Abstract
Elevated glomerular filtration rate (GFR) is a common observation in early diabetes mellitus and closely correlates to the progression of diabetic nephropathy. The hyperfiltration has been explained to be the result of a reduced load of sodium and chloride passing macula densa, secondarily to an increased proximal reabsorption of glucose and sodium by the sodium glucose co-transporters. This results in an inactivation of the tubuloglomerular feedback, leading to a reduced afferent arteriolar vasoconstriction and subsequently an increase in GFR. This hypothesis has recently been questioned due to the observation that adenosine A1-receptor knockout mice, previously shown to lack a functional tubuloglomerular feedback mechanism, still display a pronounced hyperfiltration when diabetes is induced. Leyssac demonstrated in the 1960’s (Leyssac, 1963) that GFR and proximal reabsorption can work independently of each other. Furthermore, by the use of micropuncture technique a reduced hydrostatic pressure in Bowman’s space or in the proximal tubule of diabetic rats has been observed. A reduced pressure in Bowman’s space will increase the pressure gradient over the filtration barrier and can contribute to the development of diabetic hyperfiltration. When inhibiting proximal reabsorption with a carbonic anhydrase inhibitor, GFR decreases and proximal tubular pressure increases. Measuring intratubular pressure allows a sufficient time resolution to reveal that net filtration pressure decreases before TGF is activated which highlights the importance of intratubular pressure as a regulator of GFR. Taken together, these results imply that the reduced intratubular pressure observed in diabetes might be crucial for the development of glomerular hyperfiltration.
Keywords: Diabetes, glomerular filtration rate, glucose transport, tubuloglomerular feedback, proximal pressure
Introduction
Sustained hyperglycaemia is associated with several complications, including nephropathy, neuropathy and retinopathy, and is the leading cause for end-stage renal disease (Deshpande et al., 2008). Approximately 30% of all patients with insulinopenic diabetes will develop renal disease (Hovind et al., 2004). The degree of hyperglycaemia is the major predictor for development of diabetes-induced complications according to the Diabetes Control and Complication Trial Research Group (DCCTR, 1993). Increased glomerular filtration rate (GFR) is one of the earliest indications of altered kidney function in diabetic patients (O’Donnell et al., 1988), and is commonly used as a predictor for later development of progressive renal dysfunction. The mechanism mediating diabetes-induced glomerular hyperfiltration has been subject for extensive research, and potential mechanisms have been proposed. The most commonly accepted hypothesis for the diabetes-induced glomerular hyperfiltration involves an inactivated tubuloglomerular feedback (TGF) mechanism. The TGF mechanism is mediated by adenosine acting on adenosine A1 receptors (A1AR) in the afferent arterioles causing vasoconstriction (Sun et al., 2001, Brown et al., 2001). Changes in tubular sodium-chloride concentration are detected by the macula densa (MD) cells in the distal nephron which express the sodium-potassium-2-chloride (NKCC2) isoform B. This results in corresponding adjustment of the afferent arteriolar resistance correcting GFR to match tubular transport capacity (Wright and Schnermann, 1974). This transporter has highest affinity for chloride and is adapted for transport of sodium and chloride concentrations around 25 mM. In TGF-studies, maximal response is achieved when tubules are perfused with NaCl concentration up to 60 mM. Sodium, chloride and potassium are reabsorbed from the tubules. Chloride is excreted on the basolateral side while potassium is being transported back to the tubules. The MD cells have a very low expression of the sodium/potassium-ATPase. The consequence is that sodium concentration in the MD reflects tubular sodium concentration, with a concomitant depolarization of MD when NaCl is increased. However, how the signal thereafter is transmitted is yet not fully understood, but it is probably a release of ATP, which is subsequently degraded to adenosine. The NKCC-transporter is necessary for a functional TGF-response, as well as recycling of potassium and excretion of chloride, and mice lacking NKCC2 have reduced TGF-responsiveness (Oppermann et al., 2006, Oppermann et al., 2007).
Inactivation is a result from reduced tubular sodium load to the MD due to increased tubular reabsorption secondary to increased tubular glucose load (Thomson et al., 2004). Indeed, the concentrations of sodium, potassium and chloride in the early distal nephron are reduced in diabetic rats (Vallon et al., 1999). However, this hypothesis has recently been questioned in studies using A1AR knockout mice (Sallstrom et al., 2007, Faulhaber-Walter et al., 2008). These mice lack the TGF mechanism, but still display diabetes-induced glomerular hyperfiltration. This review will summarize some of the mechanisms that have been proposed to contribute to diabetic hyperfiltration with a primary focus on the influence of tubular reabsorption as a crucial determinant of filtration pressure.
