and analyzed. The detection limit was 0.5 μg/ml. Pharmacokinetic analysis was conducted using standard methods. The plasma levels of bFGF were determined using an Enzyme-Linked ImmunoSorbent Assay kit (Oncogene, Cambridge, MA) according to manufacturer instructions. The antibody was murine monoclonal anti-bFGF antibody conjugated to horseradish peroxidase. The lower detection limit of the assay was 2.5 pg/ml.
We evaluated whether the suramin concentrations were maintained at the target range of 10–50 μM between 8 and 48 h. For logistic reasons, actual blood samples were obtained at 48 h only during the first treatment cycle, and no samples were collected at 8 h. Hence, plasma concentrations at these time points were obtained using pharmacokinetic modeling and simulations, using a 3-compartment open model with multiple dosing. The fitting was performed using WinNonlin (Pharsight Corporation, Mountain View, CA).
Results
General
Between July 2003 and January 2005, 18 patients were enrolled and received a total of 79 cycles of treatment on study (median number of cycles, 6.5; range, 1–11). Patient baseline characteristics are listed in Table 1. All 18 patients received prior platinum therapy; 16 patients also received prior therapy with paclitaxel. Sixteen patients had had disease progression while on platinum therapy and 14 patients had had disease progression while on paclitaxel therapy. Eight of 18 patients received prior suramin therapy in a separate clinical trial. All 18 patients on this study were evaluated for toxicity. Sixteen patients completed two or more treatment cycles and were evaluable for response. Patients who were non-evaluable for response included one patient with a history of peptic ulcer disease and coronary artery disease who was hospitalized for gastrointestinal bleed and atrial fibrillation during the first cycle of suramin and docetaxel and another patient with a history of hypertension and chronic obstructive pulmonary disease who was hospitalized with arrhythmia (atrial fibrillation vs. multifocal atrial tachycardia) following the first administration of suramin and gemcitabine. Due to safety concerns, no additional study treatment was offered to these patients.
Table 1.
Demographics and baseline patient characteristics
| Characteristic | Docetaxel no. | Gemcitabine no. | All patients no. |
|---|---|---|---|
| Total number of patients | 12 | 6 | 18 |
| Age (years) | |||
| Median | 62 | 56 | 60 |
| Range | 47–73 | 51–71 | 47–73 |
| Gender | |||
| Male | 4 | 4 | 8 |
| Female | 8 | 2 | 10 |
| Race/ethnicity | |||
| Caucasian | 10 | 5 | 15 |
| African–American | 2 | 1 | 3 |
| ECOG | |||
| 0–1 | 10 | 5 | 15 |
| 2 | 2 | 1 | 3 |
| Histology | |||
| Adenocarcinoma | 8 | 3 | 11 |
| Squamous cell carcinoma | 2 | 0 | 2 |
| Large cell carcinoma | 0 | 2 | 2 |
| Non-specified NSCLC | 2 | 1 | 3 |
| Stage | |||
| IIIB | 1 | 0 | 1 |
| IV | 11 | 6 | 17 |
| Sites of metastasis | |||
| Lung | 8 | 2 | 10 |
| Brain | 4 | 2 | 6 |
| Mediastinum | 5 | 1 | 6 |
| Lymph node | 4 | 2 | 6 |
| Bone | 4 | 1 | 5 |
| Adrenal | 1 | 2 | 3 |
| Prior therapies | |||
| Chemotherapya, b, c | 12 | 6 | 18 |
| Suramin-containing combinations | 4 | 4 | 8 |
| Radiation | 10 | 3 | 13 |
| Surgery | 5 | 1 | 6 |
Four of 18 patients had two prior chemotherapies; 14 patients had one prior chemotherapy treatment
All 18 patients had prior platinum therapy, of which 16 had disease progression while on therapy or within 3 months after therapy
Sixteen of 18 patients received prior paclitaxel therapy, of which 14 had disease progression while on or within 3 months after therapy
Three of the first six patients who received docetaxel at 75 mg/m2 experienced DLT of febrile neutropenia. Therefore, six additional patients were treated at the 56 mg/m2 dose (one dose level reduction) of docetaxel. The initial cohort of patients was also treated at this reduced dose of docetaxel for all subsequent cycles. At the dose of 56 mg/m2, febrile neutropenia was seen in only 1 of 30 cycles of docetaxel administered. This was seen in one of the patients who developed DLT at the 75 mg/m2 dose. This patient had two prior lines of chemotherapy, including nine cycles of paclitaxel-containing treatments. There were no DLTs observed in the six patients initiated at the starting dose of gemcitabine or in the seven patients who received gemcitabine after crossing over from the docetaxel arm. In total, twelve patients were initiated with docetaxel; seven of these patients underwent the allowed crossover and also received gemcitabine. Six patients were initiated with gemcitabine; two of these patients crossed-over to receive docetaxel. Overall, fourteen patients received docetaxel at any time during the study for a total of 37 cycles, and 13 patients received gemcitabine at any time during the study for a total of 42 cycles.
