Abstract
Background
Electronic “cigarettes” (ECs) are marketed to tobacco users as potential reduced exposure products (PREPs), albeit with little information regarding EC user toxicant exposure and effects. This information may be obtained by adapting clinical laboratory methods used to evaluate other PREPs for smokers.
Methods
Thirty-two smokers participated in four independent Latin-square ordered conditions that differed by product: own brand cigarette, “NPRO” EC (18 mg cartridge), “Hydro” EC (16 mg cartridge) or sham (unlit cigarette). Participants took 10 puffs at two separate times during each session. Plasma nicotine and carbon monoxide (CO) concentration, heart rate, and subjective effects were assessed.
Results
Own brand significantly increased plasma nicotine and CO concentration and heart rate within the first five minutes of administration while “NPRO” EC, “Hydro” EC, and sham smoking did not. Own brand, “NPRO” EC, and “Hydro” EC (but not sham) significantly decreased tobacco abstinence symptom ratings and increased product acceptability ratings. The magnitude of symptom suppression and increased acceptability was greater for own brand than for “NPRO” EC and “Hydro” EC.
Conclusions
Under these acute testing conditions, neither of the ECs exposed users to measurable levels of nicotine or CO, though both suppressed nicotine/tobacco abstinence symptom ratings.
Impact
This study illustrates how clinical laboratory methods can be used to understand the acute effects of these and other PREPs for tobacco users. The results and methods reported here will likely be relevant to the evaluation and empirically-based regulation of ECs and similar products.
Keywords: Electronic cigarette, tobacco, cigarette, nicotine
Introduction
A variety of potential reduced exposure products (PREPs) are/were marketed to cigarette smokers with explicit or implied claims that their use is associated with less exposure to lethal smoke constituents (e.g., 1, 2, 3). These PREPs for smokers include products that involve burning specially cured tobacco that contains lower levels of some toxicants, that primarily heat rather than burn tobacco, or smokeless tobacco (e.g., 4, 5, 6). As has been argued many times (1, 7, 8), objective empirical evaluation of these products is critical as a means of determining the extent to which PREP use is associated with reduced toxicant exposure (e.g., nicotine, carbon monoxide or CO) and suppression of tobacco/nicotine abstinence symptoms. Clinical laboratory work has been very revealing, and sometimes demonstrates reduced toxicant exposure and often demonstrates a failure to suppress aversive abstinence symptoms fully (9, 10). In theory, toxicant exposure reduction may be associated with long-term decreased health risk, but this theoretical decreased risk is very unlikely with a product that is not used because it fails to suppress abstinence symptoms and/or is otherwise unacceptable.
So-called electronic “cigarettes” (ECs) are one of the newest types of PREPs available, and anecdotal evidence suggests that, at least for some smokers, ECs can completely replace tobacco cigarettes (e.g., 11, 12). Many EC brands are available in retail outlets and over the internet. ECs consist of a rechargeable battery, heater, and a cartridge that contains a liquid made of propylene glycol, nicotine, and other chemicals (13). When the battery-powered heater is activated, it heats the solution to produce a vapor that can then be inhaled by the user (14, 15). ECs are marketed as PREPs for smokers, with manufacturer claims such as “alternative to smoking that will satisfy your nicotine urges and cravings” (16), “helps smokers quit, cut down or smoke healthier” (17) and “gives smokers all the pleasure and satisfaction of traditional smoking without all the health, social and economic problems” (18). However, there are, to date, few objective data to substantiate these claims. Indeed, there is little objective information describing EC toxicant content and yield or user toxicant exposure and effect.
The little available data suggest that EC cartridges and vapors may contain trace amounts of impurities and tobacco specific nitrosamines (13, 19). EC cartridge nicotine content may be less than product labeling indicates, and the vapor produced from the cartridge may yield very little nicotine (13, 19). The scant data from human laboratory studies suggest that EC use is likely to involve little nicotine exposure and tobacco abstinence symptom suppression that is far less than that produced by a tobacco cigarette (20, 21). Existing clinical models that have been used to evaluate combustible and non-combustible PREPs (e.g., 10, 22, 23) might be adapted to provide quickly and efficiently necessary information regarding EC toxicant exposure and effect.
The purpose of this study was to describe clinical laboratory methods that could be used to characterize EC users’ nicotine and CO exposure, cardiovascular response, and ratings of tobacco/nicotine abstinence symptom suppression and product acceptability. Accordingly, this within-subject study used these outcomes to compare, in 32 tobacco cigarette smokers, the effect of two marketed EC brands with own brand cigarettes and sham smoking (i.e., puffing on an unlit cigarette). Based on preliminary data presented elsewhere (20, 21) we hypothesized that, compared to own brand, ECs would deliver less nicotine and no CO and would be less effective at reducing symptoms of tobacco abstinence while producing lower acceptability ratings.
