Abstract
BACKGROUND
The need of an early and noninvasive diagnosis of AD requires the development of imaging-based techniques. As an alternative, the magnetic resonance image (MRI) relaxation time constant (T1ρ ) was measured in brains of Alzheimer’s disease (AD), mild-cognitive impairment (MCI), and age-matched controls in order to determine whether T1ρ values correlated with the neurological diagnosis.
METHODS
MRI was performed on AD (n = 48), MCI (n = 45), and age-matched control (n = 41), on a 1.5 Tesla Siemens clinical MRI scanner. T1ρ maps were generated by fitting each pixel’s intensity as a function of the duration of the spin-lock pulse. T1ρ values were calculated from the gray matter (GM) and white matter (WM) of medial temporal lobe (MTL).
RESULTS
GM and WM T1ρ values were 87.5 ± 1.2 ms and 80.5 ± 1.4 ms, respectively, in controls, 90.9 ± 1.3 ms and 84.1 ± 1.7 ms in MCI, and 91.9 ±.8 ms and 88.3 ± 1.3 ms in AD cohorts. Compared to control, AD patients showed 9% increased WM T1ρ and 5% increased GM T1ρ. Compared to control, MCI individuals showed 4% increased T1ρ both in WM and GM. A 5% increased T1ρ was found in WM of AD over MCI.
CONCLUSION
The increased T1ρ in WM and GM of MTL in AD may be associated with the pathological changes that are not evident on conventional MRI.
Keywords: Alzheimer’s disease, medial temporal lobe, MRI, T1ρ
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder associated with the disturbance of neuronal function and gradual deterioration in cognition, function, and behavior. AD is the most common cause of dementia in elderly, affecting approximately 2–4 millions individuals in the United States and more than 30 millions worldwide.1 The pathological characteristics of AD are the presence of intracellular neurofibrillary tangles (NFTs) and the extracellular deposition of amyloid-beta (Aβ) plaques.2,3 The formation of NFTs and Aβ plaques result in cellular damage as well as brain tissue loss commonly described as brain atrophy. The rates of brain atrophy within AD are most typically reported to amount to 2% per year with substantial variability among populations studied.4
The degenerative process in the AD brain starts decades (10–20 years) before the clinical onset of disease.5 During this phase, plaque and tangle loads increase and after a certain threshold the first neurological symptoms become evident. Because several pharmaceutical agents along with new agents undergoing clinical trials are available for the treatment of AD,6 the current consensus statements have emphasized the need of an early and noninvasive diagnosis of AD. The increasing demand for a noninvasive measurement has driven the development of imaging-based methods. MRI has been shown to be the most powerful noninvasive technique over other imaging techniques, such as positron emission tomography (PET). Although it has the potential to enable identification of more subtle pathological changes earlier during the disease course, the wide clinical research utility of PET is hampered by its poor resolution and requirement for the injection of radioactive tracers.
MRI differentiates normal from pathological tissues based on differences in tissue relaxation times T1 and T2. However, changes in these relaxation time parameters have not identified any significant differences in clinical AD.7 An alternate magnetic resonance (MR) contrast mechanism is T1ρ the spin lattice T1ρ in the rotating frame, which generates a different type of contrast than that seen with conventional relaxation times (T1 and T2).8 T1ρ relaxation phenomena in biological tissues have been extensively studied and shown to be sensitive to physio-chemical processes (eg, spin–spin interaction, chemical exchange). In proteins and biological tissues, exchange between protons in different environments contributes to T1ρ relaxation. Prominent exchange mechanisms may be classified as exchange between (i) water molecules in bulk and hydration water on proteins, (ii) hydration water and –OH and –NH protons on proteins (exchange between two sites), and (iii) H217O and H216O molecules (scalar relaxation). Low-frequency interactions (between macromolecules and bulk water) and changes in macromolecular content can be quantified by spatially mapping the T1ρ relaxation times.8 T1ρ MRI has been widely used to characterize breast cancer tissue, monitor the level of cartilage degeneration, and delineate brain tumors.9–11
Previously, T1ρ MRI has been used to measure the T1ρ relaxation times in normal brain and it was found that T1ρ showed a greater range of values compared to T2.8 Quantitative estimation of T1ρ in brain provides a new way to monitor the disease progression longitudinally with or without potential therapies. Our group previously reported higher medial temporal lobe (MTL) T1ρ in AD and MCI compared to control, however, the number of patients in their study was small (AD = 14, MCI = 11, and controls = 16).12 Since then, we measured T1ρ in the hippocampus and showed significantly higher values in AD patients compared to MCI and control.13 The hippocampus has been shown to be primarily affected by AD pathology followed by temporal lobe. Therefore, the current clinical study performed T1ρ measurements in MTL to determine if T1ρ can discriminate AD from MCI and control in this region as well using the significantly higher number of patients.