Toxicities
Table 2 summarizes the hematologic and non-hematologic toxicities. The most common grade 3 or 4 hematologic toxicity was neutropenia, occurring in 16 of 79 cycles (20%). Grade 3 or 4 neutropenia was more common, though not statistically significant (P 0.17), in patients receiving docetaxel [10 of 37 cycles (27%)] compared to patients receiving gemcitabine [6 of 42 cycles (14%)]. Febrile neutropenia was observed in three patients (4 cycles) receiving docetaxel at 75 mg/m2. The febrile neutropenia noted was of short duration, and two of the three patients were treated successfully with empiric oral antibiotics in the outpatient setting. The third patient was hospitalized briefly but no infectious source was found. All three patients were previously treated with carboplatin and paclitaxel (range 4–9 cycles).
Table 2.
Toxicities
| Regimen | Total no. of courses | No. of courses with | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutropenia | Anemia | Thrombocytopenia | ||||||||||||||||||
| 1 | 2 | 3 | 4 | FNa | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||||||
| A. Hematologic toxicities | ||||||||||||||||||||
| Sur + Doc 75 mg/m2 | 7 | 0 | 1 | 0 | 3 | 3 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | ||||||
| Sur + Doc 56 mg/m2 | 30 | 2 | 2 | 6 | 1 | 1 | 3 | 4 | 1 | 0 | 1 | 0 | 0 | 0 | ||||||
| Sur + Gem 1,250 mg/m2 | 42 | 1 | 3 | 5 | 1 | 0 | 1 | 9 | 0 | 0 | 8 | 0 | 4 | 1 | ||||||
| Total | 79 | 3 | 6 | 11 | 5 | 4 | 7 | 14 | 1 | 0 | 12 | 0 | 4 | 1 | ||||||
| Sur + Doc 75 mg/m2 | Sur + Doc 56 mg/m2 | Sur + Gem 1,250 mg/m2 | Total (% of courses) | |||||||||||||||||
| B. Common non-hematologic toxicitiesb | ||||||||||||||||||||
| Total patients | 6 | 13 | 13 | 18 | ||||||||||||||||
| Total no. of courses | 7 | 30 | 42 | 79 (100) | ||||||||||||||||
| No. of courses with (grade) | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | All grades | |||||||
| Fatigue | 2 | 2 | 2 | 0 | 1 | 7 | 4 | 0 | 5 | 8 | 3 | 0 | 34 (43) | |||||||
| Anorexia | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 11 (14) | |||||||
| Nausea | 1 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 11 (14) | |||||||
| Skin rash | 4 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 1 | 0 | 0 | 11 (14) | |||||||
| Vomiting | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 3 | 1 | 0 | 0 | 10 (13) | |||||||
| Stomatitis | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 8 (10) | |||||||
| Electrolytes | ||||||||||||||||||||
| Hyperglycemia | 1 | 2 | 0 | 0 | 2 | 4 | 0 | 0 | 2 | 3 | 0 | 0 | 14 (18) | |||||||
| Hypocalcemia | 2 | 2 | 0 | 0 | 1 | 3 | 0 | 0 | 4 | 1 | 0 | 0 | 13 (16) | |||||||
| Hypokalemia | 3 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 4 | 0 | 0 | 1 | 11 (14) | |||||||
| Hypophosphatemia | 0 | 2 | 2 | 0 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 0 | 10 (13) | |||||||
| Hyponatremia | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 4 | 0 | 9 (11) | |||||||
| Hepatic function | ||||||||||||||||||||
| Hypoalbuminemia | 0 | 2 | 1 | 0 | 3 | 2 | 0 | 0 | 6 | 2 | 0 | 0 | 16 (20) | |||||||
| Protime (PT) | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 (6) | |||||||
| SGPT/ALTc | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 5 (6) | |||||||
| SGOT/ASTc | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 (5) | |||||||
Eighteen patients were enrolled and treated with a total of 79 courses. All patients received suramin (Sur) with every course of therapy. Fourteen patients received docetaxel (Doc) for a total of 37 courses. Thirteen patients received gemcitabine (Gem) for a total of 42 courses. Nine patients received both docetaxel and gemcitabine (sequentially, as a result of crossover) during the course of the study
FN = Febrile Neutropenia (ANC < 500 for >5 days or with fever)
Toxicities occurring in ≥5% of all courses
SGPT/ALT = Serum glutamic pyruvic transaminase/alanine aminotransferase and SGOT/AST = Serum glutamic oxaloacetic transaminase/aspartate aminotransferase
The incidence of grade 3 or 4 anemia was low for both docetaxel and gemcitabine, 2.7 and 0%, respectively. Grade 3 or 4 thrombocytopenia was seen only in patients treated with gemcitabine, in 5 of 42 cycles (12%). One patient developed transient grade 4 thrombocytopenia during the 5th cycle of study treatment (after 3 cycles of docetaxel and 2 cycles of gemcitabine). The day 8 dose of gemcitabine was held in five patients (7 cycles) for hematologic reasons-four cycles for neutropenia, one cycle for anemia, and two cycles for thrombocytopenia. There were no dose delays due to hematologic reasons for docetaxel. Overall, the observed hematologic toxicities were comparable to those expected from single agent gemcitabine or docetaxel [43, 44].
