Abstract
Introduction
Obesity and diabetes are known risk factors for endometrial cancer; thus genetic risk factors of these phenotypes may also be associated with endometrial cancer risk. To evaluate this hypothesis, we genotyped tagSNPs and candidate SNPs in FTO and HHEX in a primary set of 417 endometrial cancer cases and 406 population-based controls, and validated significant findings in a replication set of approximately 2,347 cases and 3,140 controls from three additional studies.
Methods
We genotyped 189 tagSNPs in FTO (including rs8050136) and five tagSNPs in HHEX (including rs1111875) in the primary set and one SNP in each of FTO (rs12927155) and HHEX (rs1111875) in the validation set. Per allele odds ratios (OR) and 95% confidence intervals (CI) were calculated to estimate the association between the genotypes of each SNPs(as an ordinal variable)and endometrial cancer risk using unconditional logistic regression models, controlling for age and site.
Results
In the primary study, the most significant findings in FTO was rs12927155 (OR=1.56, 95% CI 1.21–2.01, p=5.8×10−4) and HHEX was rs1111875 (OR=0.80, 95% CI 0.66–0.97; p=0.026). In the validation studies, the pooled per allele ORs, adjusted for age and study, were for FTO rs12927155: OR=0.94, 95% CI 0.83–1.06, p=0.29 and for HHEX rs1111875: OR=1.00, 95%CI 0.92–1.10, p=0.96.
Conclusion
Our data indicate that common genetic variants in two genes previously related to obesity (FTO) and diabetes (HHEX) by genome-wide association scans are not associatedwith endometrial cancer risk.
Impact
Polymorphisms in FTO and HHEX are unlikely to have large effects on endometrial cancer risk but may have weaker effects.
Keywords: endometrial cancer, obesity, genetics, FTO, HHEX
Introduction
Obesity and type II diabetes are major risk factors for endometrial cancer. Genetic variation that is associated with obesity and diabetes may provide clues to the molecular pathways mediating endometrial carcinogenesis. Agenome-wide association study (GWAS) of body mass index (BMI) identified a 47 kb region on chromosome 16 encompassing FTO gene intron1-exon2-intron2 that is marked by tagSNP rs8050136 (and the correlated SNP rs9939609 (r2=1))to be associated with BMI(1). Another GWASof type II diabetes(2)identified the HHEX gene region on chromosome 10 marked by rs1111875also to be associated with BMI. To examine whether genetic variants of FTO and HHEX are associated with endometrial cancer risk, we genotyped tagSNPs and candidate SNPs in the Polish Endometrial Case-Control Study(PECS)of 417 endometrial cancer cases and 406 population-based controls(3). Significant findings in this set were then selected for validation in a replication set of approximately 2,347cases and 3,140controls from three additional studies.
PECS Methods
Genotyping of 189 tagSNPsin FTO (including rs8050136)and five tagSNPs in HHEX (including rs1111875)were done as part of an Illumina Infinium custom iSelect chip using aSNP selection strategy described previously(4). For FTO, four SNPs were excluded due to violations of quality control measures: concordance of 1% replicates, completion proportions, and departure of Hardy-Weinberg proportions (p<0.05), and a further 14 SNPs due to minor allele frequency among controls less than 0.05. For HHEX, all five SNPs passed quality control filters. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to estimate the association between SNPs and endometrial cancer risk using unconditional logistic regression models, controlling for matching factors, age and study site. We also conducted analyses of haplotypes, including the sequential haplotype scan (5)and the variable-sized sliding-window regularized regression association analysis (6), to localize a set of adjacent markers associated with risk. Due to the size of the FTO gene, the sequential haplotype scan was performed in three overlapping sections (section 1 (SNPs 1–65): rs8055834–rs17820875, section 2 (SNPs 60–115): rs10521303–rs8056199, and section 3 (SNPs 110–171): rs16952730–rs16953089).
