Abstract
Abnormal expression of anaplastic lymphoma kinase (ALK) gene is a key pathogenic factor for Anaplastic Large Cell Lymphoma (ALCL). To study the role of ALK, an inducible shRNA system was stably introduced into cultured human ALCL cells. Inducing shRNA expression in the generated cells resulted in cellular ALK gene silencing and led to inactivation of multiple signaling pathways and growth arrest. Interestingly, a combination of ALK gene silencing with U0126, a kinase inhibitor specific for the ERK1/2 pathway, resulted in an augmented reduction in cellular JunB expression. Functional studies indicated that combining ALK gene silencing with U0126 treatment provided a synergistic growth inhibition, which occurred faster and was more profound than with either treatment alone. This synergistic effect was also observed when measuring cell proliferation, apoptosis, and in vitro cell colony formation. Importantly, the combination of ALK gene silencing and U0126 had a prolonged inhibitory effect, preventing recovery of ALCL cell growth even after treatments were removed. Moreover, this synergistic inhibitory effect was confirmed in vivo using a mouse model with xenografted ALCL tumors. Our findings indicate that combining cellular ALK gene silencing with a low dose of U0126 may prove to be an effective and more specific therapeutic approach to treating ALCL.
Keywords: Anaplastic Large Cell Lymphoma (ALCL), Anaplastic Lymphoma Kinase (ALK), ERK1/2 pathway, kinase inhibitor U0126, small interference RNA (siRNA)-induced gene silencing, synergistic growth effect
Introduction
Anaplastic lymphoma kinase (ALK)-positive Anaplastic Large Cell Lymphoma (ALCL) is an aggressive T-cell lymphoma that carries a chromosomal translocation involving the ALK gene (Stein et al., 2000; Delsol et al., 2008). The most common chromosomal aberration is the translocation t(2;5)(p23;q35), occurring in nearly 75% of cases, which results in abnormal expression of a chimeric fusion protein, nucleophosmin (NPM)-ALK (Morris et al., 1994 and 1997; Duyster et al., 2001). Auto-phosphorylation and subsequent activation of ALK in the fusion proteins has been demonstrated to be lymphomagenic both in vitro and in vivo (Fujimoto et al., 1996; Kuefer et al., 1997; Chiarle et al., 2003; Amin and Lai, 2007). Currently, a multi-drug chemotherapy approach is the mainstream treatment for ALCL (Shulman et al., 1993; Reiter et al., 1994). However, the chemotherapy used is neither cell- nor gene-specific. The presence of a unique molecular pathogenesis (abnormal ALK expression) in ALCL provides a molecular basis to develop more specific therapeutic approaches. RNA interference, the process by which specific mRNAs are targeted for degradation by complementary small interfering RNA (siRNA), enables one to silence a single gene at the cellular level (Hannon, 2002; Zamore, 2002; ). Recent studies have shown that transient transfection of cells with synthetic siRNAs could ablate cellular ALK gene expression, cause cell growth inhibition, and augment the anticancer effects of chemotherapy in vitro, suggesting that the ALK gene is a potential target for specific treatment of ALCL (Ritter et al., 2003; Piva et al., 2006; Hsu et al., 2007).
As cellular ALK fusion proteins have a relative long half-life (at least 48 hours), repeated transfection/introduction of siRNA into cells was necessary for successful gene silencing (Ritter et al., 2003). However, repeated transfection or use of a high dose of siRNA led to pronounced levels of cytotoxicity with a subsequent reduction in cell viability (Hsu et al., 2007). An additional challenge is that the transfection/introduction rate of siRNAs into ALCL cells frequently varies in efficiency from 15% to 70% (Piva et al., 2006). Together, these issues highlight the difficulty in validating functional changes that result from ALK gene silencing, versus those induced by cell manipulations including serum starvation, transfection reagents, medium changes, temperature alteration, and cell sorting through a laser beam. To overcome this obstacle and perform reproducible studies, an assay system in which the cellular ALK gene can be silenced in a stable and inducible manner is indispensable. In this study, we established a stable cell model by introducing a tetracycline-inducible short hairpin RNA (shRNA)-expressing system into cultured human ALCL cells. Moreover, we go on to use this stable cell model to demonstrate that synergistic inhibition of ALCL cell growth could be achieved by combining ALK gene silencing with a low dose of the inhibitor U0126 specific for extracellular signal-regulated kinases 1/2 (ERK1/2) pathway (Favata et al., 1998).
Results
Establishing ALCL cells with an inducible shRNA construct that specifically silences cellular NPM-ALK gene
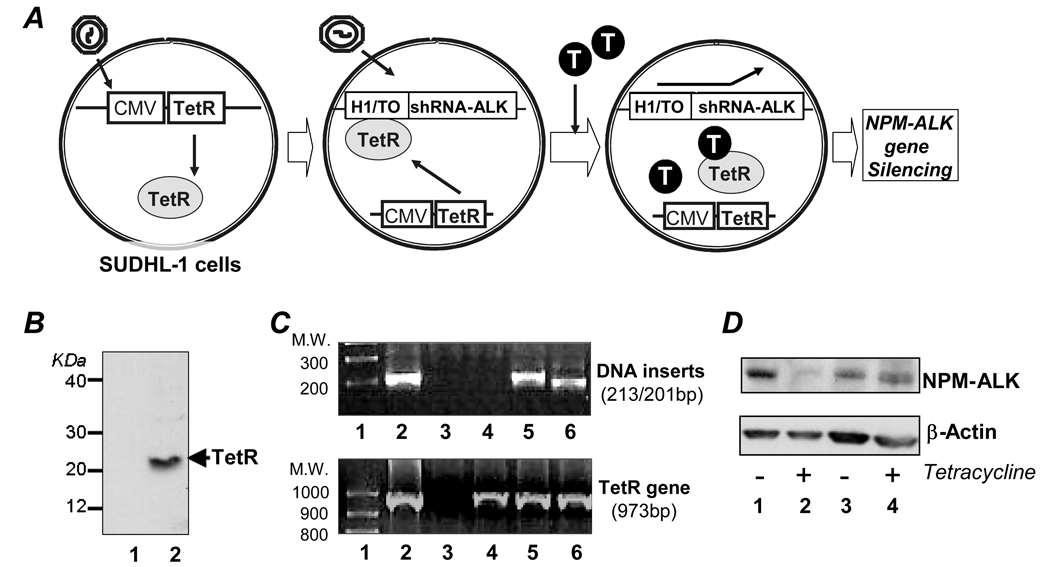
To establish stably transfected cells with an inducible shRNA for ALK gene silencing, we utilized a human ALCL cell line (SUDHL-1) (Morgan et al., 1989). Two gene-expressing constructs were introduced in a stepwise fashion as illustrated in Figure 1A. The ‘conditioned cells’ were generated to constitutively express Tetracycline Repressor (TetR) by transfecting ALCL cells with purified Lentiviral particles containing the vector pLenti6/CMV/TetR/Blasticidin. After selection for the Blasticidin-resistant ‘conditioned cells’, protein expression of TetR was confirmed. A Western blot indicates the presence of a 24-kDa protein band consistent with TetR in ‘conditioned cells’ (Figure 1B), which is not present in the parental cells. The inducible short hairpin RNA (shRNA) construct containing sequences that specifically target the C-terminal ALK portion of the NPM-ALK fusion gene were designed (shRNA-ALK), and the corresponding DNA inserts were synthesized as described in ‘Materials and Methods’. The synthesized dsDNAs encoding shRNA-ALK were cloned into a pLenti4/H1/TO/Zeocin vector and introduced into ‘conditioned cells’. To generate control cells, a non-related inducible shRNA for the Lamin gene (pLenti4/H1/TO/Zeocin/Lamin; Invitrogen) was used. Zeocin-resistant cells were examined for the presence of DNA inserts using PCR analysis of cellular DNA. As shown in the upper panel of Figure 1C, DNA inserts corresponding to shRNA-ALK (213-bp band in lane 5) and shRNA-Lamin (201-bp band in lane 6) were detected. No amplified DNA products were seen in the parental cells (lane 3) or in ‘conditioned cells’ (lane 4). The presence of the TetR gene in the same cells was confirmed by PCR with amplification of a 973-bp DNA product (lower panel of Figure 1C). TetR was detected in ‘conditioned cells’ (lane 4), as well as in the generated cells containing the shRNA-ALK construct (lane 5) or shRNA-Lamin construct (lane 6), but it was not present in the parental cells (lane 3).