Tubular hypothesis of glomerular filtration rate
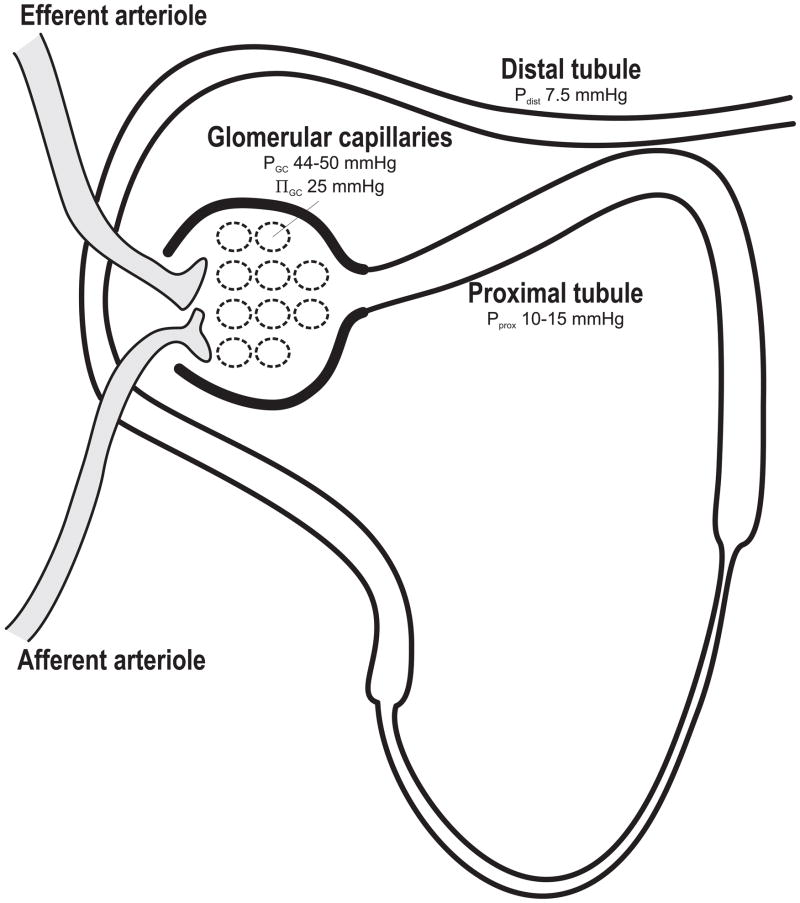
GFR is attributable to the sum of hydrostatic and colloid osmotic forces across the glomerular membrane, also known as net filtration pressure (ΔP). These forces are: hydrostatic pressure in the glomerular capillaries (Pgc), hydrostatic pressure in the Bowman’s capsule or the proximal tubule (Pprox), colloid osmotic pressure of the glomerular capillaries (πgc) and the colloid osmotic pressure of Bowman’s capsule (πBow, which under normal conditions is assumed to be zero). Net filtration (ΔP) pressure is calculated according to: ΔP = Pgc − Pprox − (πgc + πBow). These pressures have been measured by micropuncture technique. During normal physiological conditions Pgc amounts to 44–50 mmHg (Vallon et al., 1999, Leyssac et al., 1991a, Hostetter et al., 1981, Persson et al., 1984), Pprox 12.3–15 mmHg (Leyssac et al., 2000, Leyssac et al., 1991a, Leyssac et al., 1994, Thorup et al., 2000, Hostetter et al., 1981, Sorensen et al., 2004, Nordquist et al., 2009) and a mean πgc of 25 mmHg (Brenner et al., 1971). This results in a ΔP ranging from 4 to 13 mmHg (Fig. 1). Theoretically, the relative small ΔP must result in the conclusion that a minor change in Pprox that is unopposed by changes in Pgc or πgc will have an immense influence on GFR. Distal tubular pressure (Pdist) is approximately 7.5 mmHg (Leyssac et al., 1991a), which implies that the relatively high hydraulic resistance in the loop of Henle has implications for Pprox. However, increasing tubular flow rate does not cause a proportional increase in proximal tubular pressure. This phenomenon is further explained by Koh et al. showing that the loop of Henle behaves like a collapsible rubber tube (Koh and Baines, 1974). During low flow rate, the loop of Henle is collapsed leading to a high hydraulic resistance. However, if the tubular flow increases the tubules are expanding leading to reduced resistance. Thus, the resistance determining Pprox will originate from the distal tubules or collecting ducts during high tubular flow rates. Leyssac demonstrated in the 1960’s that proximal tubular reabsorption can function independently of GFR (Leyssac, 1963). He reported that the proximal reabsorption is unaltered when the renal artery is ligated and, thus, GFR is stopped. Leyssac tested his hypothesis further by inhibiting proximal tubular reabsorption with the carbonic anhydrase (CA) inhibitor acetazolamide (Leyssac et al., 1991a), which previously had been shown to reduce fractional reabsorption of sodium, chloride and bicarbonate with approximately 30% (Kunau, 1972) and reduce GFR in both rats and humans (Skott et al., 1989, Persson and Wright, 1982, Tucker and Blantz, 1980, Tucker et al., 1978). This reduced ΔP by increasing Pprox before TGF was activated. Therefore, Leyssac proposed that GFR can be determined by changes in proximal tubular reabsorption (Leyssac et al., 1991a). Reduced tubular reabsorption will leave more electrolytes and, subsequently, more fluid in the tubules, which increases the intratubular pressure in the proximal tubule and therefore reduces the pressure gradient (ΔP) across the glomerular membrane. Persson and Wright had earlier explained the reduced GFR during inhibition of proximal tubular reabsorption with a TGF-mediated afferent arteriolar vasoconstriction due to increased electrolyte load to the macula densa (MD) (Persson and Wright, 1982). These results showed that total kidney GFR was reduced by 30% and single nephron (SN) GFR by 23%, using distal collections, i.e. with an intact TGF-loop. With proximal collection (TGF not functional), SNGFR was similar for control and acetazolamide treated rats, demonstrating the influence of TGF. In contrast, Leyssac et al. showed increased Pprox by 1.7 mmHg and reduced ΔP immediately after CA inhibition before