Methods
Participants
This study was approved by the IRB of Virginia Commonwealth University and conducted in accordance with the Declaration of Helsinki. Sixty-six men and women recruited from the Richmond, VA area provided written, informed consent. Prior to participation in any experimental sessions, 18 individuals were disqualified based on health concerns (e.g., high blood pressure) or failure to meet other inclusion/exclusion criteria (see below). In addition, two participants withdrew from the study and another 14 were withdrawn due to failure to comply with study procedures (n = 9), poor venous access (n = 3), or because study enrollment was completed (n = 2). The remaining 32 participants (13 women; 18 white) completed the study proper and were included in the analyses. Participants smoked at least 15 cigarettes per day (M = 22 cigarettes per day; SD = 8.8), were between the ages of 18 to 55 (M = 33.6; SD = 12), provided an afternoon screening CO of at least 15 ppm (M = 23.5; SD = 8.8), and a urine cotinine result of at least four on a seven point scale (0-6; NicAlert, Nymox Corp., Maywood, NJ) (M = 5.9; SD = 0.2). Exclusion criteria included: self-reported history of any chronic mental or physical health condition, pregnancy or breastfeeding, active menopause, self-reported use of ECs, current smoking cessation attempt, current drug use (other than marijuana), or > 20 days self-reported marijuana or alcohol use in the past 30 days.
Study Design and Procedures
This study was conducted on an outpatient basis at the Clinical Behavioral Pharmacology Laboratory at Virginia Commonwealth University. Participants completed four laboratory sessions; each approximately 150 minutes in duration. One of four Latin-square ordered conditions was presented each session (separated by at least 48 hours) that differed by product administered: own brand (i.e. a lit cigarette of the participant’s preferred brand), sham (i.e. an unlit cigarette of the participant’s preferred brand), “NPRO” EC (NJOY, Scottsdale, AZ; 18 mg cartridge), and “Hydro” EC (Crown Seven, Scottsdale, AZ; 16 mg cartridge).
Participants were asked to refrain from cigarette smoking for at least 12 hours prior to their scheduled session. Smoking abstinence was verified upon arrival at the laboratory by an expired air CO level ≤ 10 ppm. At the start of a session, a heparinized catheter was inserted into a forearm vein, physiological monitoring equipment was attached and continuous physiological recording commenced. Thirty minutes after session onset, participants responded to the subjective effect questionnaires, 7 ml of blood was sampled, and product was administered. Product administration consisted of 10 puffs with a 30 second interpuff interval (IPI; puff number and IPI were monitored by study staff): package instructions state that ECs should be used similarly to a tobacco cigarette, and 10 puffs with a 30 second IPI approximates ad libitum cigarette smoking (26). Five, 15, 30 and 45 minutes after the first of 10 puffs of the initial product administration, participants responded to the subjective effect questionnaires and 7 ml of blood was sampled. Expired air CO was recorded at 15, 30 and 45 minutes. At 60 minutes, participants responded to the subjective effect questionnaires, 7 ml of blood was sampled and product was administered a second time (again, 10 puffs, 30 second inter puff interval). Five, 15, 30 and 45 minutes after the second product administration, participants responded to the subjective effects questionnaires and 7 ml of blood was sampled. Expired air CO was recorded at 15, 30 and 45 minutes. At session’s end, the venous catheter was removed, participants were compensated for their time and, if necessary, additional sessions were scheduled.
Materials
“NPRO” EC was purchased from NJOY in Scottsdale, AZ (24). The “NPRO” EC resembles a tobacco cigarette in length and diameter and consists of a disposable mouthpiece which houses a cartridge, a small heating element (vaporizer) that is activated when the user draws air through the device, and a rechargeable lithium battery. Cartridges come in a variety of flavors with varying nicotine content (0 to 18 mg). According to the NJOY website, cartridge ingredients include nicotine, propylene glycol, water, ethanol, glycerol, acetylpyrazine, guaiacol, mysomine, cotinine, and vanillin.
“Hydro” EC was purchased from Crown Seven in Scottsdale, AZ (25). “Hydro” EC is similar in composition to “NPRO” EC and, according to the manufacturer’s website cartridges also come in a variety of flavors and contain nicotine (0 to 16 mg), propylene glycol, water and tobacco flavoring.
For EC conditions, a new 16 (“Hydro” EC) or 18 (“NPRO” EC) mg nicotine cartridge was used and batteries were fully charged before each session. The flavor of each EC was matched to the participants’ usual brand of cigarettes (regular or menthol). Consistent with product instructions, participants were instructed to puff from the EC devices as they would a normal cigarette.
Participants’ usual brand of cigarette was used in the own brand and sham conditions. According to the Federal Trade Commission (FTC, 2001), on average, usual brand yield was 1.06 mg nicotine, 14.7 mg tar, and 14.6 mg CO (based on available data for 26 participants).
Outcome Measures
Physiological measures
Heart rate was measured every 20 seconds (Model 506, Criticare Systems, fitted with a reusable pulse oximeter sensor). Blood samples were centrifuged and plasma was stored at −70° C. Plasma samples were sent to the Bioanalytical Analysis Core Laboratories of Virginia Commonwealth University’s Department of Pharmaceutics and were analyzed for nicotine content using LC-MS-MS (limit of quantitation [LOQ] = 2.0 ng/ml, see 26 for details). Expired air CO was assessed using a BreathCO monitor (Vitalograph).