The most common non-hematologic adverse event (AE) was fatigue, occurring in 43% of all cycles. Grade 3 fatigue was noted in 9 cycles (6 during treatment with docetaxel and 3 during treatment with gemcitabine) among 9 patients. None of the patients with grade 3 fatigue required dose interruption for more than 2 weeks. Other non-hematologic AEs occurring in ≥10% of all cycles included anorexia, nausea, vomiting, skin rash, stomatitis, as well as, electrolyte abnormalities. The majority of AEs was grade 1 or 2 and did not worsen with subsequent cycles. Grade 3 non-hematologic clinical toxicities included anorexia (1), diarrhea (1), dyspnea (1), and hypotension (1). Grade 3 non-hematologic laboratory toxicities included aspartate aminotransferase (AST) elevation (1), protime abnormality (1), hyperkalemia (1), hypokalemia (1), alkalosis (1), hypoalbuminemia (1), hyponatremia (4), and hypophosphatemia (6). There was one grade 4 toxicity of uncomplicated hypokalemia in a patient with chronic hypokalemia on potassium supplementation. None of these grade 3 or 4 toxicities required dose interruption for more than 2 weeks and, therefore, was not considered to be DLT. Overall, the incidence of non-hematologic clinical toxicities was comparable to the reported toxicities related to the docetaxel or gemcitabine [43, 44]. However, we did observe more electrolyte abnormalities than was previously reported. The majority of hypokalemia and hyponatremia occurrences were seen in patients treated with gemcitabine, although these did not always correlate with the presence of emesis or diarrhea. Hyperglycemia was observed in almost a quarter of all cycles, half of which occurred for unclear reasons in patients who were on gemcitabine and did not receive premedication with dexamethasone. There were no significant neurologic, renal, adrenal, immunologic, or dermatologic abnormalities seen.
Pharmacokinetics
A total of 79 cycles were administered to 18 patients. Each 3-week cycle comprised one treatment of docetaxel plus suramin on day 1 or two treatments of gemcitabine plus suramin on days 1 and 8. The total number of treatments was 108 (37 docetaxel plus suramin treatments and 71 gemcitabine plus suramin treatments). In all, 727 blood samples were collected (271 samples for docetaxel plus suramin and 456 samples for gemcitabine plus suramin). The corresponding pharmacokinetic data were used to evaluate (a) whether the suramin pharmacokinetics was equivalent in patients receiving docetaxel or gemcitabine and (b) whether suramin concentrations were maintained in the target range.
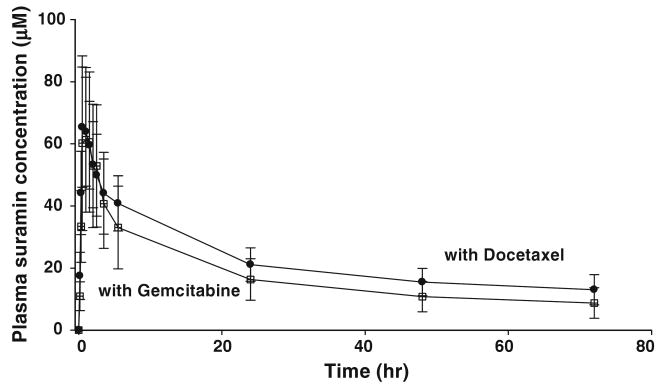
We first evaluated the suramin concentration profiles obtained after the first suramin dose, comparing the group of patients receiving docetaxel (n = 12) with the group of patients receiving gemcitabine (n = 6). For each patient, samples were collected at the time points indicated in the Methods section. Figure 2 shows the average plasma concentration–time profiles of suramin for both combinations. There were no statistically significant differences between the two profiles (P = 0.387).