the ~10 seconds time-lag for TGF-activation and afferent arteriolar vasoconstriction, indicating that alterations in Pprox will be involved in the reduction of ΔP after CA-inhibition (Leyssac et al., 1991a). TGF-activation was thereafter observed and resulted in reduced renal blood flow (RBF) and a further reduction in ΔP. The theory of a TGF independent reduction in GFR after CA-inhibition has later been supported by data from A1AR−/− mice (Hashimoto et al., 2004). However, the conclusion was that both proximal reabsorption and TGF-activation contributes to the reduced GFR after inhibition of proximal tubular reabsorption since ΔP was lowered already before the TGF was activated. Leyssac et al. went one step further and restored the RBF back to baseline by infusing dopamine, which resulted in an incomplete restoration of GFR. Approximately 30% of the acetazolamide induced decrease in GFR was reversed by dopamine, due to a parallel increase in Pprox (Leyssac et al., 1994). This finding provides further support for Pprox as a major determinant of GFR since Pprox works in parallel to Pgc and therefore results in an unaltered ΔP. This is only possible if distal hydraulic resistance is greater than the resistance for filtration across the glomerular membrane. Another aspect that needs to be considered is whether the glomerular filtration is characterized by filtration pressure equilibrium. However, with normal arterial pressure and kidney function, the glomerular filtration does not reach filtration pressure equilibrium (Kallskog et al., 1975b, Kallskog et al., 1975a). Furthermore, it has been shown in several studies that the efferent arteriole can influence GFR, which would be difficult if there was filtration pressure equilibrium across the glomerular capillary (Palm et al., 2010, Nordquist et al., 2009). During filtration pressure equilibrium, the GFR is mainly determined by the plasma flow and there are indeed several studies showing increased renal plasma flow in diabetes (Jensen et al., 1981, Hostetter et al., 1981). However, we have previously demonstrated that diabetic hyperfiltration occurs without concomitant increase in renal plasma flow (Palm et al., Nordquist et al., 2009). Accordingly, the hydrostatic pressure in Bowman’s space will influence ΔP and therefore also regulate GFR.
Figure 1.
Normal pressures in capillaries and tubules reported from studies using the micropuncture technique. Data from (Vallon et al., 1999, Leyssac et al., 1991a, Hostetter et al., 1981, Persson et al., 1984, Leyssac et al., 2000, Leyssac et al., 1994, Thorup et al., 2000, Sorensen et al., 2004, Brenner et al., 1971). π denotes osmotic pressure.
Diabetic hyperfiltration
GFR is increased in both diabetic patients and in experimental animal models of diabetes early after the onset of diabetes (O’Donnell et al., 1988). GFR and tubular function are interlinked via the tubular content of electrolytes, preferentially sodium and chloride, that reaches the MD through the TGF (Skott et al., 1989, Persson and Wright, 1982, Tucker and Blantz, 1980, Tucker et al., 1978). One of the hypotheses for diabetic hyperfiltration, commonly referred to as “The tubular hypothesis of glomerular filtration”, states that the GFR is increased due to an inactivated TGF secondary to increased tubular sodium reabsorption and therefore decreased electrolyte load to the MD (Thomson et al., 2004). Therefore, the diabetes-induced glomerular hyperfiltration would be the result of a primary tubular effect, defined as any mechanism altering tubular reabsorption upstream to the MD. A primary vascular effect is defined as any other mechanism, but proximal reabsorption. Tubular sodium load to the MD must be reduced if GFR is increased by a primary tubular effect. Conversely, sodium load to MD must be increased if GFR is increased by a primary vascular effect.