Subjective effect questionnaires
Participants responded to a computerized version of the Tiffany Drobes Questionnaire of Smoking Urges Brief (QSU Brief; 27). The questionnaire consists of 10 items rated from 0 (Strongly disagree) to 6 (Strongly agree). Items from this scale are loaded onto two previously validated factors; Factor 1 (intention to smoke) and Factor 2 (anticipation of relief from withdrawal).
Participants responded to three computerized questionnaires containing visual analog scale (VAS) items. Each word or phrase was centered above a horizontal line that represented a scale from 0 to 100 points; the left anchor was “Not at all” and the right anchor was “Extremely”. A mouse-controlled cursor produced a vertical mark on the line that could be adjusted by the participant. Scores on visual analog scale items were calculated as the distance between the vertical mark and the left anchor and expressed as a percentage of line length.
Eleven VAS items were used to assess nicotine/tobacco abstinence symptom suppression (see 28). The direct effects of nicotine were assessed using 10 VAS items sensitive to nicotine effects (29, 30). Participants also responded to 14 visual analog scale items which assessed the direct effects of tobacco.
Data Analyses
Heart rate values were averaged for five-minute periods prior to each product administration or blood sampling. One heart rate measurement was missing for one participant in one session and the missing value was replaced with an average of values before and after it. For plasma nicotine, values below the LOQ were replaced by the LOQ as in previous work (10, 31, 32).
We have reported interim findings of this study previously, in a letter that examined nicotine exposure, heart rate, and craving results (21). The first 16 participants were included in that analysis. Prior to analyzing the data from all 32 participants described here, we first compared results of the first group of 16 participants to those of the second group of 16 participants using a repeated measures analysis of variance with two within subjects factors: condition (4 levels) and time (10 levels for nicotine, subjective and HR data; 7 for CO data), and 1 between subjects factor: study group (first 16 or second 16). No significant main effects or interactions involving the study group factor were observed on any measure. Given this failure to observe any differences across the two groups, all data were combined. Thus, the data from all 32 participants for plasma nicotine, CO, heart rate, and subjective measures were analyzed using repeated measures analysis of variance with two factors: condition (4 levels) and time (10 levels for nicotine, HR data and subjective data; 7 levels for CO). Huynh-Feldt corrections were used to account for violations of sphericity. Tukey’s Honestly Significant Difference test (HSD) was used to examine differences between means (p < .05).
Results
Main effects and interactions for all measures are presented in Table 1. The primary results of interest are those for which a significant condition by time interaction were observed, indicating that changes over time observed on that measure depended upon product used in that condition.
Table 1.
Results of statistical analyses for all outcome measures.
| Measure | Condition F (p) |
Time F (p) |
Condition × Time F (p) |
|---|---|---|---|
| Heart rate a | 13.5 (> .001) | 62.0 (> .001) | 16.1 (> .001) |
| Plasma nicotine a | 112.2 (> .001) | 53.0 (> .001) | 45.2 (> .001) |
| Carbon monoxide b | 105.5 (> .001) | 107.0 (> .001) | 205.5 (> .001) |
| Hughes and Hatsukami a | |||