Fig. 2.
Pharmacokinetics of non-cytotoxic suramin in combination with gemcitabine or docetaxel. Plasma concentration–time profiles during the first cycles of treatment with suramin plus docetaxel or gemcitabine (n = 232 samples, see text). Data are from the 6 patients who received suramin + gemcitabine as the first chemotherapy (total of 78 samples in 6 cycles) and the 12 patients who received suramin + docetaxel as the first treatment (total of 154 samples in 12 cycles). Time zero refers to the initiation of suramin infusion. Data reported are mean plasma suramin concentrations ± SD. The respective area-under-the-curve from time zero to the last time point of 72 h was 1,105 ± 259: M-hr for suramin plus docetaxel and 975 ± 354: M-hr for suramin plus gemcitabine; these values are not significantly different (P = 0.387, two-tailed t test)
We then evaluated the suramin concentrations at 8 and 48 h after the start of suramin infusion for all 108 treatments; the concentrations at 8 h were estimated by pharmacokinetic fitting, and the concentrations at 48 h were obtained experimentally from patient samples (35 samples) or from model-simulated data (73 data points). The target range of 10–50 μM between 8 and 48 h was achieved in 90% of the treatments (97 of 108 treatments). The remaining 11 treatments all had concentrations below 10 μM at 48 h, 5 of which were found in one patient. These results were comparable to the results of our previous studies of non-cytotoxic suramin in combination with carboplatin and paclitaxel [37, 38].
Correlative studies
Table 3 summarizes the plasma concentrations of bFGF prior to each treatment (day 1 for docetaxel; days 1 and 8 for gemcitabine) for all 79 cycles. There were no apparent trends or relationships between pretreatment bFGF levels and treatment arms or patient response.
Table 3.
Pretreatment basic fibroblast growth factor (bFGF) levels in plasma
| Cycle | Number of patients | Range (pg/ml) | Median (pg/ml) | Mean ± SD (pg/ml) |
|---|---|---|---|---|
| 1 | 17 | Undetectable—23.3 | 2.64 | 5.41 ± 6.46 |
| 2 | 16 | 1.6–10.4 | 3.16 | 4.30 ± 2.73 |
| 3 | 13 | 0.78–67.6 | 3.22 | 10.4 ± 18.7 |
| 4 | 9 | Undetectable—25.5 | 3.11 | 7.19 ± 10.3 |
| 5 | 8 | 0.78–63.8 | 4.55 | 12.8 ± 21.1 |
| 6 | 5 | 2.99–18.5 | 9.13 | 10.1 ± 6.62 |
| 7 | 3 | 1.10–4.02 | 2.8 | 2.64 ± 1.47 |
| 8 | 3 | 2.5–35.5 | 4.02 | 14.0 ± 18.6 |
| 9 | 2 | 10.4–13.7 | 12.0 | 12.0 ± 2.31 |
| 10 | 1 | 4.72 | NA | NA |
| 11 | 1 | 22.9 | NA | NA |
Eighteen patients received a total of 79 cycles. Data shown are from 78 cycles, as the sample from one cycle was not available. Samples were obtained prior to each treatment cycle of suramin plus docetaxel on day 1, and prior to each treatment on days 1 and 8 of each cycle of suramin plus gemcitabine. bFGF level was measured using ELISA assay; the lower detection limit was 2.5 pg/ml
NA not applicable
Antitumor activity
Relevant details pertaining to the antitumor effects of the combination of suramin with either docetaxel or gemcitabine, according to the initial randomized treatment regimen, are illustrated in Table 4. Among the 16 patients evaluable for response, 11 (60%) had discernable antitumor activity. Two patients had partial response and nine had stable disease. The first patient with PR was a 47-year-old female, never smoker, with adenocarcinoma who achieved PR after 7 months on treatment in this study protocol and maintained PR for 2.3 months before progressing and survived 13 months from the time of study enrollment. She received suramin and docetaxel for 3 cycles, followed by suramin and gemcitabine for 8 cycles. The second patient was a 73-year-old female, previous 50 pack-year smoker, with adenocarcinoma who achieved PR after 3 months on treatment and maintained PR for 4.7 months and survived 12.4 months from the time of study enrollment. She received suramin and docetaxel for 3 cycles, followed by gemcitabine for 6 cycles. The EGFR and Kras mutation status are not known for these two patients.