Glucose is reabsorbed by proximal tubules by two types of sodium glucose linked transporters (SGLT), SGLT1 and SGLT2. SGLT2 is present in the S1 and S2 segments of proximal tubule, and has a high capacity, low affinity transport characteristics for glucose. SGLT1 is present in the S3 segment and has a low capacity, high affinity transport characteristics (Hediger and Rhoads, 1994). Progressive increase in tubular glucose in both diabetic and control rats results in increased net sodium and fluid reabsorption (Bank and Aynedjian, 1990). Furthermore, mRNA of both SGLT2 and SGLT1 are up regulated by 36% and 20%, respectively, in diabetic rats (Vestri et al., 2001). Hence, in combination with increased tubular glucose load, due to increased plasma glucose levels during diabetes, proximal tubular sodium reabsorption increases, which reduces sodium load to MD and deactivates the TGF (Vallon et al., 1999, Pollock et al., 1991).
So far, it has not directly been demonstrated that the increased sodium reabsorption is due to increased sodium-glucose co-transport. The theory originates from other observations. First, sodium concentration is reduced in the distal part of the nephron in diabetic rats, and is normalized when the sodium-glucose co-transport is blocked by phlorizin. Phlorizin also reduce diabetic glomerular hyperfiltration in both rats and in conscious mice (Pollock et al., 1991, Vallon et al., 1999). This may seem a bit surprising since the sodium-glucose co-transport is believed to only be a minor contributor to the total electrolyte transport in the proximal tubule. However, the sodium-glucose co-transporters are up-regulated in diabetes, and the transport maximum might increase (Vestri et al., 2001). In a control rat with blood glucose of 5 mM it can be estimated that the amount of sodium reabsorbed in the proximal tubule by sodium/glucose co-transport to approximately 5 % of total sodium reabsorption, whereas in diabetic animals with a blood glucose around 15 mM the amount of total sodium going through sodium/glucose co-transport can be estimated to 14 %. This is a considerable part of total proximal reabsorption and corresponds well with the difference in lithium clearance commonly observed in diabetes (Nordquist et al., 2009). These calculations are based on the assumptions that plasma sodium is 140 mM and that proximal reabsorption of sodium is 75% of the filtered load in control rats and 85% in diabetic rats (Nordquist et al., 2009). Furthermore, it is also assumed that the total ratio of glucose:sodium reabsorption in the proximal tubule is 1:1.1 (90% going through SGLT2 with ratio 1:1 and 10% mediated by SGLT1 with ratio 1:2 (Wright, 2001)) and that this ratio is similar in diabetic rats. These assumptions do not include the known upregulation of SGLT protein levels in the diabetic kidney that will increase the contribution of sodium reabsorption via SGLTs at glucose levels above 15 mM.
Interestingly, Noonan and co-workers could increase GFR 42% in control rats by infusing glucose, which implies that glucose per se can influence GFR (Noonan et al., 2001). They also showed correlation between the amount of filtered and reabsorbed glucose. However, it should be noted that the renal blood flow was not measured in this study. Preventing tubular growth in diabetic rats by inhibition of ornithine decarboxylase prevents hyperfiltration, but has no effect in control rats (Thomson et al., 2001). Normalizing early distal sodium delivery by inhibiting proximal reabsorption results in normal SNGFR in diabetic rats (Vallon et al., 1999). However, this leads to a 100% concomitant increase in early distal flow rate. Accordingly, this may result in increased Pprox reducing the ΔP since the distal tubule has high hydraulic resistance (Koh and Baines, 1974). Reduced Pprox in diabetic rats has been demonstrated, which indicates that the mechanism explaining the diabetic glomerular hyperfiltration must be different from the commonly accepted “Tubular hypothesis” (Thorup et al., 2000, Vallon et al., 1999, Nordquist et al., 2009). Jensen and co-workers reported reduced Pprox while Pgc was not different between control and diabetic rats. However, the renal plasma flow was increased. The authors concluded that hyperglycaemia induced simultaneous vasodilation of both the afferent and the efferent arteriole, and that this in combination with the reduced Pprox caused the glomerular hyperfiltration (Jensen et al., 1981). Reduced Pprox is contradictive to the increased tubular flow rates observed in diabetic rats as long as it not accompanied by a reduction in the distal hydraulic resistance. This is explained by the results of Koh et al. showing that resistance in the loop of Henle is reduced in parallel to increased tubular flow rates. It should also be noted that sustained hyperglycaemia is associated with increased oxidative stress (Palm et al., 2003), which itself has been shown to directly stimulate reabsorption of sodium and chloride in the thick ascending limb (Ortiz and Garvin, 2002). This could potentially reduce tubular flow rate in the distal parts of the nephron and subsequently reduce Pprox. However, antioxidant treatment of diabetic rats fail to prevent the diabetes-induced glomerular hyperfiltration (Palm et al., 2003), implying no major influence of this mechanism on GFR during diabetes.