PECS Results
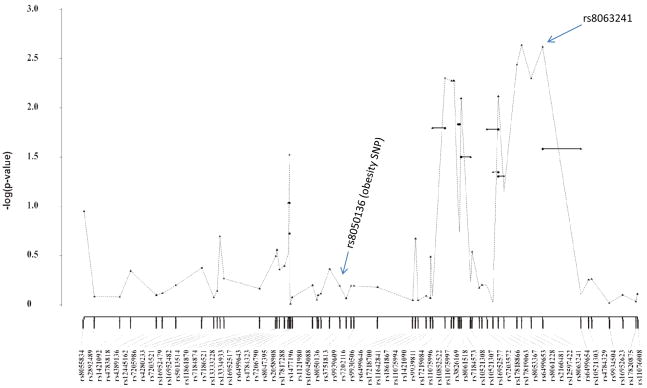
Among PECS controls, carrying increasing copies of the minor A allele of rs8050136in FTO was associated with increased mean body mass index (BMI) (Kruskal-Wallisp-value=0.015) but not prevalence of diabetes (chi-square p=0.26). rs1111875 in HHEX was not associated with BMI (p=0.16) or diabetes (p=0.56). In the PECS case-control analyses, the minor A allele of rs8050136 was not associated with endometrial cancer risk(per allele OR=1.05, 95% CI 0.86–1.28, p=0.64). However, 20 of the remaining 171 FTO tagSNPs were significantly associated with endometrial cancer risk (per allele p-values ranged from 0.00068–0.027) and represented independent SNPs (n=4) or clustered into three linkage disequilibrium blocks. Haplotype analyses identified strong signals (haplotype p-values <10−3)in two of these regions (Figures 1–2). The first region resides in intron 4 and is marked by SNP rs8063241 (OR=0.71, 95% CI 0.56–0.88, p-value=1.7×10−3) (Figure 1). The second region in intron 8 is marked by 3 correlated tagSNPs that also had the lowest p-values in the single locus analysis [rs2689264 (MAF=0.17): OR=1.57, 95% CI 1.22–2.02, p=4.5×10−4; rs12927155(MAF=0.17): OR=1.56, 95% CI 1.21–2.01, p=5.8×10−4; and, rs2540776 (MAF=0.17): OR=1.54, 95% CI 1.19–1.99, p=8.7×10−4] (Figure 2). The candidate SNP in HHEX, rs1111875, was associated with a 20% lower risk of endometrial cancer for each minor T allele (per allele OR=0.80, 95% CI 0.66–0.97; p=0.026). No other HHEX loci were associated with risk.
Figure 1.
FTO Sequential haplotype scan analysis of tagSNPs rs8055834 through rs17820875. Polish Endometrial Cancer Study (417 cases and 406 controls)
Figure 2.
FTO sequential haplotype scan analysis of tagSNPs rs16952730 through rs16953089, Polish Endometrial Cancer Study (417 cases and 406 controls)
Replication Studies
In an attempt to replicate our findings for FTO SNP rs12927155 and HHEX SNP rs1111875, we approached three independent case-control studies of women of European ancestry (5,522 subjects, Table 1), including the Study of Epidemiology and Risk Factors in Cancer Heredity (SEARCH)with 1,494endometrial cancer cases and 1,600community controls, the Australian National Endometrial Cancer Study (ANECS)with 1,048 endometrial cancer cases and 1,010population-based controls(http://www.anecs.org.au/; (7)), and the Leuven Endometrial Cancer Study (LES)(8)with 206 endometrial cancer cases and 649hospital-based controls. The distribution of age and BMI were similar for the three studies (age range (median): SEARCH, 26–71 (56); ANECS, 26–80 (62); LES, 20–80 (48); and BMI range (median): SEARCH, not reported; ANECS, 15.1–67.3 (28.0); LES, 16.4–89.0 (24.9)) and with the PECS. We excluded controls with a history of hysterectomy (including 249 for SEARCH, 95 for ANECS, and 1 for LES). The SEARCH samples were genotyped using TaqMan assays, ANECS and LES samples were genotyped using the Sequenom iPLEX platform.
Table 1.