Figure 1. Establishing a stable ALCL cell model that contains an inducible shRNA construct for specifically silencing the ALK gene. A, Overview of cell model.
First, ‘conditioned cells’ are generated by stable introduction of pLenti/CMV/TetR/Blasticidin vector into cultured human ALCL cells (SUDHL-1) to constitutively express Tetracycline Repressor (TetR) under CMV promoter control. Subsequentially, the pLenti/H1/TO/shRNA-ALK/Zeocin construct is introduced to express shRNA-ALK that specifically targets the ALK portion of the NPM-ALK gene. The H1/TO promoter of shRNA-ALK construct is normally suppressed by the presence of TetR. As desired, addition of tetracycline (T) into cell media will lead to competitive binding of tetracycline to TetR, resulting in activation of H1/TO promoter. As a gene silencing control, pLenti/H1/TO/shRNA-Lamin vector is stably introduced into ‘conditioned cells’ to generate control cells that carry an inducible shRNA-Lamin construct. B, Stable TetR protein expression in ‘conditioned cells’. Western blot analysis confirmed stable protein expression of TetR (24-kDa band) in ‘conditioned cells’ (lane 2). Parental ALCL cells are shown in lane 1 as a negative control. C, DNA inserts encoding shRNAs and TetR gene. Upper panel: PCR analysis of DNA inserts encoding shRNAs. Lane 1: molecular weight markers; Lane 2: plasmid DNA control of pLenti/H1/TO/shRNA-ALK/Zeocin with a 213-bp band; Lane 3: parental ALCL cells with no amplified DNA products; Lane 4: ‘conditioned cells’ with no amplified DNA products; Lane 5: generated cells containing shRNA-ALK construct with a 213-bp band identical to that seen in plasmid DNA control (lane 2); and Lane 6: control cells with a 201-bp band corresponding to the DNA insert encoding shRNA-Lamin. Lower panel: PCR analysis of cellular TetR gene. Lane 1: molecular weight markers.; Lane 2: control plasmid of pLenti6/CMV/TetR/Blasticidin with a 973-bp band; Lane 3: parental SUDHL-1 cells with no amplified DNA products; Lane 4: ‘conditioned cells’ containing TetR gene with a 973-bp band identical to that seen in plasmid DNA control (lane 2); Lane 5: generated cells containing both shRNA-ALK and TetR gene with a 973-bp band identical to that seen in plasmid DNA control (lane 2); and Lane 6: control cells with a 973-bp band identical to that seen in plasmid DNA control (lane 2). D, Inducing shRNA-ALK suppresses NPM-ALK protein expression. Cells were treated with 3 µg/ml tetracycline for 6 days and ALK gene silencing was monitored by detecting cellular NPM-ALK protein expression using Western blotting. Lanes 1 and 2: generated cells containing an inducible shRNA-ALK. Lanes 3 and 4: control cells carrying an inducible shRNA-Lamin. Expression of cellular β-actin was used as an internal control for equivalent cellular protein loading. Experiments were repeated three times with similar results.
To induce shRNA expression and silence the cellular ALK gene, the generated cells were treated with tetracycline (3 µg/ml) for 6 days. Western blot assays indicated that the protein expression of NPM-ALK was significantly reduced in tetracycline treated cells containing the inducible shRNA-ALK construct (Figure 1D, lanes 1 and 2), but was not altered in control cells carrying the inducible shRNA-Lamin construct (lanes 3 and 4).
Silencing the cellular ALK gene results in growth arrest, apoptosis, and death of ALCL cells
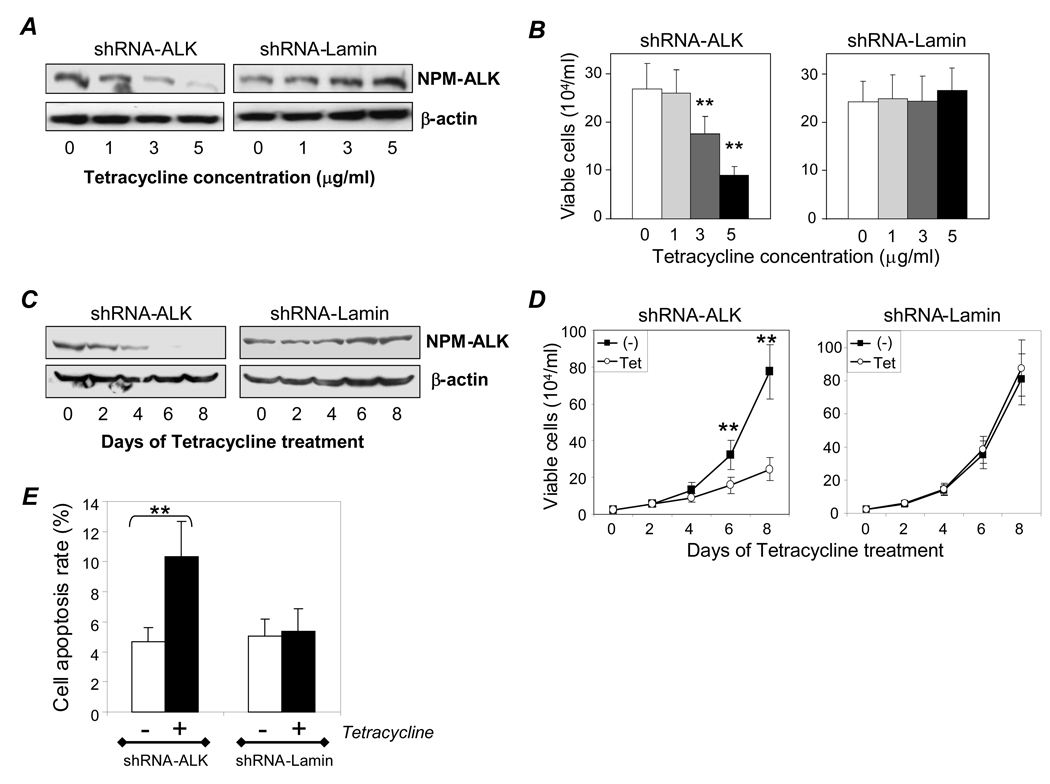
To obtain optimal ALK gene silencing conditions, the cells were treated with different concentrations of tetracycline for 6 days. Western blot assays indicated that the level of ALK gene silencing was tetracycline dose-dependent and reached maximal inhibition in the presence of 5 µg/ml tetracycline (Figure 2A). No change in NPM-ALK protein expression was observed in the control cells containing the inducible shRNA-Lamin construct. To evaluate the corresponding cellular effects that resulted from ALK gene silencing, the treated cells were simultaneously stained with trypan blue and viable cells were counted. A tetracycline dose-dependent growth inhibition was observed in cells containing the inducible shRNA-ALK construct (Figure 2B, p < .01), which was not present in the control cells with inducible shRNA-Lamin. As NPM-ALK fusion proteins have a relative long half-life time of over 48 hours (Ritter et al., 2003), a time course of ALK gene silencing was performed by treating cells with tetracycline (3 µg/ml) for 8 days. Western blot assays demonstrated that cellular NPM-ALK protein levels were significantly decreased after 4 days of tetracycline treatment, and nearly abolished after 6 days (Figure 2C). In addition, simultaneous studies on cell growth were carried out by counting the number of viable cells present at each time-point. Significant inhibition in the number of viable cells was seen in cells containing the inducible shRNA-ALK construct after tetracycline treatment for 6 days (Figure 2D, p < .01), a timeline that closely matches the observed changes in cellular NPM-ALK protein expression. In contrast, tetracycline treatment had no effect on the growth rate of the control cells carrying inducible shRNA-Lamin.