There is some evidence for a restrictive role of TGF for mediating the elevated GFR during diabetes. Pollock et al. collected tubular fluid from proximal nephron segments (TGF nonfunctional) and distal nephron segments (TGF intact) using micropuncture to measure SNGFR (Pollock et al., 1991). They reported a larger increase in proximally versus distally measured SNGFR in diabetic rats compared to controls. This implies that the TGF works towards abating diabetic hyperfiltration. Administering phlorizin, an SGLT antagonist reduced blood glucose and SNGFR, both proximally and distally determined to a level corresponding to SNGFR of untreated rats with comparable blood glucose. Proposing that the TGF is protective against diabetic hyperfiltration implies a resetting of the TGF response curve leftward with an increased sensitivity since the increased proximal tubular electrolyte reabsorption in diabetes results in decreased sodium delivery load to MD (Vallon et al., 1999, Pollock et al., 1991). However, there is a possible link between diabetes and a sensitized TGF mechanism. Hyperglycaemia has been shown to increase angiotensin II (Ang II) production in the kidney (Onozato et al., 2008, Price et al., 1999, Zimpelmann et al., 2000), which is at least partly mediated by reactive oxygen species (ROS) (Hsieh et al., 2002). Ang II is the major modulator in increasing TGF-responsiveness to adenosine (Deray et al., 1990, Traynor et al., 1998) Ang II, acting on AT1-receptors stimulates ROS production by the NADPH oxidase (Shinozaki et al., 2004, Onozato et al., 2002). Finally, ROS decrease nitric oxide (NO) bioavailability through an irreversible reaction between oxygen radicals and NO forming peroxynitrite. It has been shown in several studies that NO blunts the TGF-response to increased sodium load to the MD (Welch et al., 1999, Ren et al., 2000, Ito and Ren, 1993). Interstingly, Ren and co-workers demonstrated that oxidative stress influences the inhibitory effect of NO on the TGF response also under normal situations (Ren et al., 2002). Tempol, a superoxide dismutase mimetic, attenuated the TGF response in afferent arterioles from normoglycaemic Sprague-Dawley rats, and the effect was abolished after specific nNOS inhibition by 7-NI. Indeed, it has been shown that the TGF responsiveness is elevated in diabetic rats (Thorup et al., 2000), and mice lacking NADPH oxidase-mediated ROS production have reduced contraction of the afferent arteriole in response to Ang II (Carlstrom et al., 2009). Finally, superoxide has been demonstrated to directly increase the efficiency of the 5′-nucleotidase, which is responsible for the majority of the hydrolysis of adenosine monophosphate to free adenosine (Chen et al., 2001), the latter being a crucial mediator for the TGF mechanism over the adenosine A1 receptor (Brown et al., 2001, Sun et al., 2001).
Knowledge from genetically modified animal models
The TGF-mediated afferent arteriolar vasoconstriction is mediated by adenosine acting on A1AR (Brown et al., 2001, Sun et al., 2001, Osswald et al., 1980). Two groups independently developed A1AR knockout mice (A1AR−/−) and both groups reported that these mice completely lack the classic TGF-response (Brown et al., 2001, Sun et al., 2001). Administration of the CA inhibitor benzolamide to A1AR−/− resulted in a drop of GFR by 21.1% and RBF by 15.9%, which was similar to that of the wild type animals, which is a direct evidence that a reduction in electrolyte transport in the proximal segment of the nephron can alter GFR via other mechanisms than TGF. Interestingly, benzolamide increased plasma renin concentration and acute Ang II receptor blockade by candesartan diminished the effect of benzolamide on GFR and RBF, indicating a critical role for Ang II in reducing renal blood flow during CA inhibition (Hashimoto et al., 2004). The development of A1AR−/− mice has provided the opportunity to further study the specific role of TGF in health and disease. Interestingly, Sallstrom et al. showed that diabetic A1AR−/− mice display diabetes-induced glomerular hyperfiltration to the same extent as wild-type mice (Sallstrom et al., 2007), indicating no involvement of TGF for the increased GFR in these animals. These results were later confirmed by Faulhaber-Walter and co-workers where A1AR−/− mice were crossed with the diabetic Ins2+/− Akita mice (Faulhaber-Walter et al., 2008). Similar to the results by Sallstrom et al. the diabetic Ins2+/−/A1AR−/− mice lacking TGF displayed pronounced glomerular hyperfiltration. However, an interesting finding was that the diabetic mice lacking the A1AR presented with a more exaggerated increase in GFR compared with Akita having a functional TGF mechanism. These results indicate that the role of TGF is to attenuate, rather than cause the diabetes-induced glomerular hyperfiltration. Importantly, both these studies reported that the absence of a functional TGF mechanism did not alter baseline GFR in the normoglycemic mice. However, it should be noted that conflicting results do exist. The paper by Vallon et al. reported that diabetic A1AR−/− mice do not present with diabetic hyperfiltration while their diabetic wild type littermates do (Vallon et al., 2009).