| Anxious | 4.8 (.01) | 5.7 (> .001) | 1.8 (.05) |
| Craving a cigarette | 37.7 (> .001) | 25.7 (> .001) | 10.6 (> .001) |
| Depression/feeling blue | 0.5 (ns) | 1.5 (ns) | 1.1 (ns) |
| Difficulty concentrating | 0.5 (ns) | 3.4 (.02) | 0.8 (ns) |
| Drowsy | 0.6 (ns) | 3.9 (.007) | 1.6 (ns) |
| Hunger | 2.6 (ns) | 7.8 (> .001) | 1.8 (ns) |
| Impatient | 4.3 (.009) | 3.7 (.006) | 2.5 (.005) |
| Irritability/frustration/anger | 5.8 (.004) | 2.3 (ns) | 3.3 (.001) |
| Restless | 1.5 (ns) | 2.5 (.05) | 1.9 (.025) |
| Desire for sweets | 1.7 (ns) | 1.7 (ns) | 1.7 (ns) |
| Urge to smoke | 40.8 (> .001) | 28.2 (> .001) | 9.7 (> .001) |
| QSU a | |||
| Factor 1 | 40.6 (> .001) | 35.7 (> .001) | 16.9 (> .001) |
| Factor 2 | 19.5 (> .001) | 16.6 (> .001) | 7.6 (> .001) |
| Direct effects of nicotine a | |||
| Confused | 1.0 (ns) | 1.4 (ns) | 1.4 (ns) |
| Dizzy | 1.5 (ns) | 5.2 (.002) | 4.1 (.002) |
| Headache | 0.7 (ns) | 0.9 (ns) | 1.6 (ns) |
| Heart pounding | 0.4 (ns) | 2.9 (.05) | 1.9 (ns) |
| Lightheaded | 1.2 (ns) | 7.6 (> .001) | 5.8 (> .001) |
| Nausea | 0.1 (ns) | 0.8 (ns) | 1.1 (ns) |
| Nervous | 1.0 (ns) | 1.0 (ns) | 1.2 (ns) |
| Salivation | 0.5 (ns) | 0.2 (ns) | 1.0 (ns) |
| Sweaty | 0.8 (ns) | 0.9 (ns) | 1.3 (ns) |
| Weak | 0.1 (ns) | 0.8 (ns) | 1.1 (ns) |
| Direct effects of tobacco a | |||
| Satisfying | 76.2 (> .001) | 61.6 (> .001) | 17.2 (> .001) |
| Pleasant | 66.5 (> .001) | 68.5 (> .001) | 17.6 (> .001) |
| Taste good | 47.7 (> .001) | 66.8 (> .001) | 14.4 (> .001) |
| Dizzy | 19.2 (> .001) | 17.6 (> .001) | 7.2 (> .001) |
| Calm | 42.0 (> .001) | 31.1 (> .001) | 10.3 (> .001) |
| Concentrate | 17.9 (> .001) | 15.8 (> .001) | 5.5 (> .001) |
| Awake | 27.0 (> .001) | 22.0 (> .001) | 6.6 (> .001) |
| Reduce hunger | 24.2 (> .001) | 19.4 (> .001) | 5.6 (> .001) |
| Sick | 3.6 (.02) | 3.6 (.01) | 0.9 (ns) |
| Taste like own brand | 115.5 (> .001) | 60.0 (> .001) | 26.9 (> .001) |
| Feel like own brand | 65.9 (> .001) | 67.0 (> .001) | 21.6 (> .001) |
| Harsh as own brand | 64.9 (> .001) | 49.2 (> .001) | 21.3 (> .001) |
| Mild as own brand | 62.4 (> .001) | 54.0 (> .001) | 20.5 (> .001) |
| Smoke another cigarette RIGHT NOW | 6.5 (> .001) | 38.3 (> .001) | 4.8 (> .001) |
dfCondition=(3,93), dfTime=(9,279), dfCondxtime=(27,837)
dfCondition=(3,93), dfTime= (6,186),dfCondxtime= (18,558)
Physiological Measures
Plasma nicotine
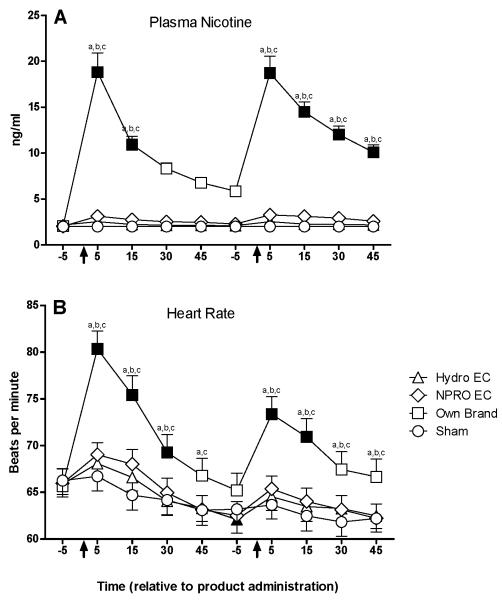
A significant condition by time interaction was observed for plasma nicotine (Figure 1, Panel A). Mean (SD) plasma nicotine increased from a pre-administration level of 2.1 (0.32) ng/ml to a peak of 18.8 (11.8) ng/ml five minutes after the first administration under the own brand condition. No significant changes in plasma nicotine were observed for the “Hydro” EC, “NPRO” EC, or sham conditions.
Figure 1.
Mean data for nicotine blood plasma (panel A) and heart rate (panel B) as a function of condition and time. X-axes: time in minutes relative to product administration. Arrows represent the first and second product administrations. Y-axes: Top: nicotine blood plasma concentration (ng/ml), Bottom: heart rate (beats per minute). Filled symbols indicate a significant difference from baseline. An “a”, “b”, or “c” indicates that own brand was significantly different from sham, “Hydro” EC, or “NPRO” EC at that time point. A “d” indicates that “Hydro” EC was significantly different from sham at that time point. An “e” indicates that “NPRO” EC was significantly different from sham at that time point (Tukey’s HSD, p < 0.05). Unidirectional error bars represent one S.E.M.