Materials and Methods
Subject
The Institutional Review Board of University of Pennsylvania approved the study protocols. In this study, we included 48 AD patients (mean age ± SD = 76.8 ± 9.1 years), 45 MCI patients (mean age ± SD = 71.93 ± 8.7 years), and 41 age-matched controls (mean age ± SD = 70.2 ± 9.4 years). All patients underwent a standardized clinical assessment including medical history, physical and neurological examination, psychometric evaluation, and brain MRI. The Mini-Mental State Examination (MMSE) was used as a measure of general cognitive function. Diagnoses were made in conference by a team of neurologist, neuropsychologists, a neurophysiologist, and a psychiatrist. Diagnoses of MCI was made according to the Petersen criteria for MCI14 and the National Institute of Neurological and Communicative Diseases and Stroke/AD and Related Disorders Association criteria (NINCDS–ADRDA) for probable AD.15 Patients were excluded if they had a history of irritable bowel syndrome, chronic diarrhea, peptic ulcer, or gastroesophageal reflux disease; a history of cardiac disease; significant electrocardiographic abnormalities; hematologic disorders; hepatic or renal disease; active malignancy within 5 years; or clinically important depressive, neuropsychiatric, cerebrovascular, or respiratory disease.
MRI Protocol
All these patients underwent a standard MRI protocol on a 1.5 Tesla Siemens Sonata clinical scanner (Siemens Medical Solutions USA Inc., Malvern, PA) using the vendor-supplied head coil. The written informed consent was obtained from each patient before they underwent for MRI. For T1ρ -weighted images, a fluid-attenuated T1ρ preencoded Turbo Spin-Echo pulse sequence was used. The imaging parameters were: TE/TR = 12/2,000 ms, TSL (duration of spin-lock pulse) = 10, 20, 30, and 40 ms, with a spin-lock frequency of 500 Hz, slice thickness = 2 mm, field of view (FOV) = 22 cm, matrix size = 256 × 128, bandwidth = 130 Hz/pixel, echo train length = 4 for a total imaging time of 6 minute for four images. The inversion time was fixed at 860 ms to remove the contribution of the cerebrospinal fluid to the T1ρ maps. An oblique coronal T1ρ -weighted image of a slice perpendicular to the anterior–posterior commissure plane was obtained. The slice was chosen to include the head of the hippocampus. Immediately after T1ρ MRI, the entire volume of each subject’s brain was imaged in the coronal plane using a T1-weighted 3-dimensional volumetric magnetization-prepared rapid acquisition gradient echo (MPRAGE) pulse sequence with 124 continuous slices. The parameters were TR/TE = 3.5 ms/3,000 ms, slice thickness = 1.2 mm, FOV of 24 cm and 192 phase encode steps, and flip angle = 8° for a total imaging time of 10 minute.
Data Processing
T1ρ maps were generated by fitting each pixel’s intensity as a function of the duration of the spin-lock pulse (TSL) by a linear least-square algorithm.8 Pixels whose intensities correlated poorly (R2 < .95) with the fitting equation were set to zero. Pixels outside of the brain were also set to zero. The images were transferred to a Dell PC computer (Dell Inc., Austin, TX) and images were processed in the MatLab (The MathWorks Inc., Natick, MA) programming language. T1ρ values were calculated from the gray matter (GM) and white matter (WM) of right and left MTL. For GM and WM segmentation, a previously developed method was used to partition the volumetric MPRAGE scans into 92 region of interests (ROIs) incorporating all major cortical and subcortical regions.16 This method deforms MRI scans of different brains into anatomical coregistration with each other, and into coregistration with a standardized template. The template’s labels are then transformed to individual scans by applying the elastic transformation that was found to coregister the respective images. For quantitative analysis the 4 ROIs were defined on T1ρ images, ie, left and right MTL, WM and GM. A program written in IDL was used to automatically calculate T1ρ values only from pixels that were classified as GM and WM located either in left or right MTL.
Statistical Analysis
All the statistical computations were performed using the SPSS (SPSS Inc., Chicago, IL). T1ρ values from the left and right side were averaged for both GM and WM. Descriptive statistics were performed to calculate the mean value of T1ρ from GM and WM for different groups (controls, MCI, and AD). ANOVA Bonferroni post hoc multiple comparisons was performed in order to compare T1ρ among the different groups. A P-value of less than .05 was considered to be statistically significant. Pearson correlation between T1ρ values versus age and T1ρ values versus MMSE scores was performed both for GM and WM separately for three cohorts.