Age-adjusted odds ratios (OR) and 95% confidence intervals for the association between candidate polymorphisms and endometrial cancer risk in four independent case-control studies
| Study | Cases | Controls | FTO rs12927155 | Cases | Controls | HHEX rs1111875 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per allele | Per allele | |||||||||||
| N | N | MAF1 | OR | (95% CI) | p-value | N | N | MAF1 | OR | (95% CI) | p-value | |
| PECS | 417 | 406 | 0.17 | 1.53 | (1.19–1.97) | 0.001 | 417 | 406 | 0.42 | 0.80 | (0.66–0.97) | 0.026 |
| SEARCH | 1121 | 1596 | 0.16 | 1.01 | (0.85–1.21) | 0.88 | 1119 | 1591 | 0.41 | 1.01 | (0.88–1.14) | 0.93 |
| ANECS | 1022 | 896 | 0.15 | 0.89 | (0.74–1.08) | 0.24 | 992 | 898 | 0.40 | 1.01 | (0.88–1.15) | 0.91 |
| LES2 | 204 | 648 | 0.16 | 1.08 | (0.70–1.68) | 0.72 | 0 | 0 | ||||
| Pooled3 | 2347 | 3140 | 0.15 | 0.94 | (0.83–1.06) | 0.29 | 2111 | 2489 | 0.40 | 1.00 | (0.92–1.10) | 0.96 |
MAF=minor allele frequency among controls
rs1111875 was not genotyped in LES
Pooled estimates were calculated for the three replication studies (SEARCH, ANECS, and LES); therefore, models were also adjusted for study. Numbers do not sum to the total samples available because of missing genotype data.
Among these three studies, the pooled per allele ORs, adjusted for continuous age and study, were for FTO rs12927155: OR=0.94, 95% CI 0.83–1.06, p=0.29andfor HHEX rs1111875: OR=1.00, 95%CI 0.92–1.10, p=0.96 (Table 1). Between-study heterogeneity was not evident among these studies(p=0.23 and 0.74, respectively), and the CIs for both SNPs excluded the ORs from the PECS.
Conclusion
Our data indicate that common genetic variants in two genes previously related to obesity (FTO) and diabetes (HHEX) by genome-wide association scans are not associated with endometrial cancer risk.
Acknowledgments
For the PECS, the authors would like to thank Neonila Szeszenia-Dabrowska of the Nofer Institute of Occupational Medicine (Lodz, Poland) and Witold Zatonski of the M. Sklodowska-Curie Institute of Oncology and Cancer Center (Warsaw, Poland) for their contribution to the Polish Endometrial Cancer Study. Anita Soni (Westat, Rockville, MD, USA) and Pei Chao (IMS, Silver Spring, MD, USA) have been invaluable to the management of the study. This work would not be possible without the dedicated efforts of the physicians, nurses, interviewers and study participants. The authors thank all the women who participated in the SEARCH research as well as Caroline Baynes, Don Conroy, Craig Luccarini, and Mitul Shah for work within the SEARCH study. The ANECS Group comprises: AB Spurdle, P Webb, J Young (Queensland Institute of Medical Research); Consumer representative: L McQuire; Clinical Collaborators: NSW: S Baron-Hay, D Bell, A Bonaventura, A Brand, S Braye, J Carter, F Chan, C Dalrymple, A Ferrier (deceased), G Gard, N Hacker, R Hogg, R Houghton, D Marsden, K McIlroy, G Otton, S Pather, A Proietto, G Robertson, J Scurry, R Sharma, G Wain, F Wong; Qld: J Armes, A Crandon, M Cummings, R Land, J Nicklin, L Perrin, A Obermair, B Ward; SA: M Davy, T Dodd, J Miller, M Oehler, S Paramasivum, J Pierides, F Whitehead; Tas: P Blomfield, D Challis; Vic: D Neesham, J Pyman, M Quinn, R Rome, M Weitzer; WA: B Brennan, I Hammond, Y Leung, A McCartney, C Stewart, J Thompson; Project Managers: S O’Brien, S Moore; Laboratory Manager: K Ferguson; Pathology Support: M Walsh; Admin Support: R Cicero, L Green, J Griffith, L Jackman, B Ranieri; Laboratory Assistants: M O’Brien, P Schultz; Research Nurses: B Alexander, C Baxter, H Croy, A Fitzgerald, EHerron, C Hill, M Jones, J Maidens, A Marshall, K Martin, J Mayhew, E Minehan, D Roffe, H Shirley, H Steane, A Stenlake, A Ward, S Webb, J White. ANECS would also like to gratefully acknowledge the cooperation of the following institutions: NSW: John Hunter Hospital, Liverpool Hospital, Mater Misericordiae