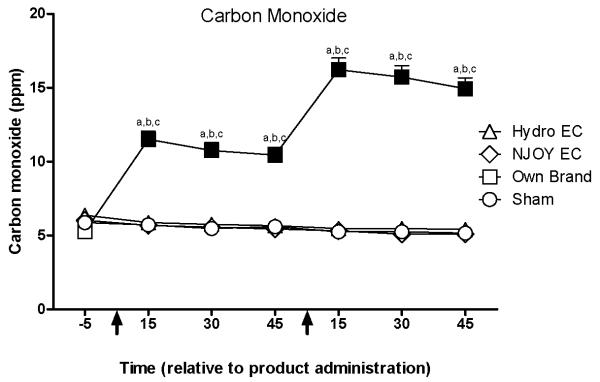
Expired air CO
A significant condition by time interaction was observed for expired air CO levels (Figure 3). Mean (SD) CO increased from a pre-administration level of 5.3 (2.1) ppm to a peak of 16.2 (4.5) ppm fifteen minutes after the second administration under the own brand condition. No significant changes in CO level were observed for the “Hydro” EC, “NPRO” EC, or sham conditions.
Figure 3.
Mean data for carbon monoxide (CO) as a function of condition and time. X-axis: time in minutes relative to product administration. Arrows represent the first and second product administrations. Y-axis: CO in parts per million (ppm). Filled symbols indicate a significant difference from baseline. An “a”, “b”, or “c” indicates that own brand was significantly different from sham, “Hydro” EC, or “NPRO” EC at that time point. A “d” indicates that “Hydro” EC was significantly different from sham at that time point. An “e” indicates that “NPRO” EC was significantly different from sham at that time point (Tukey’s HSD, p < 0.05). Unidirectional error bars represent one S.E.M.
Heart rate
A significant condition by time interaction was also observed for heart rate (Figure 1, Panel B). Heart rate increased from an average (SD) of 65.7 (10.4) bpm at baseline to a peak of 80.3 (10.9) bpm five minutes after the first administration under the own brand condition. No significant changes in heart rate were observed for the “Hydro” EC, “NPRO” EC, or sham conditions.
Subjective effect questionnaires
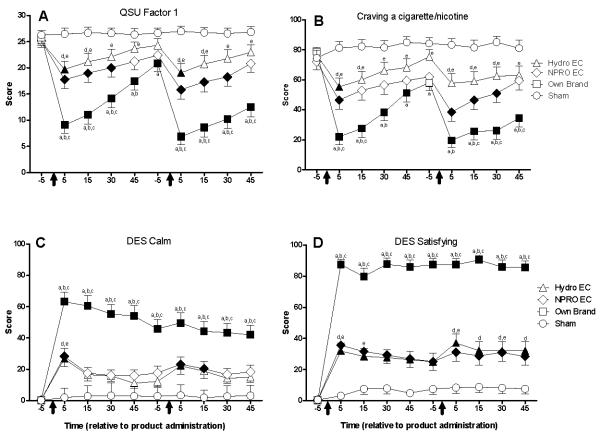
QSU Brief
A significant condition by time interaction was observed for both factors of the QSU brief. Figure 2, Panel A shows data for Factor 1 (intention to smoke; the QSU factor with the greater condition by time F value). “Hydro” EC and “NPRO” EC significantly decreased scores on both factors (relative to sham and/or baseline) at several time points. In contrast, own brand significantly decreased scores (relative to baseline and sham) on both factors at nearly every time point. In addition, the scores observed for own brand after product administration were significantly lower than “Hydro” EC and “NPRO” EC at almost every time point. A nearly identical pattern of results was observed for Factor 2, though ratings were generally lower than those observed on Factor 1.
Figure 2.
Mean data for QSU Factor 1 ratings (panel A), ratings of “craving a cigarette” (panel B), ratings of “calm” (panel C), and ratings of “satisfying” (panel D) as a function of condition and time. X-axes: time in minutes relative to product administration. Arrows represent the first and second product administrations. Y-axes: subjective ratings. Filled symbols indicate a significant difference from baseline. An “a”, “b”, or “c” indicates that own brand was significantly different from sham, “Hydro” EC, or “NPRO” EC at that time point. A “d” indicates that “Hydro” EC was significantly different from sham at that time point. An “e” indicates that “NPRO” EC was significantly different from sham at that time point (Tukey’s HSD, p < 0.05). Unidirectional error bars represent one S.E.M.
Nicotine/tobacco abstinence symptom suppression
Significant condition by time interactions were observed on ratings of “anxious”, “craving a cigarette/nicotine”, “impatient”, “irritability/frustration/anger”, “restless”, and “urge to smoke a cigarette”. Own brand generally decreased ratings on all of these measures. “NPRO” EC and “Hydro” EC produced some abstinence symptom suppression on two measures; “craving a cigarette” and “urge to smoke”. Figure 2, Panel B depicts data for ratings of “craving a cigarette” (the measure with the largest F-value). Ratings of “urge to smoke” decreased significantly 5 minutes following the first “Hydro” EC administration and 5, 15, and 30 minutes following the first and second “NPRO” EC administrations relative to baseline. Ratings of “urge to smoke” decreased significantly at all time points following the first and second own brand administrations relative to baseline and own brand was significantly different from “Hydro” EC and “NPRO” EC at several time points. Similar effects of own brand, “Hydro” EC, and “NPRO” EC were observed on ratings of “craving a cigarette” (see Figure 2, Panel B).