Results
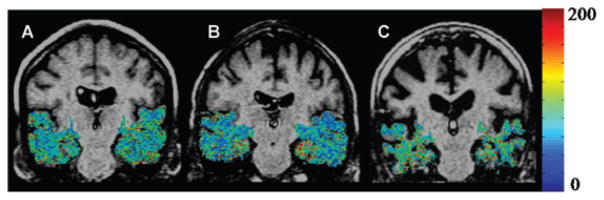
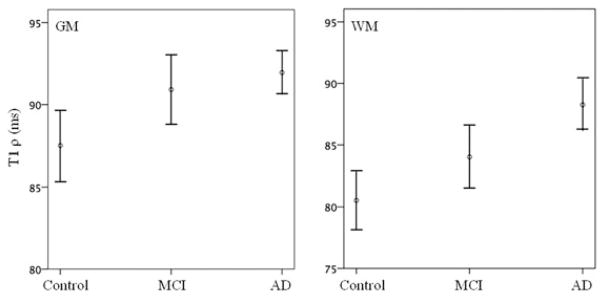
The mean MMSE scores in control, MCI, and AD were 29.213 ± 1.24, 24.98 ± 2.15, and 19.24 ± 4.68, respectively. Figure 1 shows T1ρ maps in MTL of control, MCI, and AD. Higher T1ρ pixels (in red) were found in the AD subjects compared to control and MCI. AD patients also showed a greater degree of sulcal space, which was more prominent with increasing age, suggestive of higher brain atrophy (Fig 1C). T1ρ values in GM and WM of controls, MCI, and AD are reported in Table 1 and shown in Figure 2. The ANOVA showed that T1ρ values were significantly changed (GM, P <.05: WM, P <.001) among the groups. Bonferroni multiple comparisons showed that T1ρ was significantly increased both in GM and WM of AD patients compared to control. In MCI individuals, no significant difference in T1ρ value was observed compared to control and AD (Table 2). T1ρ was increased by 9% in WM whereas it was increased by 5% in GM of AD over control. In MCI, a 4% increase in T1ρ was present both in GM and WM compared to control. However, a 5% increase in T1ρ was found in WM of AD over MCI but it did not reach to the statistical significant level.
Fig 1.
T1ρ maps of the medial temporal lobe (MTL) region in the brain (in color) overlaid on fluid-attenuated T1ρ MRI of control (70 Y female, A), MCI (69 Y male, B), and AD patient (84 Y female, C). Pixels with higher T1ρ (red) are more prominent in MTL of AD patient. Increase in sulcal space in AD patients suggesting greater degree of brain atrophy. A lack of signal from CSF implies that the higher T1ρ values are not due to fluid.
Table 1.
T1ρ Values in MTL of the Control, MCI, and AD
| Diagnosis | Number | GM T1ρ (mean ± SE) | WM T1ρ (mean ± SE) |
|---|---|---|---|
| Controls | 41 | 87.5 ± 1.2 | 80.5 ± 1.4 |
| MCI | 45 | 90.9 ± 1.3 | 84.1 ± 1.7 |
| AD | 48 | 91.9 ± 0.8 | 88.3 ± 1.3 |
| ANOVA (P ) | .013* | .001* |
GM = gray matter; WM = white matter; MCI = mild cognitive impairment; AD = Alzheimer disease; ANOVA = analysis of variance.
P indicates value at significant level.
Fig 2.
The error bars (at 95% confidence interval) show mean T1ρ value in control, MCI, and AD cohorts in gray matter (GM) and white matter (WM). A 9% increase in T1ρ value was present in WM, while it was only 5% in GM in AD over control. In MCI, both GM and WM showed a 4% increase in T1ρ over control.
Table 2.
Bonferroni Multiple Comparisons among Different Groups
| Region | Group | Group | Mean Difference (T1ρ) | P-Value | 95% CI |
|
|---|---|---|---|---|---|---|
| LB | UB | |||||
| GM | Control versus | MCI | −3.41 | .120 | −7.39 | .58 |
| Control versus | AD | −4.45 | .013* | −8.14 | −75 | |
| MCI versus | AD | −1.04 | .948 | −4.86 | 2.79 | |
| WM | Control versus | MCI | −3.53 | .266 | −8.54 | 1.47 |
| Control versus | AD | −7.74 | .000* | −12.38 | −3.09 | |
| MCI versus | AD | −4.20 | .107 | −9.00 | .60 | |
GM = gray matter; WM = white matter; MCI = mild cognitive impairment; AD = Alzheimer’s disease; CI = confidence interval.