Figure 2. ALK gene silencing by inducing shRNA-ALK results in cell growth arrest, apoptosis, and death. A, Dose-dependent effect of tetracycline treatment on NPM-ALK expression.
Cells were treated with the indicated concentration of tetracycline for 6 days, and NPM-ALK expression was detected by Western blot (upper panel). β-actin expression was used as internal control for equivalent cellular protein loading (lower panel). B, Corresponding effect on cell growth. Treated cell growth was monitored using trypan blue staining. C, Time course study of ALK gene silencing. Cells were treated with 3 µg/ml tetracycline for up to 8 days and NPM-ALK expression was monitored by Western blot. D, Corresponding effect on cell growth. Cell growth was examined by counting viable cells. E, Cell apoptosis. Cells were treated with 3 µg/ml tetracycline (solid bars) for 4 days, and apoptotic cells were detected by flow cytometry. Apoptosis rates (%) among cells containing shRNA-ALK (left) and control cells carrying an inducible shRNA-Lamin (right) are displayed. Experiments were repeated three times with similar results. Student’s t-test: ** p < .01 versus control.
To study the effect of ALK gene silencing on cell apoptosis, the generated cells were treated with tetracycline (3 µg/ml) for 4 days and stained with FITC-conjugated Annexin V. Flow cytometry analysis illustrated that silencing of the ALK gene markedly stimulated cell apoptosis from a basal level of 4.7% (Figure 2E, open bar) to 10.4% (closed bar, p < .01). In contrast, no change in cell apoptosis was seen in control cells carrying inducible shRNA-Lamin. Taken together, our results confirm that NPM-ALK protein expression is indispensible for ALCL cell growth and survival, and the generated cells are a useful model for studying ALK gene silencing.
Combining ALK gene silencing and the kinase inhibitor U0126 leads to synergistic inhibition of cellular JunB protein expression and ALCL cell growth
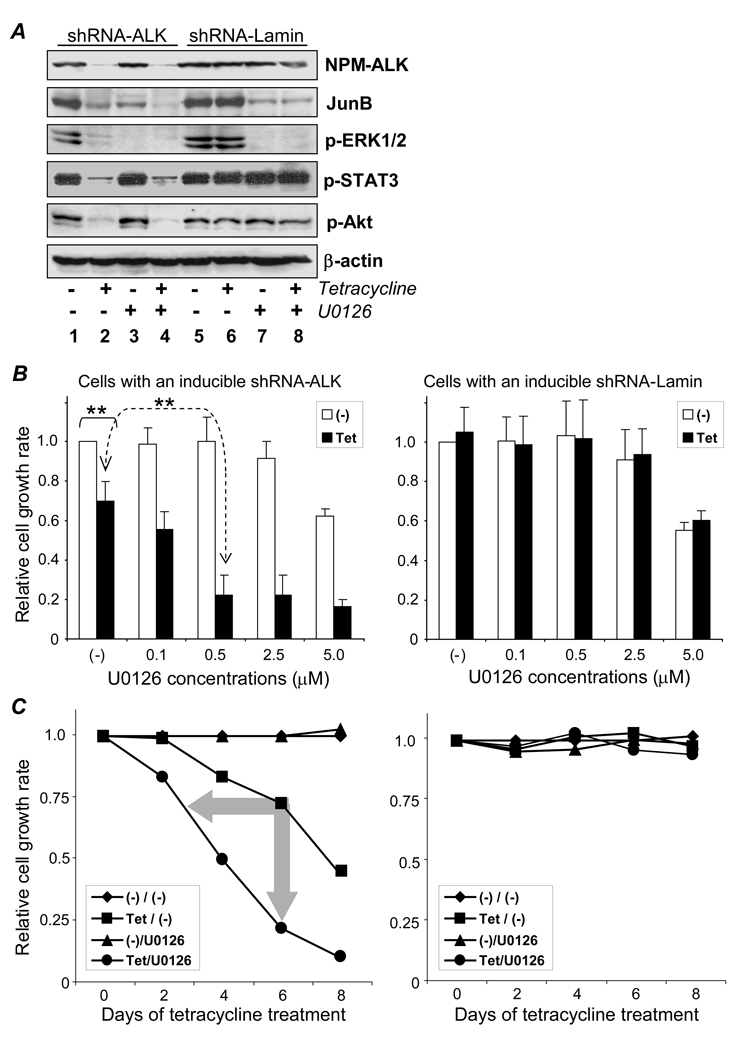
To study ALK-regulated cellular signaling pathways (Bai et al., 2000; Zamo et al., 2002; Chiarle et al., 2005; Amin et al., 2007; ), the generated cells were treated with tetracycline to induce gene silencing for 6 days as described above. Resultant changes in the activity of cellular ERK1/2, STAT3, and Akt were examined by Western blot using antibodies that recognize their phosphorylated/activated forms (p-ERK1/2, p-STAT3, and p-Akt). In addition, changes in protein expression levels of cellular JunB were studied. Simultaneously, the status of ALK gene silencing was followed by monitoring expression levels of NPM-ALK protein. As an internal control for the Western blot studies, equivalent protein loading β-actin was examined. Silencing of the ALK gene by shRNA induction with tetracycline resulted in a marked decrease of cellular p-ERK1/2, p-STAT3, p-Akt, and JunB (Figure 3A, lanes 1 and 2). In contrast, tetracycline treatment had no effect on control cells with inducible shRNA-Lamin (lanes 5, and 6). The abnormal expression of JunB protein mediated by CD30 activation in ALCL cells has been suggested to act via the ERK1/2 pathway (Watanabe et al., 2005). To block the ERK1/2 pathway, cells were exposed for 2 days to the kinase inhibitor U0126 which is specific for MEK1/2, an upstream kinase of ERK1/2 (Favata et al., 1998). As expected, exposure to U0126 (10 µM) completely inhibited ERK1/2 phosphorylation/activity and resulted in a significant decrease of JunB protein expression, while having no effect on p-STAT3, p-Akt, and NPM-ALK (lanes 3 and 7). Thus, silencing of the ALK gene or down-regulating the ERK1/2 pathway by U0126 resulted in a decrease of cellular JunB protein expression in ALCL cells. We then examined whether altering both signaling pathways (ALK and ERK1/2) would have synergistic effects. To this end, cells were treated with tetracycline for 6 days to induce ALK gene silence and were also exposed to U0126 during the last 2 days of tetracycline treatment. Changes in the activity of individual signaling pathways were examined as described above. Inhibition of both ALK gene expression (by the induced shRNA-ALK) and the ERK1/2 pathway (by addition of U0126) led to an augmented suppression of cellular JunB protein expression, but did not alter the level of p-STAT3 or p-Akt (lane 4). Furthermore, the enhanced inhibition on JunB protein expression was not seen in the control cells with inducible shRNA-Lamin (lanes 5–8). These findings suggest that although the activity of ERK1/2 pathway is partially dependent on ALK fusion protein expression in ALCL cells, it appears the ALK and ERK1/2 signaling pathways may cooperate, and independent inhibition of both pathways leads to synergistic effects on JunB protein expression.