Table 4.
Best tumor response according to RECIST
| Sur + Doc 75 mg/m2 | Sur + Doc 56 mg/m2 | Sur + Gem 1,250 mg/m2 | Total | |
|---|---|---|---|---|
| Evaluable for responsea | ||||
| No. of assessable patients | 5 | 6 | 5 | 16 |
| Progressive disease | 0 | 3 | 2 | 5 |
| Partial response (PR) | 1 | 1 | 0 | 2 |
| Time to PR (mo) | 6 | 3 | NA | NA |
| Duration of PR (mo) | 2.3 | 4.7 | NA | NA |
| Stable disease (SD) | 4 | 2 | 3 | 9 |
| Evaluable for time to progression and overall survivala | ||||
| Assessable patients | 6 | 6 | 6 | 18 |
| Median time to progression in months | 3.5 | 3.5 | 4.0 | 3.7 95% CI (2.5,5.4) |
| Median overall survival in months | 7.9 | 11.5 | 5.6 | 9.3 95% CI (4.2,13.5) |
Sixteen of 18 patients completed ≥2 cycles and were evaluable for response. All 18 patients were evaluable for time to progression and overall survival
All 18 patients were evaluable for disease progression and survival. For all patients treated, the median TTP and median overall survival (OS) were 3.7 months [95% CI (2.5,5.4)] and 9.3 months [95% CI (4.2,13.5)], respectively. The TTP and OS according to initial randomization to docetaxel or gemcitabine are noted in Table 3. Four patients who received only gemcitabine plus suramin showed a median overall survival of 4.1 months (range 2–6.8 months), while the remaining 14 patients who received at least one cycle of docetaxel plus suramin showed a median overall survival of 11.8 months. The 1-year survival rate was 33% for all 18 patients (50% for the 14 patients who received at least one treatment of docetaxel plus suramin and 0% for the 4 patients who received only gemcitabine plus suramin). The longest survivor was a 56-year-old female, previous 30 pack-year smoker, with adenocarcinoma previously treated with carboplatin, paclitaxel, and suramin, who lived 26.4 months from the time of study enrollment. She received suramin plus gemcitabine for 3 cycles, followed by suramin plus docetaxel for 3 cycles, and obtained SD as her best response on study treatment. She was taken off study due to disease progression and subsequently received treatment with gefitinib for 7 months, pemetrexed for 6 months, erlotinib for 5 months, and temozolomide for 1 month (for recurrent brain metastasis). Her EGFR and Kras mutational status are not known.
Discussion
The treatment of advanced lung cancer remains challenging, despite important therapeutic advancements in the past decade. Although the utility of single agent suramin as a cytotoxic agent has been limited by severe toxicities observed at the high concentrations necessary for antitumor activity, our previous data in chemotherapy-naïve and chemo-refractory patients with advanced NSCLC have suggested that at lower and non-cytotoxic doses, suramin may be an effective chemosensitizer without adding to the toxicity profile of carboplatin and paclitaxel [37, 38]. The present study investigated the feasibility of suramin as a modulator of docetaxel and gemcitabine in previously treated patients with NSCLC.
This study showed that the combination of non-cytotoxic doses of suramin with either docetaxel or gemcitabine was generally well-tolerated and easy to administer in the outpatient setting. The hematologic and non-hematologic toxicities were consistent with those previously reported with either docetaxel or gemcitabine alone. The non-hematologic toxicities were generally transient and manageable. The electrolyte abnormalities noted in this study were generally without associated clinical sequelae and short-lived. The mechanism for these abnormalities is not clear. We did not observe any severe neurologic, renal, or dermatologic toxicity which have previously been reported with cytotoxic doses of suramin [25–29].
Suramin has complex pharmacology, including an extremely long half-life of 30 to 50 days. Our group has previously validated the use of a nomogram to guide appropriate dosing of suramin [39]. The pharmacokinetic data confirm that the dosing nomogram correctly predicted the targeted suramin concentration in 90% of all treatment cycles and demonstrate that the use of this nomogram is feasible in the outpatient setting.