The conclusion so far from using A1AR−/− mice, it seems that TGF does not mediate the diabetes-induced glomerular hyperfiltration, and that GFR remains remarkably stable in the absence of a functional TGF mechanism.
In conclusion, several factors affect GFR during the course of diabetes. The kidneys grow, glucose transporters are up regulated and hemodynamics are altered. Recent reports confirm the importance of tubular reabsorption and proximal tubule pressure as determinants of GFR and support the hypothesis that increased proximal tubular electrolyte reabsorption with a subsequent reduction in proximal tubular pressure is a major mechanism for the commonly observed glomerular hyperfiltration in early diabetes. Better understanding of the mechanisms mediating the glomerular hyperfiltration may present new therapeutical targets and improve the treatment of diabetic nephropathy.
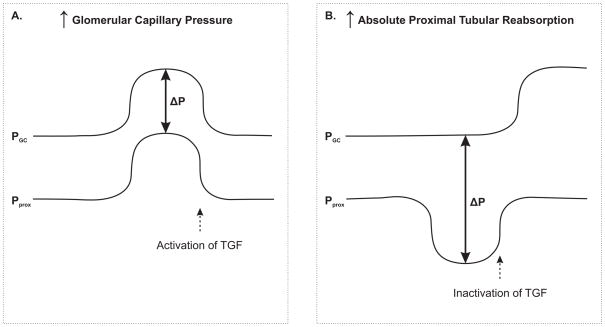
Figure 2.
Situation with a distal resistance greater than resistance for filtration across the glomerular membrane. A: Increased glomerular capillary pressure (Pgc) results in a parallel increase in proximal tubular pressure (Pprox), leaving ΔP unaltered. B: Increased absolute proximal reabsorption reduces Pprox, which increases ΔP. The subsequent TGF-inactivation due to decreased load to MD will increase Pgc and Pprox in parallel. Modified from Leyssac et al. (Leyssac et al., 1991b).
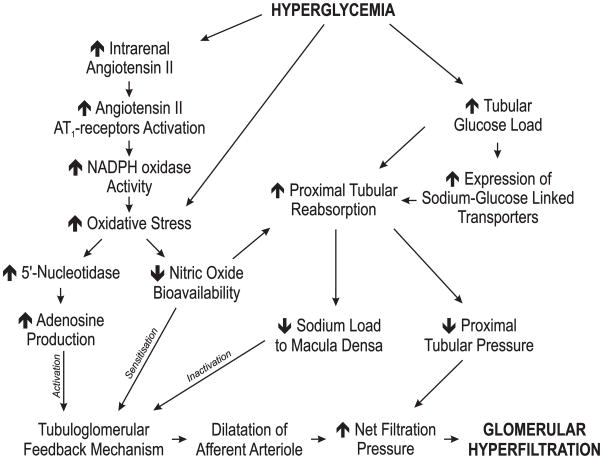
Figure 3.
Summary of factors influencing GFR during sustained hyperglycaemia. See text for further details.
Acknowledgments
The work from our laboratory presented herein was supported by the Swedish Society for Medical Research, the Swedish Diabetes Foundation, the Swedish Research Council and the NIH/NIDDK K99/R00 grant (DK077858).
Footnotes
Conflict of interest: There are no conflicts of interests in this paper.