Figure 3. Combining ALK gene silencing and U0126 had a synergistic effect on cellular JunB protein expression and cell growth. A, Cooperative effect of ALK and EKR1/2 pathways on cellular JunB expression.
Cells were treated with 3 µg/ml tetracycline alone for gene silencing (lanes 2 and 6), 10 µM U0126 alone for down-regulating the ERK1/2 pathway (lanes 3 and 7), or both (lanes 4 and 8) for 6 days, and changes in the activity of signaling pathway proteins were examined. NPM-ALK and JunB expression was detected by Western blot, and ERK1/2, Akt, and STAT3 activity was detected by evaluating their phosphorylated forms (p-ERK1/2, p-STAT3, and p-Akt). Lanes 1–4: cells containing shRNA-ALK. Lanes 5–8: control cells carrying shRNA-Lamin. NPM-ALK expression (top) confirms ALK gene silencing, while β-actin expression (bottom) verifies equivalent cellular protein loading. B, Synergistic effects of ALK gene silencing and U0126 on cell growth. Cells were treated with 3 µg/ml tetracycline for ALK gene silencing (solid bars) or without (open bars). In addition, cells were exposed to different concentrations of U0126. After treatment(s) for 6 days, viable cells in each condition were counted, and relative cell growth rates were calculated. Control cells carrying shRNA-Lamin were exposed to the same treatment(s). C, Time course study of synergistic effects of ALK gene silencing and low dose U0126 on cell growth. Cells were treated with 3 µg/ml tetracycline [Tet/(−)] and 0.5 µM U0126 [(−)/U0126], individually and in combination, for 8 days, and relative cell growth rates were determined. Arrows indicate synergistic inhibition of cell growth. Control cells carrying shRNA-Lamin were exposed to the same treatment(s) (right panel). Experiments were repeated three times with similar results. Student’s t-test: ** p < .01 versus control.
Observation of the cooperative effect on cellular JunB expression suggested that the combination of treatments might also synergistically regulate other ALCL cell functions. To this end, the cells were treated with tetracycline to induce ALK gene silencing and were simultaneously exposed to increasing doses of U0126 (0.1–5 µM) for 6 days. Cell growth rates were then calculated by the ratio of viable cells in treated conditions versus viable cells in untreated controls. As shown in Figure 3B, silencing of the ALK gene alone with tetracycline treatment (solid bars) led to approximately a 31% reduction in cell growth (p < .01). Exposure of cells to low doses (≤ 0.5 µM) of U0126 alone (open bars) had no effect on cell growth, although the presence of 5 µM U0126 resulted in about 37% inhibition. A significantly enhanced inhibition of cell growth (~80% reduction) was achieved by combining ALK gene silencing with a low dose (0.5 µM) of U0126 (p < .01). In contrast, this synergistic inhibitory effect was not observed with the control cells carrying inducible shRNA-Lamin, although they did respond in a similar manner to a high dose of U0126 (5 µM) with an approximately 43% reduction observed in cell growth . To further dissect the observed synergistic effect, an extended time-course study (8 days) with 0.5 µM of U0126 was performed and resultant changes in cell growth rates were examined. Silencing of the ALK gene alone via tetracycline treatment resulted in cell growth inhibition, which was initially evident at day 4 [Figure 3C, Tet/(−)]. When the low dose of U0126 treatment was combined with ALK gene silencing a synergistic inhibition of cell growth was observed (Tet/U0126), with the effects occurring faster (evident at day 2 when used in combination vs. day 4 for ALK gene silencing alone) and being more pronounced (~80% suppression with the combination treatments vs. 30% with ALK gene silencing alone at day 6). It is notable that the presence of 0.5 µM of U0126 alone had no effect on cell growth [(−)/U0126], with growth rates being comparable to cells receiving no treatment(s) [(−)/(−)]. In addition, the control cells with inducible shRNA-Lamin had no change in their growth rate even though they underwent similar treatments.
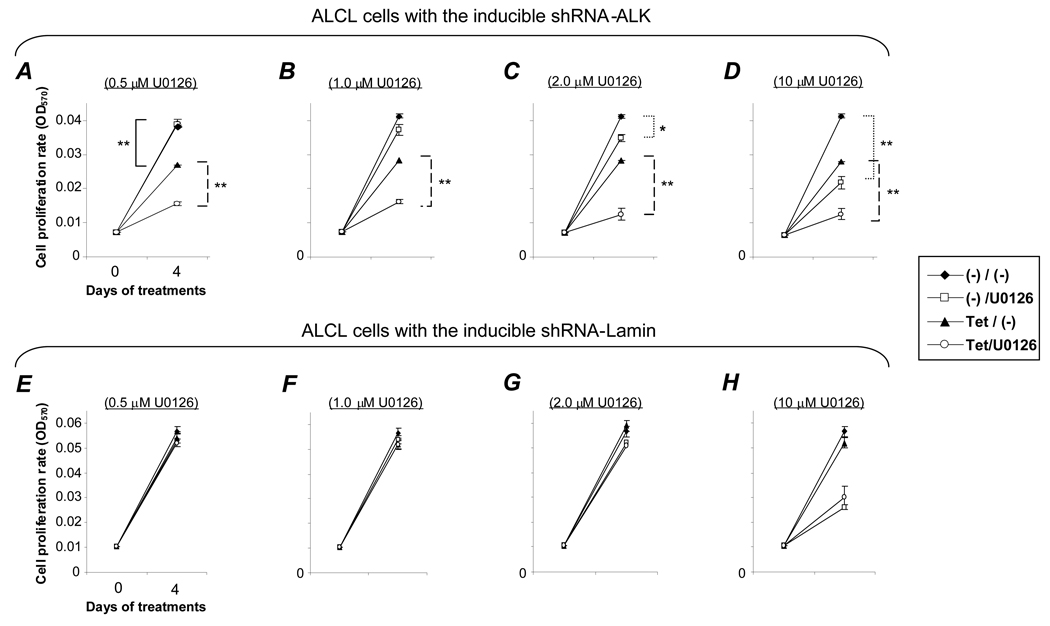
To verify the observed synergistic effects on cell growth, we performed MTT cell proliferation assays. The cells were treated with tetracycline to induce ALK gene silencing and simultaneously were exposed to different doses of U0126 as indicated for 4 days (Figure 4). As was observed with viable cell counting experiments, the MTT assays showed silencing of the ALK gene alone caused a moderate decrease in cell proliferation [Figures 4A–D, Tet/(−), p < .01]. This ALK gene silencing-induced effect was significantly augmented by U0126 at a concentration as low as 0.5 µM (Tet/U0126, p < .01). It is important to note that at this low concentration, when used alone, U0126 had no effect on cell proliferation [(−)/U0126]. Moreover, although higher concentrations of U0126 (> 2 µM) suppressed cell proliferation, this effect could be further enhanced when combined with ALK gene silencing. In the control cells with inducible shRNA-Lamin, exposure to U0126 alone showed a similar effect on cell proliferation to the pattern seen with cells containing the inducible shRNA-ALK construct [Figures 4E–H, (−)/U0126]. However, the control cells showed no change in cell proliferation with tetracycline treatment alone [Tet/(−)], and no synergistic effect with tetracycline and U0126 (Tet/U0126). Taken together, these findings demonstrate that a combination of ALK gene silencing along with a low dose of U0126 can achieve a synergistic inhibition of ALCL cell growth/proliferation.