Although it is beyond the scope of this phase I feasibility study, there was some preliminary evidence of antitumor activity of the combination of non-cytotoxic suramin with either docetaxel or gemcitabine in patients with platinum-refractory NSCLC. Eleven of sixteen (69%) patients evaluable for response attained SD and PR. Patients who received at least one cycle of docetaxel plus suramin showed a longer OS (11.8 vs. 4.1 months) and higher 1-year survival rate (50 vs. 0%), compared to patients who received only gemcitabine plus suramin. Patients who had progression on prior paclitaxel therapy derived benefit from treatment with docetaxel and suramin. In these patients, the overall survival time observed was 11.6 months. Another interesting observation was that patients who previously received suramin in an earlier trial had longer median overall survival (18.9 months) compared with patients who did not receive any prior suramin therapy (9.3 months).
Our preclinical findings that FGF induces chemoresistance [31–35] have led to the current evaluation of non-cytotoxic suramin, a non-specific inhibitor of FGF signaling, as a chemosensitizer in patients with NSCLC. The collection of plasma bFGF levels in this study was exploratory, and no firm conclusions can be derived from the data observed. Studies to evaluate the combined data from this study and a separate trial in pretreated breast cancer patients are ongoing. The actual mechanism of interaction between fibroblast growth factors and suramin is unknown and more complicated than initially thought. Additional FGF-independent mechanisms are also likely.
In conclusion, non-cytotoxic doses of suramin can safely be combined with docetaxel 56 mg/m2 or gemcitabine 1,250 mg/m2 in previously treated patients with NSCLC. The manageable toxicity profile, predictable pharmacokinetics, and early evidence of antitumor activity encourage further evaluation of the combination of suramin with docetaxel or gemcitabine in future efficacy trials.
Acknowledgments
This study was supported by the National Institutes of Health Research Project Cooperative Agreement U01 CA 76576 (PI, Grever) and Research Project Grant R01 CA 93871 (PI, Au). Au and Wientjes have been awarded patents on the use of suramin as a chemosensitizer.
Footnotes
Preliminary results of this study were presented in part at the following meetings:
Abstract 1: American Society of Clinical Oncology Annual Meeting, May 13–17, 2005, Orlando, FL. Modulation of chemotherapy resistance with low dose suramin in refractory non-small cell lung cancer (NSCLC) patients: A phase I study of sequential non-cross resistant chemotherapy. Citation: Journal of Clinical Oncology, 2005 ASCO Annual Meeting Proceedings. Vol 23, No. 16S, Part I of II (June 1 Supplement), 2005: 2104. Authors: T. Olencki, G. Wientjes, G. Otterson, T. Bekaii-Saab, A. Grainger, T. Yeh, R. Jensen, D. Young, J. Au, M. Villalona-Calero.
Abstract 2: Twelfth World Conference on Lung Cancer, September 2–6, 2007, Seoul, South Korea. A Phase I Study of Nontoxic Suramin As A Chemosensitizer In Pretreated/Refractory Non-Small Cell Lung Cancer (NSCLC) Patients: P2-230 [Poster Abstracts: NSCLC: Cytotoxic Chemotherapy: NSCLC: Cytotoxic Chemotherapy.]. Citation: Journal of Thoracic Oncology: Vol 2(8) Supplement 4 August 2007 pp S663–4. Authors: J. Au, T. Olencki, M. Wientjes, G. Otterson, T. Bekaii-Saab, A. Grainger, T. Yeh, R. Jensen, D. Young, M. Villalona-Calero.
Contributor Information
Elaine T. Lam, Department of Internal Medicine, Division of Hematology and Oncology, The Ohio State University, Columbus, OH, USA
Jessie L.- S. Au, College of Pharmacy, The Ohio State University, Columbus, OH, USA
Gregory A. Otterson, Department of Internal Medicine, Division of Hematology and Oncology, The Ohio State University, Columbus, OH, USA
M. Guillaume Wientjes, College of Pharmacy, The Ohio State University, Columbus, OH, USA.
Ling Chen, College of Pharmacy, The Ohio State University, Columbus, OH, USA.
Tong Shen, College of Pharmacy, The Ohio State University, Columbus, OH, USA.
Yong Wei, College of Pharmacy, The Ohio State University, Columbus, OH, USA.
Xiaobai Li, Center for Biostatistics, The Ohio State University, Columbus, OH, USA.
Tanios Bekaii-Saab, Department of Internal Medicine, Division of Hematology and Oncology, The Ohio State University, Columbus, OH, USA.
Anthony J. Murgo, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, The National Cancer Institute, Bethesda, MD, USA
Rhonda R. Jensen, Department of Internal Medicine, Division of Hematology and Oncology, The Ohio State University, Columbus, OH, USA
Michael Grever, Department of Internal Medicine, Division of Hematology and Oncology, The Ohio State University, Columbus, OH, USA.