P indicates value at significant level.
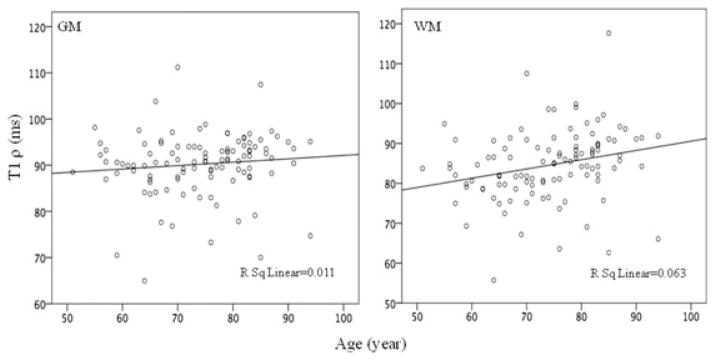
Five control patients showed significantly increased T1ρ (10–15 ms) in both GM and WM. We will follow these patients for the next 3 years to correlate these early changes in T1ρ with the cognitive normal and MCI who might have the probability to develop AD in future. Further, we examined the age-related changes in T1ρ value. None of the group (control, MCI, and AD) showed any age-dependent changes in T1ρ value in either GM or WM (Fig 3). On correlating the MMSE score with T1ρ we did not find any significant correlation in any of the group (control [r = −.129, P = .378], MCI [r = −.101, P = .487], and AD [r = −.084, P = .581]).
Fig 3.
Plot of T1ρ in gray matter (GM) and white matter (WM) with age of the subjects. No significant correlation was found between T1ρ and age in either of the tissue.
Discussion
T1ρ provides information about the low-frequency interactions (100 Hz to a few kHz) in biological systems. In biological tissues, the T1ρ relaxation may have contributions from several interactions. The interactions that are studied using this methodology can be broadly categorized into (i) dipole–dipole, (ii) chemical exchange, and (iii) scalar-coupling processes. Depending upon the tissue type, more than one mechanism may be operative simultaneously but with different relative contributions.17 In biological tissues, frequency dependence of relaxation rates or relaxation dispersion may arise from (i) rotational motion of a fraction of water bound to proteins, (ii) exchange of protons on macromolecules with bulk water, and (iii) the non-averaged residual dipolar interaction of spin associated with oriented macromolecules in the tissue. It is possible for T1ρ to probe protein content in an indirect manner in living tissues. In this study, both the WM and GM showed significantly higher T1ρ in MTL of AD compared to control. The MCI individuals showed a nonsignificantly increased T1ρ in WM and GM of MTL compared to control, whereas it was nonsignificantly decreased compared to AD.
Nonsignificant difference in T1ρ between AD and MCI might be due to the onset of AD-like pathology in MCI individuals. Also, the possibility of variation in T1ρ data cannot be ruled out. In other words, some MCI individuals may have AD-like pathology resulting in increased T1ρ without significant cognitive impairment to be classified as AD by a neurologist. Similarly, five cognitive controls that exhibited higher T1ρ values may have a greater probability to convert into the MCI or AD in future. We speculate that a follow-up study may provide the exact nature of the disease progression in these groups (AD, MCI, and controls). This study will follow these patients for the next 3 years to correlate the early increase in T1ρ with cognitive impairment.
In this study, we observed that increase in T1ρ was higher in WM (9%) of AD patients whereas in case of MCI both WM (4%) and GM (4%) showed similar trend. This suggests that individual with higher changes in T1ρ value in WM may have more probability to convert into AD in future. This was also supported by an increased T1ρ (5%) in WM of AD patients over MCI.
Atrophy of the MTL on MRI has been found to be an early and sensitive marker for AD and is assumed to reflect underlying neuronal loss of the hippocampus and the temporal lobe.18 However, atrophy may also be present in other types of dementia and absence of medial temporal lobe atrophy (MTA) does not exclude the diagnosis AD especially in the early stages. Any normal age-related brain atrophy may also confound a specific diagnosis of AD. In this study, the lack of correlation between age and T1ρ value suggests that the discrimination of cohorts based on T1ρ is due to an underlying pathology instead of any normal age-related changes in brain. No significant correlation between MMSE score and T1ρ further suggests that these two markers may be independent to each other in providing the nature of disease. However, the specificity of T1ρ to plaque burden or to other AD pathology remains to be determined.
Although high-resolution MRI can be used to image Aβ-plaques in transgenic mice model of AD or brain-tissue specimens, it is currently not possible to achieve this resolution in the clinical settings.19 However, by incorporating the measurement of T1ρ relaxation time into the current clinical MRI acquisition protocols, it may possible to measure the changes in T1ρ due to the presence of AD-related pathology. We suggest that it is better to follow-up an individual with T1ρ MR imaging during the disease course and measure changes in T1ρ.