Figure 4. Synergistic inhibition of cell proliferation by combining ALK gene silencing and low dose U0126.
Cells containing an inducible shRNA-ALK construct (A–D) were treated with 3 µg/ml tetracycline alone [Tet/(−)], different concentrations of U0126 alone [(−)/U0126], or both tetracycline and U0126 (Tet/U0126) for 4 days, and cell proliferation was measured using an MTT assay. Control cells carrying an inducible shRNA-Lamin (E–H) were exposed to the same treatments. Experiments were repeated three times with similar results. Student’s t-test: * p < .05 and ** p < .01 versus controls.
Synergistic effects of ALK gene silencing and low dose U0126 on cell apoptosis and in vitro cell colony formation
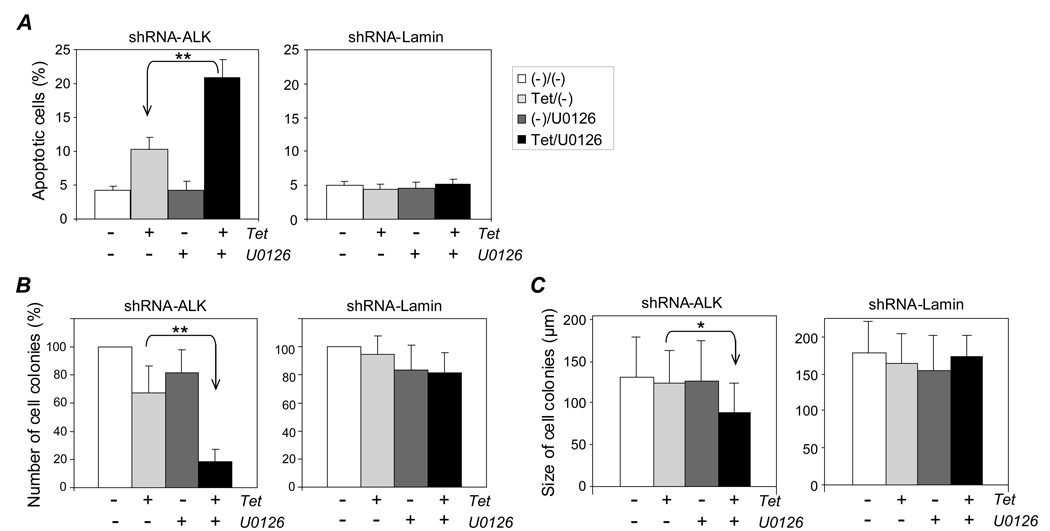
To determine if the observed changes in cell viability were due in part to an increase in apoptosis, the cells were treated with tetracycline to induce the ALK gene silencing alone or also received a low dose of U0126 (0.5 µM) for 4 days. Apoptotic cells were stained with Annexin-V and detected by flow cytometry. Silencing of the ALK gene alone induced about a 1-fold increase in the rate of cell apoptosis (Figure 5A, lighter gray bar). Although a low dose (0.5 µM) of U0126 alone had no effect on the cell apoptosis rate (darker gray bar), when used in combination with ALK gene silencing the cell apoptosis rate increased by 4-fold (solid bar, p < .01). In contrast, this marked increase in cell apoptosis rate was not observed in control cells carrying the inducible shRNA-Lamin construct undergoing the same treatment(s).
Figure 5. Synergistic effects of ALK gene silencing and low dose U0126 on cell apoptosis and in vitro cell colony formation. A, Cell apoptosis.
Cells were treated with 3 µg/ml tetracycline (lighter gray bars), 0.5 µM U0126 (darker gray bars), or both (solid bars) for 4 days and stained with Annexin-V. Control cells carrying shRNA-Lamin exposed to the same treatments are shown in the right panel. B, Cell colony formation. Cells were cultured in semi-solid culture medium containing tetracycline (lighter gray bars), 0.5 µM U0126 (darker gray bars), or both (solid bars) for 7 days, and cell colonies were counted under a light microscope. The relative cell colony formation rate (%) is shown, with non-treated cells as a negative control (open bars). Control cells carrying shRNA-Lamin exposed to the same treatments are shown in the right panel. C, Cell colony size. The diameter of colonies (n =50) was measured under a light microscope equipped with ruler lens. Combination of ALK gene silencing with low dose U0126 (left panel) and control cells carrying shRNA-Lamin (right panel). Experiments were repeated three times with similar results. Student’s t-test: * p < .05 and ** p < .01 versus controls.
To evaluate the potential therapeutic value of the combination treatment, we examined the effects on in vitro cell colony formation. Cells were cultured in semi-solid methylcellulose medium containing tetracycline (3 µg/ml) to induce ALK gene silencing and/or 0.5 µM U0126 for 7 days. The formed cell colonies were then counted using light microscopy. As shown in Figure 5B, ALK gene silencing alone induced a moderate inhibition of cell colony formation (33% decrease; lighter gray bar). This ALK gene silencing-induced inhibition was significantly enhanced when combined with U0126 treatment (> 80% decrease; solid bar, p < .01). It should be noted that U0126 alone had only a mild inhibitory effect (darker gray bar). In the control cells carrying inducible shRNA-Lamin, no inhibition of cell colony formation was observed with tetracycline treatment alone, while a similar mild inhibitory effect was observed when U0126 was given. However, no synergistic inhibition was noted in the control cells receiving a combination of tetracycline treatment and U0126. In addition, we also examined the effects of the combination treatment on the size (diameter) of formed cell colonies using a microscope equipped with ruler lens. Neither treatment alone, tetracycline to induce ALK gene silencing nor 0.5 µM U0126 to inhibit the ERK1/2 pathway, had any effect on the size of cell colonies (Figure 5C, lighter and darker gray bars). However, when used together the combination treatment resulted in a significant inhibition in the size of formed cell colonies in cells containing inducible shRNA-ALK (solid bar, p < .05). In contrast, this synergistic effect was not seen in the control cells carrying an inducible shRNA-Lamin.