Hospital (Sydney), Mater Misericordiae Hospital (Newcastle), Newcastle Private Hospital, North Shore Private Hospital, Royal Hospital for Women, Royal Prince Alfred Hospital, Royal North Shore Hospital, Royal Prince Alfred Hospital, St George Hospital; Westmead Hospital, Westmead Private Hospital; Qld: Brisbane Private Hospital, Greenslopes Hospital, Mater Misericordiae Hospitals, Royal Brisbane and Women’s Hospital, Wesley Hospital, Queensland Cancer Registry; SA: Adelaide Pathology Partners, Burnside Hospital, Calvary Hospital, Flinders Medical Centre, Queen Elizabeth Hospital, Royal Adelaide Hospital, South Australian Cancer Registry; Tas: Launceston Hospital, North West Regional Hospitals, Royal Hobart Hospital; Vic: Freemasons Hospital, Melbourne Pathology Services, Mercy Hospital for Women, Royal Women’s Hospital, Victorian Cancer Registry; WA: King Edward Memorial Hospital, St John of God Hospitals Subiaco & Murdoch, Western Australian Cancer Registry. We also thank Felicity Lose, Jyotsna Batra, Xiaoqing Chen and Jonathan Beesley from The Molecular Cancer Epidemiology and Cancer Genetic laboratories at QIMR for technical assistance. The Leuven Endometrial Cancer Study (LES) gratefully acknowledges the contribution of Drs Evelyn Despierre and Gilian Peuteman for assistance.
Funding support: PECS was funded by the intramural research program at the National Cancer Institute, Division of Cancer Epidemiology and Genetics in the Hormonal and Reproductive Epidemiology Branch. Analysis and conduct of the SEARCH study was funded by Cancer Research UK grants [C20/A3084, C1287/A10118, C490/A11021, C8197/A10123, C1287/A7497]. AMD was funded by Cancer Research UK grant [C8197/A10865]. ANECS was supported by a project grants from the National Health and Medical Research Council (NHMRC) of Australia (ID #339435), The Cancer Council Queensland (ID #4196615) and Cancer Council Tasmania (ID #403031 and ID #457636). Amanda Spurdle is supported by NHMRC Senior Research Fellowship. Tracy O’Mara is supported by an Australian Postgraduate Award, an Institute of Health and Biomedical Innovation PhD Top-Up and a Smart State PhD Award. LES was supported by the Verelst Foundation for Endometrial Cancer.
References
- 1.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–5. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudet MM, Lacey JV, Jr, Lissowska J, Peplonska B, Brinton LA, Chanock S, Garcia-Closas M. Genetic variation in CYP17 and endometrial cancer risk. Hum Genet. 2008;123:155–62. doi: 10.1007/s00439-007-0454-8. [DOI] [PubMed] [Google Scholar]
- 4.Yang HP, Bosquet JG, Li Q, Platz EA, Brinton LA, Sherman ME, Lacey JV, Jr, Gaudet MM, Burdette L, Figueroa JD, Ciampa J, Lissowska J, Peplonska B, Chanock S, Garcia-Closas M. Common genetic variation in the sex hormone metabolic pathway and endometrial cancer risk: pathway-based evaluation of candidate genes. Carcinogenesis. doi: 10.1093/carcin/bgp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Z, Schaid DJ. Sequential haplotype scan methods for association analysis. Genet Epidemiol. 2007;31:553–64. doi: 10.1002/gepi.20228. [DOI] [PubMed] [Google Scholar]
- 6.Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet. 2007;16:2844–53. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
- 7.Spurdle A, Webb P. Re: Excess of early onset multiple myeloma in endometrial cancer probands and their relatives suggests common susceptibility. Gynecol Oncol. 2008;109:153. doi: 10.1016/j.ygyno.2007.12.010. author reply 154. [DOI] [PubMed] [Google Scholar]
- 8.Kalogiannidis I, Lambrechts S, Amant F, Neven P, Van Gorp T, Vergote I. Laparoscopy-assisted vaginal hysterectomy compared with abdominal hysterectomy in clinical stage I endometrial cancer: safety, recurrence, and long-term outcome. Am J Obstet Gynecol. 2007;196:248, e1–8. doi: 10.1016/j.ajog.2006.10.870. [DOI] [PubMed] [Google Scholar]