Miguel A. Villalona-Calero, Email: Miguel.Villalona@osumc.edu, Department of Internal Medicine, Division of Hematology and Oncology, The Ohio State University, Columbus, OH, USA, Department of Pharmacology, The Ohio State University, Columbus, OH, USA, Arthur G. James Cancer Hospital, The Ohio State University, B406 Starling-Loving Hall, 320 West 10th Avenue, Columbus, OH 43210-1240, USA.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a southwest oncology group trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 5.Weick JK, Crowley J, Natale RB, et al. A randomized trial of five cisplatin-containing treatments in patients with metastatic non-small cell lung cancer: a southwest oncology group study. J Clin Oncol. 1991;9:1157–1162. doi: 10.1200/JCO.1991.9.7.1157. [DOI] [PubMed] [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Cardenal F, Lopez-Cabrerizo MP, Anton A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small cell lung cancer. J Clin Oncol. 1999;17:12–18. doi: 10.1200/JCO.1999.17.1.12. [DOI] [PubMed] [Google Scholar]
- 8.Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic nonsmall cell lung cancer. J Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 9.Crinò L, Mosconi AM, Scagliotti G, et al. Gemcitabine as second-line treatment for advanced non-small-cell lung cancer: a phase II trial. J Clin Oncol. 1999;17:2081–2085. doi: 10.1200/JCO.1999.17.7.2081. [DOI] [PubMed] [Google Scholar]
- 10.Van Putten JW, Baas P, Codrington H, et al. Activity of single-agent gemcitabine as second-line treatment after previous chemotherapy or radiotherapy in advanced non-small cell lung cancer. Lung Cancer. 2001;33:289–298. doi: 10.1016/s0169-5002(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 11.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 12.Dancey J, Shepherd FA, Gralla RJ, Kim YS. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer. 2004;43:183–194. doi: 10.1016/j.lungcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 14.Hawking F. Suramin: with special reference to onchocerciasis. Adv Pharmacol Chemother. 1978;15:289–322. doi: 10.1016/s1054-3589(08)60486-x. [DOI] [PubMed] [Google Scholar]
- 15.Hosang M. Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem. 1985;29:265–273. doi: 10.1002/jcb.240290310. [DOI] [PubMed] [Google Scholar]
- 16.Garrett JS, Coughlin SR, Niman HL, et al. Blockade of autocrine stimulation in simian sarcoma virus transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci USA. 1984;81:7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak M, Richard M. Suramin blockade of insulin-like growth factor I-stimulated proliferation of human osteosarcoma cells. J Natl Cancer Inst. 1990;82:1349–1352. doi: 10.1093/jnci/82.16.1349. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CW, Lui R, Fanta P, Salmon SE. Effects of suramin on in vitro growth of fresh human tumors. J Natl Cancer Inst. 1992;84:489–494. doi: 10.1093/jnci/84.7.489. [DOI] [PubMed] [Google Scholar]
- 19.Rubio GJ, Pinedo HM, Virizuela J, et al. Effects of suramin on human lung cancer cell lines. Eur J Cancer. 1995;31A:244–251. doi: 10.1016/0959-8049(94)00444-a. [DOI] [PubMed] [Google Scholar]
- 20.Rosen PJ, Mendoza EF, Landaw EM, et al. Suramin in hormone-refractory metastatic prostate cancer: a drug with limited efficacy. J Clin Oncol. 1996;14:1626–1636. doi: 10.1200/JCO.1996.14.5.1626. [DOI] [PubMed] [Google Scholar]
- 21.Small EJ, Meyer M, Marshall ME, et al. Suramin therapy for patients with symptomatic hormone-refractory prostate cancer: results of a randomized phase III trial comparing suramin plus hydrocortisone to placebo plus hydrocortisone. J Clin Oncol. 2000;18:1440–1450. doi: 10.1200/JCO.2000.18.7.1440. [DOI] [PubMed] [Google Scholar]
- 22.Mirza MR, Jakobsen E, Pfeiffer P, et al. Suramin in non-small cell lung cancer and advanced breast cancer. Two parallel phase II studies. Acta Oncol. 1997;36:171–174. doi: 10.3109/02841869709109226. [DOI] [PubMed] [Google Scholar]