In this study, the measurement of T1ρ was done by using the 2-dimensional T1ρ -weighted MRI pulse sequence which is limited to a single-slice acquisition since the SL pulse cannot be made slice selective. We have developed and implemented a 3-dimensional T1ρ -weighted pulse sequence on a 1.5 Tesla Siemens clinical scanner.20 T1ρ values measured with this sequence agree with those obtained by a previously validated 2-dimensional T1ρ -imaging sequence.8 Furthermore, the 3-dimensional images will allow for direct comparison of T1ρ values and brain atrophy rates in several regions of the brain, such as the entire hippocampus and entorhinal cortex.
Conclusions
The high T1ρ in MTL in brain of AD patients can be used as a discriminator from other groups (MCI and control). A follow-up study may provide additional information to use T1ρ as a predictor of AD pathology. Furthermore, T1ρ MRI might be used as a biological marker to test the efficacy of putative therapeutic agents in clinical trials in humans in addition to neurological testing, as well as in preclinical trials in transgenic animal models of AD.
Acknowledgments
This work was performed at an NIH supported resource center (NIH RR02305) and from a grant from the Pennsylvania State Tobacco Settlement.
References
- 1.Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. Radiology. 2003;226:315–336. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- 2.Nagy Z, Esiri MM, Joachim C, et al. Comparison of pathological diagnostic criteria for Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:182–189. doi: 10.1097/00002093-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 4.Schott JM, Price SL, Frost C, et al. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology. 2006;66:233–235. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- 5.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Doraiswamy PM, Xiong GL. Pharmacological strategies for the prevention of Alzheimer’s disease. Expert Opin Pharmacother. 2006;7:1–10. doi: 10.1517/14656566.7.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Campeau NG, Petersen RC, Felmlee JP, et al. Hippocampal transverse relaxation times in patients with Alzheimer disease. Radiology. 1997;205:197–201. doi: 10.1148/radiology.205.1.9314985. [DOI] [PubMed] [Google Scholar]
- 8.Borthakur A, Wheaton AJ, Gougoutas AJ, et al. In vivo measurement of T1rho dispersion in the human brain at 1.5 tesla. J Magn Reson Imaging. 2004;19:403–409. doi: 10.1002/jmri.20016. [DOI] [PubMed] [Google Scholar]
- 9.Aronen HJ, Ramadan UA, Peltonen TK, et al. 3D spin-lock imaging of human gliomas. Magn Reson Imaging. 1999;17:1001–1010. doi: 10.1016/s0730-725x(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 10.Markkola AT, Aronen HJ, Ramadan UA, et al. Determination of T1rho values for head and neck tissues at 0.1 T: a comparison to T1 and T2 relaxation times. Magn Reson Imaging. 1998;16:377–383. doi: 10.1016/s0730-725x(98)00013-7. [DOI] [PubMed] [Google Scholar]
- 11.Santyr GE. MR imaging of the breast Imaging and tissue characterization without intravenous contrast. Magn Reson Imaging Clin N Am. 1994;2:673–690. [PubMed] [Google Scholar]
- 12.Borthakur A, Sochor M, Davatzikos C, et al. T1rho MRI of Alzheimer’s disease. Neuroimage. 2008;41:1199–1205. doi: 10.1016/j.neuroimage.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haris M, McArdle E, Fenty M, et al. Early marker for Alzheimer’s disease: hippocampus T1rho estimation. J Magn Reson Imaging. 2009;29:1008–1012. doi: 10.1002/jmri.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;5:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Davatzikos C, Fan Y, Wu X, et al. Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiol Aging. 2008;29:514–523. doi: 10.1016/j.neurobiolaging.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mäkelä HI, De Vita E, Gröhn OH, et al. B0 dependence of the on-resonance longitudinal relaxation time in the rotating frame (T1rho) in protein phantoms and rat brain in vivo. Magn Reson Med. 2004;51:4–8. doi: 10.1002/mrm.10669. [DOI] [PubMed] [Google Scholar]
- 18.Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology. 2008;71:1986–1992. doi: 10.1212/01.wnl.0000336925.79704.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Wengenack TM, Reyes DA, et al. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer’s transgenic mice. J Neurosci. 2005;25:10041–10048. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witschey WR, Borthakur A, Elliott MA, et al. T1rho-prepared balanced steady-state free precession for rapid 3D T1rho-weighted MRI. J Magn Reson Imaging. 2008;28:744–754. doi: 10.1002/jmri.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]