Combination treatment of ALK gene silencing and U0126 has a synergistic effect on recovering cell growth post-treatment
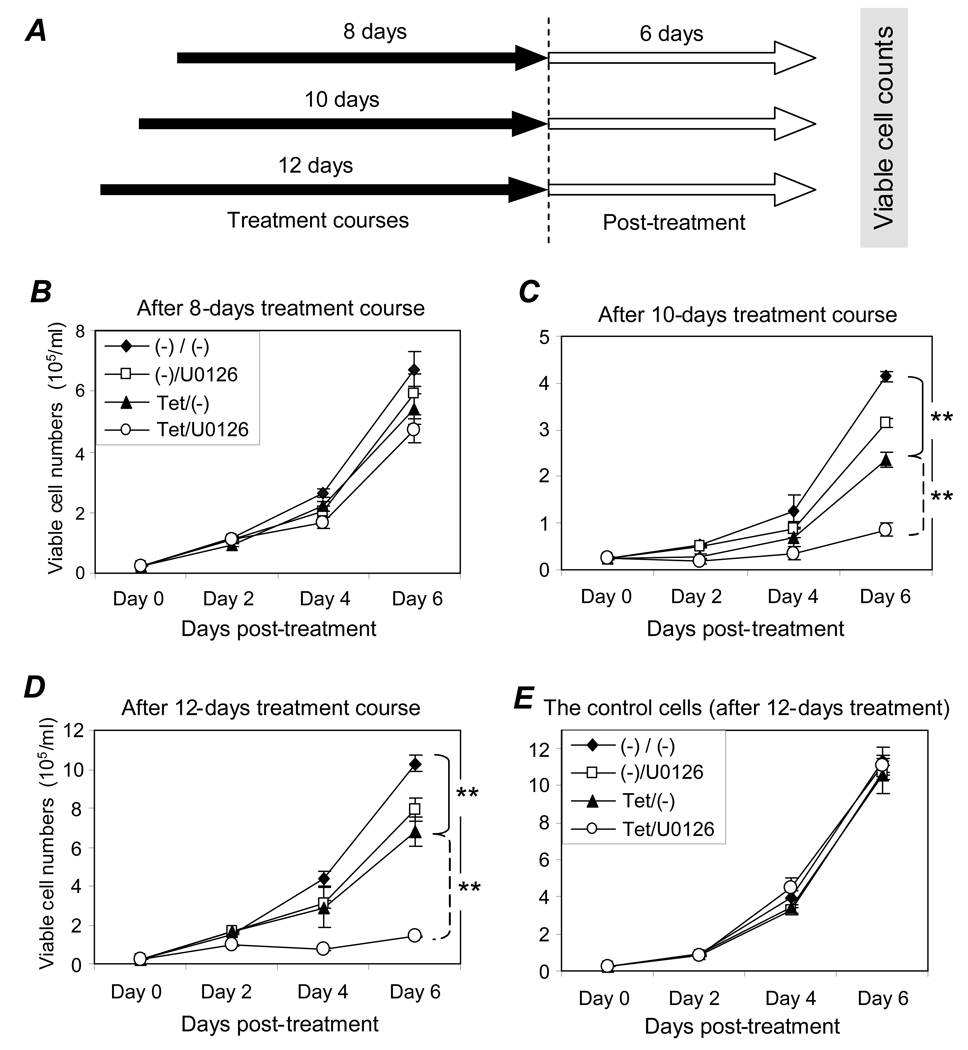
To evaluate potential post-treatment effects of the combination treatment, the cells were treated with tetracycline to induce gene silencing and/or 0.5 µM U0126 to inhibit cellular ERK1/2 pathway. As illustrated in Figure 6A, after 8, 10, or 12 days, the cells were washed with fresh medium to remove all treatment agents and an equal number of viable cells from each testing condition were cultured in the absence of treatment(s) for an additional 6 days. Although no significant effect on recovering cell growth was seen after an 8-day treatment course (Figure 6B), silencing of the ALK gene alone with tetracycline treatment for 10 days resulted in a significant inhibition in cell growth post-treatment [Figure 6C, Tet/(−), p < .01]. This inhibition was markedly augmented by the presence of U0126 (Tet/U0126, p < .01). Moreover, this augmented suppression on growth recovery was even more apparent after a 12-day course of the combination treatment with recovering cell growth post-treatment being nearly eliminated (Figure 6D, Tet/U0126, p < .01). In contrast, no change in cell growth post-treatment was detected in the control cells carrying an inducible shRNA-Lamin even after a 12-day course of the combination treatment (Figure 6E). These findings demonstrate that a minimum of a 10-day course with the combination treatment of ALK gene silencing and 0.5 µM U0126 is necessary to trigger prolonged post-treatment cell growth inhibition and a ≥12-day course of the combination treatment may prevent ALCL cell growth recovery post-treatment.
Figure 6. Prolonged inhibition of cell growth by combination of ALK gene silencing and low dose U0126. A, Overview of analysis.
Cells were treated with 3 µg/ml tetracycline and/or 0.5 µM U0126 for 8, 10, and 12 days (solid arrows). Treatments were then removed by cell washing, and equal numbers of viable cells from each condition were cultured in fresh medium with no treatment added for 6 days (open arrows). B, Cell growth after 8-day treatment. Cells were treated for 8 days with tetracycline [Tet/(−)], 0.5 µM U0126 [(−)/U0126], or both (Tet/U0126), then washed, and cultured in fresh medium with no treatment. Cell growth post-treatment was monitored by counting viable cells every other day. C, Cell growth after 10-day treatment. Same as for panel B but cells were treated for 10 days. D, Cell growth after 12-day treatment. Same as for panel B but cells were treated for 12 days. E, Control cell growth after 12-day treatment. Control cells carrying an shRNA-Lamin were exposed to the same treatments as described above for 12 days. All experiments were repeated three times with similar results. Student’s t-test: ** p < .01 versus controls.
Combination treatment of ALK gene silencing and U0126 has a synergistic effect on ALCL tumor growth in vivo
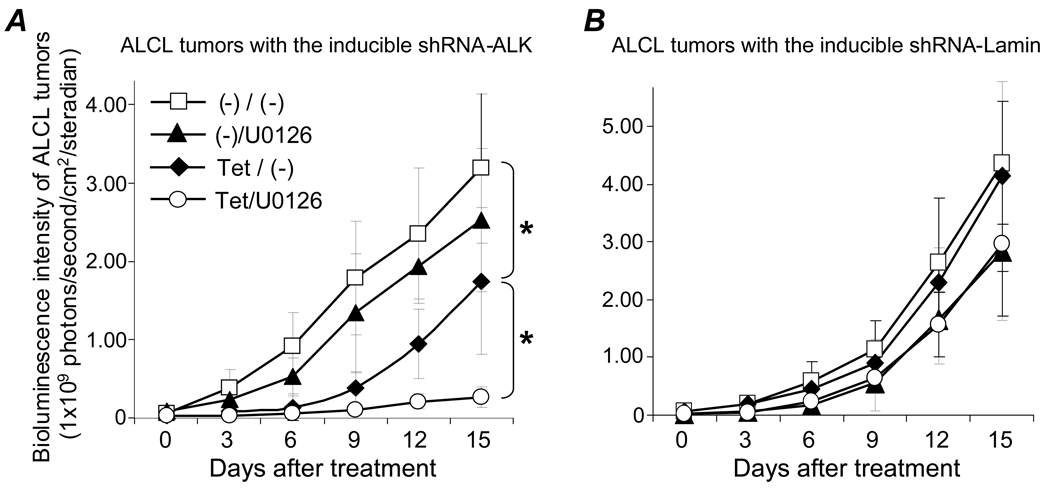
For in vivo studies, a mouse model with xenografted tumors was established by inoculating traceable ALCL cells containing shRNA-ALK subcutaneously in the right hind limb and control cells carrying shRNA-Lamin in the left hind limb of each mouse. After confirmation of tumor development, tumor-bearing mice were treated (n=5/condition) and resultant changes in tumor mass were monitored by whole body bioluminescence scanning to detect luciferase activity in traceable ALCL tumor cells (Figure S2). Inducing shRNA-ALK for specific gene silencing by feeding mice tetracycline resulted in a significant decrease in ALCL tumor growth [Figure 7A, Tet/(−), p < .05] and treatment with U0126 alone showed a minor effect on tumor growth [(−)/U0126]. Interestingly, the combined treatment with both ALK gene silencing and U0126 resulted in a synergistic inhibition of ALCL tumor growth (Tet/U0126, p < .05). In contrast, inducing shRNA-Lamin alone or in combination with U0126 treatment had no additional effect on control ALCL tumor growth (Figure 7B).
Figure 7. Synergistic inhibition of xenografted ALCL tumor growth by combination of ALK gene silencing and U0126 treatment.
Mice bearing tumors derived from ALCL cells containing shRNA-ALK (A) and carrying shRNA-Lamin (B) were fed tetracycline [Tet/(−)], treated with U0126 [(−)/U0126], treated with both (Tet/U0126), or none [(−)/(−)]. Tumor mass was monitored by whole body bioluminescence scanning every 3 days, and the strength of bioluminescence signals from the xenografted tumors were measured as photons in a digital format (photons/second/cm2/steradian). n=5 mice per condition. Student’s t-test: * p < .05 versus control.