Direct effects of nicotine
Significant condition by time interactions were observed on VAS items assessing “dizzy” and “lightheaded”. Own brand significantly increased ratings of “lightheaded” and “dizzy” within the first five minutes following the first administration. “NPRO” EC, “Hydro” EC, and sham did not alter ratings significantly on these measures.
Direct effects of tobacco
Significant condition by time interactions were observed for ratings of “satisfying”, “pleasant”, “taste good”, “dizzy”, “calm”, “concentrate”, “awake”, “reduce hunger”, “taste like own brand”, “feel like own brand”, “harsh as own brand”, “mild as own brand”, and “smoke another cigarette right now”. Figure 2, Panels C and D depict ratings of “calm” and “satisfying”. Ratings of “satisfying” and “pleasant” increased significantly at all time points under the “Hydro” EC, “NPRO” EC, and own brand conditions (relative to baseline). Own brand increased ratings of “satisfying” and “pleasant” to a significantly greater degree than “Hydro” EC or “NPRO” EC. Ratings of “taste good”, “calm”, “concentrate”, “awake”, and “reduce hunger” increased significantly at all time points after own brand and at several time points after “Hydro” EC and “NPRO” EC (relative to baseline). Ratings of “taste like own brand”, “feel like own brand”, “harsh as own brand”, and “dizzy” increased significantly at all time points under the own brand condition (relative to baseline and sham). Ratings of “mild as own brand” increased significantly at all time points under the own brand condition (relative to baseline and sham) and at nearly all time points under the “Hydro” EC condition (relative to baseline). Ratings of “smoke another cigarette right now” increased significantly relative to baseline at all time points under the “Hydro” EC and “NPRO” EC conditions and 30 and 45 minutes post administration one and 45 minutes post administration two under the own brand condition.
Discussion
The purpose of this acute study was to describe clinical laboratory methods that could be used to understand ECs better by examining users’ toxicant exposure, cardiovascular response, and subjective reports. Results of this study demonstrate the usefulness of the clinical model and suggest that, unlike puffing from a tobacco cigarette, two 10-puff bouts with the two ECs described here exposes users to no measurable nicotine or CO and does not increase heart rate. Despite the failure to deliver nicotine, acute use of the two products tested in this study produced some tobacco abstinence symptom suppression and increased subjective ratings of acceptability. Relative to the effects produced by an own brand tobacco cigarette, these subjective effects of ECs were modest.
With regard to nicotine exposure, the observation that 20 puffs from “Hydro” EC (16 mg cartridge) and “NPRO” EC (18 mg cartridges) did not increase plasma nicotine concentration significantly is entirely consistent with our preliminary report regarding these products (21). The plasma nicotine results are also consistent with the heart rate data reported here. That is, heart rate increases are observed when nicotine is administered via pharmaceutical products (e.g., 33, 34) or tobacco products (e.g., 31, 32, 35) and we observed increased heart rate when participants in this study smoked their own brand of cigarette (that also delivered nicotine). However, no heart rate increases were observed in either EC condition (where no significant increases in plasma nicotine were observed). Finally, the mean peak change from baseline in plasma nicotine concentration observed for “NPRO” EC (1.4 ng/ml) and “Hydro” EC (0.5 ng/ml) during the first product administration were similar in magnitude to the maximum nicotine concentration reported for a third EC brand with a 16 mg cartridge (1.3 ng/ml; 20). The consistency of results across participants, measures, and laboratories highlights the reliability of the clinical methods reported here (see also 26), while also calling into question the ability of the three different ECs that have been tested to date to approximate the nicotine delivery of a tobacco cigarette under acute conditions.
In spite of delivering no measureable nicotine, both ECs tested in this study reduced ratings of “craving a cigarette” and “urge to smoke” and increased subjective ratings of product acceptability (e.g. “satisfying”, “taste good”, “pleasant”). These results are consistent with anecdotal reports from long-term EC users and support the notion that ECs may provide an alternative – perhaps a substitute – to cigarette smoking in some cases (11, 12). Interestingly, de-nicotinized cigarettes have also been shown to suppress tobacco abstinence symptoms for as long as 96 hours (40). However, under the acute conditions reported here, the two ECs did not suppress nicotine/tobacco abstinence symptoms fully, relative to own brand smoking. Other PREPs that failed to suppress abstinence symptoms fully have been shown to supplement rather than substitute for cigarette smoking (e.g., 9, 26, 39, 40). Further controlled evaluation is needed to determine the extent to which the effects reported here are sufficient for ECs to substitute for tobacco cigarettes, and in what proportion of smokers and under what conditions this substitution effect might occur.