- 23.Falcone A, Pfanner E, Cianci C, et al. Suramin in patients with metastatic colorectal cancer pretreated with fluoropyrimidine-based chemotherapy. A phase II study. Cancer. 1995;75:440–443. doi: 10.1002/1097-0142(19950115)75:2<440::aid-cncr2820750205>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Dreicer R, Smith DC, Williams RD, See WA. Phase II trial of suramin in patients with metastatic renal cell carcinoma. Invest New Drugs. 1999;17:183–186. doi: 10.1023/a:1006331518952. [DOI] [PubMed] [Google Scholar]
- 25.La Rocca RV, Meer J, Gilliatt RW, et al. Suramin-induced polyneuropathy. Neurology. 1990;40:954–960. doi: 10.1212/wnl.40.6.954. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Weiss RE, Vogelzang NJ, et al. Mineralocorticoid insufficiency due to suramin therapy. Cancer. 1996;78:2411–2420. doi: 10.1002/(sici)1097-0142(19961201)78:11<2411::aid-cncr20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.May E, Allolio B. Fatal toxic epidermal necrolysis during suramin therapy. Eur J Cancer. 1991;27:1338. doi: 10.1016/0277-5379(91)90118-w. [DOI] [PubMed] [Google Scholar]
- 28.Horne MK, III, Wilson OJ, Cooper M, et al. The effect of suramin on laboratory tests of coagulation. Thromb Haemost. 1992;67:434–439. [PubMed] [Google Scholar]
- 29.Figg WD, Cooper MR, Thibault A, et al. Acute renal toxicity associated with suramin in the treatment of prostate cancer. Cancer. 1994;74:1612–1614. doi: 10.1002/1097-0142(19940901)74:5<1612::aid-cncr2820740519>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Song S, Wientjes MG, Gan Y, Au JL. Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc Natl Acad Sci USA. 2000;97:8658–8663. doi: 10.1073/pnas.140210697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Song S, Yang F, Au JL, Wientjes MG. Nontoxic doses of suramin enhance activity of doxorubicin in prostate tumors. J Pharmacol Exp Ther. 2001;299:426–433. [PubMed] [Google Scholar]
- 32.Song S, Wientjes MG, Walsh C, Au JL. Nontoxic doses of suramin enhance activity of paclitaxel against lung metastases. Cancer Res. 2001;61:6145–6150. [PubMed] [Google Scholar]
- 33.Lu Z, Wientjes TS, Au JL. Nontoxic suramin treatments enhance docetaxel activity in chemotherapy-pretreated non-small cell lung xenograft tumors. Pharm Res. 2005;22:1069–1078. doi: 10.1007/s11095-005-6038-1. [DOI] [PubMed] [Google Scholar]
- 34.Gan Y, Wientjes MG, Au JL. Expression of basic fibroblast growth factor correlates with resistance to paclitaxel in human patient tumors. Pharm Res. 2006;23:1324–1331. doi: 10.1007/s11095-006-0136-6. [DOI] [PubMed] [Google Scholar]
- 35.Behrens C, Yin HY, Lee JJ, et al. Immunohistochemical expression of basic fibroblast growth factor and fibroblast growth factor receptors 1 and 2 in the pathogenesis of lung cancer. Clin Cancer Res. 2008;14:6014–6022. doi: 10.1158/1078-0432.CCR-08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Villalona-Calero MA, Wientjes MG, Otterson GA, et al. Phase I study of low-dose suramin as a chemosensitizer in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2003;9:3303–3311. [PubMed] [Google Scholar]
- 38.Villalona-Calero MA, Otterson GA, Wientjes MG, et al. Noncytotoxic suramin as a chemosensitizer in patients with advanced non-small cell lung cancer: a phase II study. Ann Oncol. 2008;19:1903–1909. doi: 10.1093/annonc/mdn412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Song SH, Wientjes MG, et al. Nontoxic suramin as a chemosensitizer in patients: dosing nomogram development. Pharm Res. 2006;23:1265–1274. doi: 10.1007/s11095-006-0165-1. [DOI] [PubMed] [Google Scholar]
- 40.Walsh CT, Wei Y, Wientjes MG, Au JL. Quantitative image analysis of intra-tumoral bFGF level as a molecular marker of paclitaxel resistance. J Transl Med. 2008;6(4) doi: 10.1186/1479-5876-6-4. Published online 2008 January 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 42.Kassack M, Nickel P. Rapid, highly sensitive gradient narrow-bore high-performance liquid chromatographic determination of suramin and its analogues. J Chromatogr B Biomed Appl. 1996;686:275–284. doi: 10.1016/s0378-4347(96)00214-9. [DOI] [PubMed] [Google Scholar]
- 43.Eli Lilly and Company. Gemcitabine (Gemzar®) Prescribing Information. 1996 and 2007. [Google Scholar]
- 44.Sanofi-Aventis US LLC. Docetaxel (Taxotere®) Prescribing Information. 2008. [Google Scholar]