Discussion
ALCL is currently treated with a multi-drug chemotherapy, with the most prevalent being the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) (Shulman et al., 1993; Reiter et al., 1994). However, CHOP chemotherapy is neither cell- nor gene-specific. In ALCL, the unique underlying pathogenesis of a chromosomal translocation of the ALK gene with subsequent abnormal expression of ALK fusion proteins provides a novel molecular target at which to direct specific therapeutics. Recent studies have shown that transient transfection of cells with synthetic siRNAs could ablate cellular ALK gene expression and led to cell growth inhibition, suggesting the ALK gene may indeed provide a potential target for specific treatment of ALCL. Since ALK fusion proteins have a relative long half-life (> 48 hours), it is difficult to reliably silence ALK gene expression through transient transfection (Ritter et al., 2003). Thus, we have established a stable ALCL cell line containing tetracycline inducible shRNA that can be used to silence the ALK gene at will (Figure 1). Using this cell model, we found that among multiple active signaling pathways in ALCL cells, the ERK1/2 and ALK pathways co-operate in regulating JunB protein expression (Figure 3A). To block the ERK1/2 pathway we used the kinase inhibitor U0126, which is specific for MEK1/2, an upstream kinase of ERK1/2. MEK1/2 inhibitors inhibit in vitro growth of leukemic cells (Milella et al., 2001), development of human tumors in mouse xenografts (Horiuchi et al., 2003; Dai et al., 2008; Wentz et al., 2008), and are being tested in clinical trials for a variety of malignancies (Rinehart et al., 2004; Lorusso et al., 2005; Adjei et al., 2008). Interestingly, exposure of cells to U0126 significantly enhanced ALK gene silencing-induced inhibition of ALCL cell growth even at concentrations that showed little to no effect when used alone (Figure 3 and Figure 4). The resultant synergistic inhibition of cell growth by the combination of ALK gene silencing with a low dose of U0126 treatment occurred faster and was more acute than that found with ALK gene silencing alone (Figure 3C). We also noted that if ALCL cells were exposed to the combination treatment for ≥10 days the cell growth post-treatment was significantly decreased and after a 12-day combination treatment course, cell growth post-treatment was almost completely inhibited (Figure 6). Our findings indicate that a combination of ALK gene silencing with a low dose of U0126 can not only inhibit ALCL cell growth, but may also be able prevent lymphoma recurrence post-treatment. In summary, this study provides solid evidence that a more sensitive and specific therapeutic approach for ALCL may be achieved by silencing the ALK gene with shRNA treatment in combination with a low dose of U0126 to block the ERK1/2 pathway. Finally, the generated tetracycline inducible shRNA-ALK SUDHL-1 cell line is a valuable tool for future studies directed towards understanding the role of ALK in ALCL as well as providing a useful model in which to test potential treatments.
Materials and Methods
Cell cultures and reagents
In this study, a human ALCL cell line, SUDHL-1, was used in collaboration with Dr. Mark Raffeld at the National Cancer Institute (NCI)/National Institutes of Health (NIH). The cell line carries chromosomal translocation t(2;5)(p23;q35) with resultant expression of NPM-ALK fusion protein (Morgan et al., 1989). Cells were routinely maintained in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS, GIBCO, Rockville, MD). The kinase inhibitor U0126 was purchased from Tocris Cookson (Ellisrille, MO). Zeocin, Blasticidin, and tetracycline were from Invitrogen (Carlsbad, CA).
Generation of stably transfected ALCL cells with inducible shRNA constructs specific for ALK
Briefly, the pBlock-It inducible H1 Lentiviral shRNA expressing system (Invitrogen) was utilized to stably introduce first a tetracycline repressor gene and then tetracycline responsive shRNA-ALK constructs into SUDHL-1 cells. Adding tetracycline into the media of the generated cells induced shRNA expression, which in turn specifically silenced the ALK gene (please see Supplemental Text for more detailed methods).
Western blotting assay of cellular proteins
Mouse antibodies for human NPM/ALK fusion protein were purchased from BD Biosciences (San Diego, CA). Rabbit antibodies for ERK1/2 protein, phosphorylated forms of ERK1/2 (Thr202/Tyr204), STAT3, and Akt were obtained from Cell Signaling (Beverly, MA). TetR antibody was acquired from GeneTex (San Antonio, TX). JunB (sc-46) antibody was acquired from Santa Cruz Biotechnology (Santa Cruz, CA), and β-actin was acquired from Sigma (St. Louis, MO). For Western blotting, cells (5 × 106 cells/sample) were lysed in 60 µl of Laemmli sample buffer (BioRad, Mississauga, ON). Cellular proteins (25 µl) were separated by 10% SDS-PAGE and then transferred to nitrocellulose membrane. Primary antibodies to the proteins of interest were incubated with the blots overnight at 4°C, followed by a peroxidase-conjugated goat anti-rabbit or mouse secondary antibody (Sigma). Protein bands were visualized using Luminol chemiluminescent detection reagents.
Functional assays
Cell treatments
To switch on the shRNAs and silence the ALK gene, cells were exposed to tetracycline (Sigma), which competitively binds to TetR and triggers activation of the H1/TO promoter to transcribe shRNA-ALK. The induced shRNA selectively interacts with and silences the ALK gene. To down-regulate the ERK1/2 signaling pathway, cells were treated with the kinase inhibitor U0126, which is specific for MEK1/2, an upstream kinase in the ERK cascade (Favata et al., 1998).
Cell growth assay
The generated cells containing shRNA-ALK and the control cells with shRNA-Lamin (1 × 105/ml) were cultured with or without addition of tetracycline (3 µg/ml). Cells were harvested and stained in PBS buffer containing 0.1% trypan blue (Sigma) for 15 minutes at room temperature, and viable cells were counted using a hemocytometer. The relative cell growth rate was derived by calculating the ratio of viable cell number in cultures with treatment(s) to the cell number in control culture (without treatment). Synergistic effects were tested by treating cells (2.5 × 105/ml) with both tetracycline and U0126, and viable cells were counted as described above. To examine if there were prolonged growth inhibition effects post-treatment, cells were treated with tetracycline (3 µg/ml) and/or 0.5 µM U0126 for 8–12 days. At the various end points, the treatments were removed by washing cells twice with fresh medium. Subsequentially, equal numbers of viable cells from each condition were re-seeded and cultured in fresh medium with no treatments for an additional time period (6 days).
Cell apoptosis assay
Cell apoptosis assays were performed using a kit from BD Biosciences (San Diego, CA). Cells (1 × 105/ml) were treated with tetracycline (3 µg/ml) with or without 0.5 µM U0126 for 4 days and stained with 5 µl of FITC-conjugated Annexin V and 5 µl of Propidium Iodide. Apoptotic cells were detected using flow cytometry.
MTT cell proliferation assay
Cells (5 × 104/ml) were treated with tetracycline (3 µg/ml) and different concentrations of U0126 for 4 days. Aliquots of cells (100 µl/sample) were transferred to 96-well plates, mixed with 10 µl of assay buffer (AB solution of the MTT assay kit, Chemicon International, Temecula, CA), and incubated at 37°C for 4 hours per the manufacturer’s instructions. The cells were then lysed by the addition of 100 µl Detergent Reagent solution and the plate was analyzed using a BioRad microplate reader. The relative cell proliferation rate is represented by the detected absorbance at OD570 in each specimen using a reference wavelength of 630 nm. Final results represent the mean number from triplicate samples.