Importantly, neither of the ECs tested in this study were associated with any measurable CO exposure. Long-term CO exposure has been linked to cardiovascular disease caused by tobacco cigarette smoking (36). In part for this reason, substituting non-combustible tobacco or nicotine products for cigarettes has been suggested as a potentially effective strategy for reducing the harm of tobacco smoking (37). Following this same logic, and taking into account the trace levels of tobacco specific nitrosamines found in some EC products (13), ECs may also warrant careful empirical examination by those interested in harm reduction for smokers. Clinical laboratory methods have an important role to play in this empirical examination, and can reveal carcinogen exposure and abstinence symptoms suppression over several days’ PREP use (e.g., 22, 26, 38, 40). These methods will likely be extremely important to any future regulation of ECs either as tobacco products or drug delivery devices in the U.S. (i.e., by the Food and Drug Administration) and elsewhere.
Methodological considerations of the current study include the brief EC exposure period, rigorous control over some aspects of smoking behavior, use of two brands of EC with similar cartridge nicotine content, and inclusion of EC-naïve participants who may be representative of cigarette smokers sampling an EC for the first time, but not of a more experienced EC user population. Results of some outcome measures might be influenced by longer-term use, different puffing profiles, other EC models/cartridge strengths, and/or the user’s previous experience with ECs. In future studies, control conditions other than own brand and sham smoking might be of interest. Indeed, a recently published study compared the subjective and physiological effects of another brand of EC, the Ruyan® EC, to that of a pharmaceutical nicotine inhalator and found that the two products were associated with similar low levels of nicotine exposure, incomplete withdrawal suppression, and moderate acceptability (20).. In addition, behavioral studies that assess the reinforcing/rewarding properties of ECs could reveal the extent to which ECs might be used or abused. (41). Finally, the adverse event profile associated with long-term use of ECs is uncertain, and must be explored empirically. Many of these methodological issues and research questions can be addressed parametrically and conveniently in the clinical laboratory, highlighting the strength of these efficient and reliable evaluation methods (see also 26).
In sum, this study revealed that two EC brands do not expose EC-naïve users to nicotine or carbon monoxide under the acute testing procedures described here, but do produce some tobacco abstinence symptom suppression and positive ratings of product acceptability. While these results are necessarily a function of the products tested and procedures used, they suggest that EC-naïve individuals may require substantial motivation if they are to learn whatever product/procedure combination maximizes these outcomes for them. Future clinical laboratory evaluation can build on these methods and results to establish the extent to which ECs might be expected to substitute for tobacco cigarettes, and help to identify under what conditions this substitution might occur. Parallel studies addressing the abuse liability and long-term adverse event profile of ECs are also required to ensure safety and appropriate labeling and marketing of these products.
Acknowledgements
The authors wish to acknowledge the expert technical and medical assistance provided by the staff of VCU’s Behavioral Pharmacology Laboratory (Janet Austin, M.S. and Barbara Kilgalen, R.N.) as well as the staff at VCU’s Bioanalytical Core Laboratory Service Center. This research was supported by USPHS grants R01CA103827, R01CA120142, and T32DA007027-34. The results of this study were presented in poster form at the annual Society for Research on Nicotine and Tobacco meeting held in Baltimore, MD February 24-27, 2010.
Funding: US NCI
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1).Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction—executive summary. Tob Control. 2001;10(2):189–195. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).O’Connor RJ, Cummings KM, Rees VW, et al. Surveillance methods for identifying, characterizing, and monitoring tobacco products: potential reduced exposure products as an example. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3334–3348. doi: 10.1158/1055-9965.EPI-09-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Pederson LL, Nelson DE. Literature review and summary of perceptions, attitudes, beliefs, and marketing of potentially reduced exposure products: communication implications. Nicotine Tob Res. 2007;9(5):525–534. doi: 10.1080/14622200701239548. [DOI] [PubMed] [Google Scholar]
- 4).O’Connor RJ, Hyland A, Giovino GA, Fong GT, Cummings KM. Smoker awareness of and beliefs about supposedly less-harmful tobacco products. Am J Prev Med. 2005;29(2):85–90. doi: 10.1016/j.amepre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 5).Caraballo RS, Pederson LL, Gupta N. New tobacco products: do smokers like them? Tob Control. 2006;15(1):39–44. doi: 10.1136/tc.2005.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Rogers JD, Biener L, Clark PI. Test marketing of new smokeless tobacco products in four U.S. Nicotine Tob Res. 2010;12(1):69–72. doi: 10.1093/ntr/ntp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Eissenberg T. The time for tobacco industry sponsored PREP evaluation has arrived. Tob Control. 2006;15(1):1–2. doi: 10.1136/tc.2005.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Hanson K, O’Connor R, Hatsukami D. Measures for assessing the subjective effects of potential reduced-exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3209–3224. doi: 10.1158/1055-9965.EPI-09-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Buchhalter AR, Schrine L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine Tob Res. 2001;3(2):111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- 10).Cobb CO, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tob Control. doi: 10.1136/tc.2008.028993. Epub 2010 Apr 2 . doi: 10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Heavner K, Dunworth J, Bergen P, Nissen C, Phillips CV. [cited 2010 Feb 9];Electronic cigarettes (e-cigarettes) as potential tobacco harm reduction products: results of an online survey of e-cigarette users. Tob Harm Reduction [Internet] 2009 Nov; working paper 011: [about 15 pp.]. Available from: http://www.tobaccoharmreduction.org/wpapers/011.htm.