References
- Bank N, Aynedjian HS. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Invest. 1990;86:309–316. doi: 10.1172/JCI114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971;50:1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- Carlstrom M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AE. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol. 2009;296:R72–79. doi: 10.1152/ajpregu.90718.2008. [DOI] [PubMed] [Google Scholar]
- Chen YF, Li PL, Zou AP. Oxidative stress enhances the production and actions of adenosine in the kidney. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1808–1816. doi: 10.1152/ajpregu.2001.281.6.R1808. [DOI] [PubMed] [Google Scholar]
- DCCTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Deray G, Sabra R, Herzer WA, Jackson EK, Branch RA. Interaction between angiotensin II and adenosine in mediating the vasoconstrictor response to intrarenal hypertonic saline infusions in the dog. J Pharmacol Exp Ther. 1990;252:631–635. [PubMed] [Google Scholar]
- Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol. 2008;19:722–730. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Huang YG, Castrop H, Hansen PB, Mizel D, Briggs J, Schnermann J. Effect of carbonic anhydrase inhibition on GFR and renal hemodynamics in adenosine-1 receptor-deficient mice. Pflugers Arch. 2004;448:621–628. doi: 10.1007/s00424-004-1330-1. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981;19:410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, Binder C, Parving HH. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- Ito S, Ren Y. Evidence for the role of nitric oxide in macula densa control of glomerular hemodynamics. J Clin Invest. 1993;92:1093–1098. doi: 10.1172/JCI116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PK, Christiansen JS, Steven K, Parving HH. Renal function in streptozotocin-diabetic rats. Diabetologia. 1981;21:409–414. [PubMed] [Google Scholar]
- Kallskog O, Lindbom LO, Ulfendahl HR, Wolgast M. Kinetics of the glomerular ultrafiltration in the rat kidney. A theoretical study. Acta Physiol Scand. 1975a;95:191–200. doi: 10.1111/j.1748-1716.1975.tb10041.x. [DOI] [PubMed] [Google Scholar]
- Kallskog O, Lindbom LO, Ulfendahl HR, Wolgast M. Kinetics of the glomerular ultrafiltration in the rat kidney. An experimental study. Acta Physiol Scand. 1975b;95:293–300. doi: 10.1111/j.1748-1716.1975.tb10053.x. [DOI] [PubMed] [Google Scholar]
- Koh YG, Baines AD. Pressure-flow relationships in Henle’s loops and long collapsible rubber tubes. Kidney Int. 1974;5:30–38. doi: 10.1038/ki.1974.4. [DOI] [PubMed] [Google Scholar]
- Kunau RT., Jr The influence of the carbonic anhydrase inhibitor, benzolamide (CL-11,366), on the reabsorption of chloride, sodium, and bicarbonate in the proximal tubule of the rat. J Clin Invest. 1972;51:294–306. doi: 10.1172/JCI106814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssac PP. Dependence of glomerular filtration rate on proximal tubular reabsorption of salt. Acta Physiol Scand. 1963;58:236–242. doi: 10.1111/j.1748-1716.1963.tb02644.x. [DOI] [PubMed] [Google Scholar]
- Leyssac PP, Holstein-Rathlou NH, Skott O. Renal blood flow, early distal sodium, and plasma renin concentrations during osmotic diuresis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1268–1276. doi: 10.1152/ajpregu.2000.279.4.R1268. [DOI] [PubMed] [Google Scholar]
- Leyssac PP, Karlsen FM, Holstein-Rathlou NH, Skott O. On determinants of glomerular filtration rate after inhibition of proximal tubular reabsorption. Am J Physiol. 1994;266:R1544–1550. doi: 10.1152/ajpregu.1994.266.5.R1544. [DOI] [PubMed] [Google Scholar]
- Leyssac PP, Karlsen FM, Skott O. Dynamics of intrarenal pressures and glomerular filtration rate after acetazolamide. Am J Physiol. 1991a;261:F169–178. doi: 10.1152/ajprenal.1991.261.1.F169. [DOI] [PubMed] [Google Scholar]
- Leyssac PP, Karlsen FM, Skott O. Role of proximal tubular reabsorption for the intrarenal control of GFR. Kidney Int Suppl. 1991b;32:S132–135. [PubMed] [Google Scholar]
- Noonan WT, Shapiro VM, Banks RO. Renal glucose reabsorption during hypertonic glucose infusion in female streptozotocin-induced diabetic rats. Life Sci. 2001;68:2967–2977. doi: 10.1016/s0024-3205(01)01090-6. [DOI] [PubMed] [Google Scholar]
- Nordquist L, Brown R, Fasching A, Persson P, Palm F. Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. Am J Physiol Renal Physiol. 2009;297:F1265–1272. doi: 10.1152/ajprenal.00228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MP, Kasiske BL, Keane WF. Glomerular hemodynamic and structural alterations in experimental diabetes mellitus. FASEB J. 1988;2:2339–2347. doi: 10.1096/fasebj.2.8.3282959. [DOI] [PubMed] [Google Scholar]
- Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes. 2008;57:172–180. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Mizel D, Huang G, Li C, Deng C, Theilig F, Bachmann S, Briggs J, Schnermann J, Castrop H. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na,K,2Cl co-transporter. J Am Soc Nephrol. 2006;17:2143–2152. doi: 10.1681/ASN.2006040384. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J Am Soc Nephrol. 2007;18:440–448. doi: 10.1681/ASN.2006091070. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol. 2002;283:F957–962. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- Osswald H, Nabakowski G, Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem. 1980;12:263–267. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- Palm F, Fasching A, Hansell P, Kallskog O. Nitric oxide originating from NOS1 controls oxygen utilization and electrolyte transport efficiency in the diabetic kidney. Am J Physiol Renal Physiol. 298:F416–420. doi: 10.1152/ajprenal.00229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm F, Fasching A, Hansell P, Kallskog O. Nitric oxide originating from NOS1 controls oxygen utilization and electrolyte transport efficiency in the diabetic kidney. Am J Physiol Renal Physiol. 2010;298:F416–420. doi: 10.1152/ajprenal.00229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AE, Gushwa LC, Blantz RC. Feedback pressure-flow responses in normal and angiotensin-prostaglandin-blocked rats. Am J Physiol. 1984;247:F925–931. doi: 10.1152/ajprenal.1984.247.6.F925. [DOI] [PubMed] [Google Scholar]
- Persson AE, Wright FS. Evidence for feedback mediated reduction of glomerular filtration rate during infusion of acetazolamide. Acta Physiol Scand. 1982;114:1–7. doi: 10.1111/j.1748-1716.1982.tb06945.x. [DOI] [PubMed] [Google Scholar]
- Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol. 1991;260:F946–952. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- Price DA, Porter LE, Gordon M, Fisher ND, De’Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10:2382–2391. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- Ren Y, Carretero OA, Garvin JL. Mechanism by which superoxide potentiates tubuloglomerular feedback. Hypertension. 2002;39:624–628. doi: 10.1161/hy0202.103299. [DOI] [PubMed] [Google Scholar]
- Ren YL, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int. 2000;58:2053–2060. doi: 10.1111/j.1523-1755.2000.00377.x. [DOI] [PubMed] [Google Scholar]
- Sallstrom J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 2007;190:253–259. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Ayajiki K, Nishio Y, Sugaya T, Kashiwagi A, Okamura T. Evidence for a causal role of the renin-angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension. 2004;43:255–262. doi: 10.1161/01.HYP.0000111136.86976.26. [DOI] [PubMed] [Google Scholar]
- Skott P, Hommel E, Bruun NE, Arnold-Larsen S, Parving HH. The acute effect of acetazolamide on glomerular filtration rate and proximal tubular reabsorption of sodium and water in normal man. Scand J Clin Lab Invest. 1989;49:583–587. doi: 10.3109/00365518909089139. [DOI] [PubMed] [Google Scholar]
- Sorensen CM, Leyssac PP, Salomonsson M, Skott O, Holstein-Rathlou NH. ANG II-induced downregulation of RBF after a prolonged reduction of renal perfusion pressure is due to pre- and postglomerular constriction. Am J Physiol Regul Integr Comp Physiol. 2004;286:R865–873. doi: 10.1152/ajpregu.00424.2003. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004;286:F8–15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- Thorup C, Ollerstam A, Persson AE, Torffvit O. Increased tubuloglomerular feedback reactivity is associated with increased NO production in the streptozotocin-diabetic rat. J Diabetes Complications. 2000;14:46–52. doi: 10.1016/s1056-8727(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Traynor T, Yang T, Huang YG, Arend L, Oliverio MI, Coffman T, Briggs JP, Schnermann J. Inhibition of adenosine-1 receptor-mediated preglomerular vasoconstriction in AT1A receptor-deficient mice. Am J Physiol. 1998;275:F922–927. doi: 10.1152/ajprenal.1998.275.6.F922. [DOI] [PubMed] [Google Scholar]
- Tucker BJ, Blantz RC. Studies on the mechanism of reduction in glomerular filtration rate after benzolamide. Pflugers Arch. 1980;388:211–216. doi: 10.1007/BF00658483. [DOI] [PubMed] [Google Scholar]
- Tucker BJ, Steiner RW, Gushwa LC, Blantz RC. Studies on the tubulo-glomerular feedback system in the rat. The mechanism of reduction in filtration rate with benzolamide. J Clin Invest. 1978;62:993–1004. doi: 10.1172/JCI109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) Receptors Determine Glomerular Hyperfiltration and the Salt Paradox in Early Streptozotocin Diabetes Mellitus. Nephron Physiol. 2009;111:30–38. doi: 10.1159/000208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Wilcox CS, Thomson SC. Nitric oxide and tubuloglomerular feedback. Semin Nephrol. 1999;19:251–262. [PubMed] [Google Scholar]
- Vestri S, Okamoto MM, de Freitas HS, Aparecida Dos Santos R, Nunes MT, Morimatsu M, Heimann JC, Machado UF. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol. 2001;182:105–112. doi: 10.1007/s00232-001-0036-y. [DOI] [PubMed] [Google Scholar]
- Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974;53:1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimpelmann J, Kumar D, Levine DZ, Wehbi G, Imig JD, Navar LG, Burns KD. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int. 2000;58:2320–2330. doi: 10.1046/j.1523-1755.2000.00416.x. [DOI] [PubMed] [Google Scholar]