In vitro cell colony formation assay
Cells were mixed with Methylcellulose Medium, a semi-solid culture medium (MethoCult® H4100, StemCell Technologies, Vancouver, BC, Canada) containing 5% FCS (final concentration) with or without tetracycline (3 µg/ml) and 0.5 µM U0126. The cell/Methylcellulose mixtures were then seeded into a 48-well culture plate (300 cells/0.5 ml/well) and cultured at 37°C for 7 days. Formed cell colonies were stained with 0.5 ml of cell staining solution overnight (Chemicon International) and counted. Meanwhile, the size of formed cell colonies was also calculated (50 colonies/specimen) by measuring their diameters under a light microscope equipped with a ruler lens.
In vivo tumor growth analysis
For in vivo imaging monitoring, ALCL cells carrying shRNA constructs were transfected with pcDNA6.2-Luci/eGFP to stably express luciferase reporter (see Figure S1 for details). Subsequently, a mouse model with traceable xenografted ALCL tumors was established using SCID (SHO) nude mice (Charles River). The traceable ALCL cells (6 × 106) containing shRNA-ALK were inoculated subcutaneously in the right hind limb and cells carrying shRNA-Lamin in the left hind limb of each mouse. Tumor development was monitored by whole body bioluminescence scanning using a XENOGEN IVIS 200 IMAGING system (Figure S2) after mice were anesthetized with isoflurane and injected with δ-luciferin (75mg/kg body weight, i.p., 100 µl). Tumor-bearing mice (n=5/test condition) were fed with tetracycline water (1.5 mg/ml tetracycline and 5% sucrose) to induce shRNA for ALK gene silencing, treated with a low dose of U0126 (0.5 mg/kg body weight, i.p., 100µl), individually and in combination, and mice that did not receive either treatment were used as background controls. Changes in tumor growth were monitored by whole body scanning every 3 days as indicated. The strength of bioluminescence signal from tumors was measured as photons in a digital format (photons/second/cm2/steradian), and paired Student's t-tests were performed. Histological confirmation of the xenografted ALCL tumor was carried out with H&E staining and CD30 immunohistochemical study of tissue section after experiments were completed.
Supplementary Material
Acknowledgments
This study was supported in part by research funding from the Methodist Hospital Research Institute (CTSA) and grants from the National Cancer Institutes (1K22CA113493 and 5P50CA126752).
Footnotes
Conflict of interest: the authors declare no conflict of interest.
Supplementary information is available at the Cancer Gene Therapy’s website
REFERENCES
- Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110:2259–2267. doi: 10.1182/blood-2007-04-060715. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai RY, Ouyang T, Miething C, Morris SW, Peschel C, Duyster J. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96:4319–4327. [PubMed] [Google Scholar]
- Chiarle R, Gong JZ, Guasparri I, Pesci A, Cai J, Liu J, et al. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood. 2003;101:1919–1927. doi: 10.1182/blood-2002-05-1343. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- Crockett DK, Lin Z, Elenitoba-Johnson KS, Lim MS. Identification of NPM-ALK interacting proteins by tandem mass spectrometry. Oncogene. 2004;23:2617–2629. doi: 10.1038/sj.onc.1207398. [DOI] [PubMed] [Google Scholar]
- Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, et al. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112:2439–2449. doi: 10.1182/blood-2008-05-159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsol G, Jaffe ES, Faini B, Gascoyne RD, Muller-Hermelink HK, Stein H, et al. Anaplastic large cell lymphoma (ALCL), ALK positive. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumors of Haematopoietic and Lymphod Tissues. Lyon: WHO PRESS; 2008. pp. 312–316. [Google Scholar]
- Duyster J, Bai RY, Morris SW. Translocations involving anaplastic lymphoma kinase (ALK) Oncogene. 2001;20:5623–5637. doi: 10.1038/sj.onc.1204594. Review. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, et al. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5) Proc Natl Acad Sci USA. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. Review. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Kawamata H, Fujimori T, Kuroda Y. A MEK inhibitor (U0126) prolongs survival in nude mice bearing human gallbladder cancer cells with K-ras mutation: analysis in a novel orthotopic inoculation model. Int J Oncol. 2003;23:957–963. [PubMed] [Google Scholar]
- Hsu FY, Zhao Y, Anderson WF, Johnston PB. Downregulation of NPM-ALK by shRNA causes anaplastic large cell lymphoma cell growth inhibition and augments the anti cancer effects of chemotherapy in vitro. Cancer Invest. 2007;25:240–248. doi: 10.1080/07357900701206372. [DOI] [PubMed] [Google Scholar]
- Kuefer MU, Look AT, Pulford K, Behm FG, Pattengale PK, Mason DY, et al. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood. 1997;90:2901–2910. [PubMed] [Google Scholar]
- Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R, Smith SD, Hecht BK, Christy V, Mellentin JD, Warnke R, et al. Lack of involvement of the c-fms and N-myc genes by chromosomal translocation t(2;5)(p23;q35) common to malignancies with features of so-called malignant histiocytosis (SUDHL-1) Blood. 1989;73:2155–2164. [PubMed] [Google Scholar]
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- Piva R, Chiarle R, Manazza AD, Taulli R, Simmons W, Ambrogio C, et al. Ablation of oncogenic ALK is a viable therapeutic approach for anaplastic large-cell lymphomas. Blood. 2006;107:689–697. doi: 10.1182/blood-2005-05-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter A, Schrappe M, Tiemann M, Parwaresch R, Zimmermann M, Yakisan E, et al. Successfull treatment strategy for Ki-1 anaplastic large-cell lymphoma of childhood: A prospective analysis of 62 patients enrolled in three consecutive Berlin-Frankfurt- Munster group studies. J Clin Oncol. 1994;12:899–908. doi: 10.1200/JCO.1994.12.5.899. [DOI] [PubMed] [Google Scholar]
- Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- Ritter U, Damm-Welk C, Fuchs U, Bohle RM, Borkhardt A, Woessmann W. Design and evaluation of chemically synthesized siRNA targeting the NPM-ALK fusion site in anaplastic large cell lymphoma (ALCL) Oligonucleotides. 2003;13:365–373. doi: 10.1089/154545703322617041. [DOI] [PubMed] [Google Scholar]
- Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, et al. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. [PubMed] [Google Scholar]
- Shulman LN, Frisard B, Antin JH, Wheeler C, Pinkus G, Magauran N, et al. Primary Ki-1 anaplastic large-cell lymphoma in adults: Clinical characteristics and therapeutic outcome. J Clin Oncol. 1993;11:937–942. doi: 10.1200/JCO.1993.11.5.937. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sasaki M, Itoh K, Higashihara M, Umezawa K, Kadin ME, et al. JunB induced by constitutive CD30-extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase signaling activates the CD30 promoter in anaplastic large cell lymphoma and reed-sternberg cells of Hodgkin lymphoma. Cancer Res. 2005;65:7628–7634. doi: 10.1158/0008-5472.CAN-05-0925. [DOI] [PubMed] [Google Scholar]
- Wentz SC, Wu H, Yip-Schneider MT, Hennig M, Klein PJ, Sebolt-Leopold J, et al. Targeting MEK is effective chemoprevention of hepatocellular carcinoma in TGF-alpha-transgenic mice. J Gastrointest Surg. 2008;12:30–37. doi: 10.1007/s11605-007-0396-4. [DOI] [PubMed] [Google Scholar]
- Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- Zamore PD. Ancient pathways programmed by small RNAs. Science. 2002;296:1265–1269. doi: 10.1126/science.1072457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.