- 12).Zezima K. [cited 2009 Dec 23];Cigarettes without smoke, or regulation. The New York Times. 2009 June; Available from: http://nytimes.com/2009/06/02/us/02cigarette.html?_r=1.
- 13).U.S. Food and Drug Administration [Cited 2009 Dec 10];“Evaluation of e-cigarettes”. FDA. 2009 May 4; Available from: http://www.fda.gov/downloads/Drugs/ScienceResearch/UCM173250.pdf.
- 14).Wollsheid KA, Kremzner ME. Electronic cigarettes: safety concerns and regulatory issues. Am J Health-Syst Pharm. 2009;66:1740–1742. doi: 10.2146/ajhp090127. [DOI] [PubMed] [Google Scholar]
- 15).Laugesen M. [cited 2009 Dec 10];Second safety report on the Ruyan e-cigarette. Health New Zealand. (version 8). 2008 Apr; [about 28 pp]. Available from: http://www.healthnz.co.nz/2ndSafetyReport_9Apr08.pdf.
- 16). [cited 2010 Jan 26];Crown Seven Hydro. Available from: http://www.crown7.com/
- 17).E-puffer® International Inc. [cited 2010 Jan 27];Extending Smoker’s Life!™. c 2007-2010. Available from: http://www.epuffer.com/
- 18). [cited 2010 Jan 26];Njoy Electronic Cigarettes-Electronic Cigarettes and Accessories. Available from: http://www.njoycigarettestore.com/faq.
- 19).Laugessen M. Ruyan e-cigarette bench-top tests. Poster presented to the joint conference of the Society for Research on Nicotine and Tobacco and Society for Research on Nicotine and Tobacco-Europe; 2009 Apr 30. [Google Scholar]
- 20).Bullen C, McRobie H, Thornley S, Glover M, Laugesen M. Effect of an electronic cigarette on desire to smoke and withdrawal, user preferences and nicotine delivery: randomized cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 21).Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19(1):87–88. doi: 10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Gray JN, Breland AB, Weaver M, Eissenberg T. Potential reduced exposure products (PREPs) for smokeless tobacco users: clinical evaluation methodology. Nicotine Tob Res. 2008;10(9):1441–1448. doi: 10.1080/14622200802323258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced –exposure products for smokers: clinical laboratory methodology. Nicotine Tob Res. 2002;4(S2):S131–140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- 24).NJOY [Internet] NJOY®; Scottsdale, AZ: Jan 26, 2010. c 2010. Available from: http://www.njoythefreedom.com/index.php. [Google Scholar]
- 25).Crown Seven [Internet] Crown Seven; Scottsdale, AZ: Jan 26, 2010. c 2010. Available from: http://crown7.com/index.shtml. [Google Scholar]
- 26).Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8(6):727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- 27).Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 28).Eissenberg T, Griffiths RR, Stitzer ML. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology. 1996;127(4):328–336. doi: 10.1007/s002130050094. [DOI] [PubMed] [Google Scholar]
- 29).Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311:363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Exp Clin Psychopharmacol. 2008;16:99–112. doi: 10.1037/1064-1297.16.2.99. [DOI] [PubMed] [Google Scholar]
- 31).Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37(6):518–523. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Blank MD, Sams C, Weaver MF, Eissenberg T. Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine Tob Res. 2008;10(3):417–421. doi: 10.1080/14622200801901880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Perkins KA, Epstein LH, Stiller RL, Marks BL, Jacob RG. Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am J Clin Nutr. 1989;50(3):545–550. doi: 10.1093/ajcn/50.3.545. [DOI] [PubMed] [Google Scholar]
- 34).Evans SE, Blank MD, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14(2):121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control. 1999;8(4):387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Lakier JB. Smoking and cardiovascular disease. Am J Med. 1992;93(Suppl 1):S8–12. doi: 10.1016/0002-9343(92)90620-q. [DOI] [PubMed] [Google Scholar]
- 37).Hatsukami DK, Henningfield JE, Kotlyar M. Harm reduction approaches to reducing tobacco-related mortality. Annu Rev Pulic Health. 2004;25:377–395. doi: 10.1146/annurev.publhealth.25.102802.124406. [DOI] [PubMed] [Google Scholar]
- 38).Blank MD, Eissenberg T. Evaluating oral noncombustible potential reduced exposure products for smokers. Nicotine Tob Res. 2010;12(4):336–343. doi: 10.1093/ntr/ntq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Hughes JR, Keely JP. The effect of a novel smoking system—Accord—on ongoing smoking and toxin exposure. Nicotine Tob Res. 2004;6(6):1021–1027. doi: 10.1080/14622200412331296011. [DOI] [PubMed] [Google Scholar]
- 40).Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- 41).Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3241–3262. doi: 10.1158/1055-9965.EPI-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]