Abstract
Purpose
Numerous cross-sectional studies have observed an inverse association between CRP and physical activity. Exercise-training trials have produced conflicting results, but none of these studies were specifically designed to examine CRP. The objective of the Inflammation and Exercise study (INFLAME) was to examine whether aerobic exercise training without dietary intervention can reduce CRP in individuals with elevated C-reactive protein (CRP).
Methods
The study was a randomized, controlled trial of 162 sedentary men and women with elevated CRP (≥2.0 mg/L). Participants were randomized into a non-exercise control group or an exercise group that trained for 4 months. The primary outcome was change in CRP.
Results
The study participants had a mean (SD) age of 49.7 (10.9) years and a mean body mass index of 31.8 (4.0) kg/m2. The median (IQR) and mean baseline CRP levels were 4.1 (2.5, 6.1) and 4.8 (3.4) mg/L, respectively. In the exercise group, median exercise compliance was 99.9%. There were no differences in median (IQR) change in CRP between the control and exercise groups (0.0 [−0.5, 0.9] versus 0.0 [−0.8, 0.7] mg/L, p=0.4). The mean (95% CI) change in CRP adjusted for gender and baseline weight was similar in the control and exercise groups with no significant difference between groups (0.5 [−0.4, 1.3] versus 0.4 [−0.5, 1.2] mg/L, p=0.9). Change in weight was correlated with change in CRP.
Conclusions
Exercise training without weight loss is not associated with a reduction in CRP.
Keywords: cardiorespiratory fitness, physical activity, inflammatory markers, Inflammation and Exercise study
INTRODUCTION
C-reactive protein (CRP), a marker of systemic inflammation, is an independent predictor of cardiovascular disease (CVD) in both women and men.(11;13) Recently, Ridker et al examined the benefit of rosuvastatin on CVD risk in individuals with elevated CRP (≥2.0 mg/L) but normal low-density lipoprotein (LDL) cholesterol (<130 mg/dL).(12) This large (n=17,802), multinational, randomized, prospective trial (the JUPITER trial) found that the treatment group had a large reduction in CRP (4.2 to ~2.2 mg/L) and in CVD events (approximately 50%) over a median follow-up of 1.9 years. While it is impossible to determine whether the use of statin medication or the lowering of CRP was responsible for the observed CVD benefits, the JUPITER trial reinforces the potential clinical significance of inflammation as a therapeutic target. To this end, lifestyle strategies such as exercise and/or weight loss may be promising strategies to improve CRP.
Numerous cross-sectional reports have observed an inverse relation between physical activity and CRP (4;5) while exercise intervention studies have had conflicting results.(4;5) In addition, it has been suggested that weight loss, concurrent with exercise, may be responsible for the reduction in CRP, not exercise training per se.(4) The primary limitation of existing studies of the CRP response to exercise training is that they were not designed specifically with CRP as an outcome. In addition, these studies were unable to control for potential confounding variables, were underpowered to examine changes in markers of inflammation, and included populations with normal CRP levels. Finally, there may be misclassification of the exercise intervention because exercise was not tightly controlled to account for the dose administered.
The Inflammation and Exercise study (INFLAME) was designed to investigate the effect of exercise training on elevated CRP concentrations in men and women ages 30–75. The goal of INFLAME was to answer the question: Can aerobic exercise training without dietary intervention reduce CRP in individuals with an elevated CRP level? The findings of the INFLAME study will inform the development of future clinical guidelines focused on reducing plasma CRP concentrations.
METHODS
Study Participants
A complete description of the INFLAME design and methods has been published.(21) The INFLAME study was approved by the Institutional Review Board (IRB) of The Cooper Institute and reviewed quarterly by the IRB and a Data Safety and Monitoring Board (DSMB).
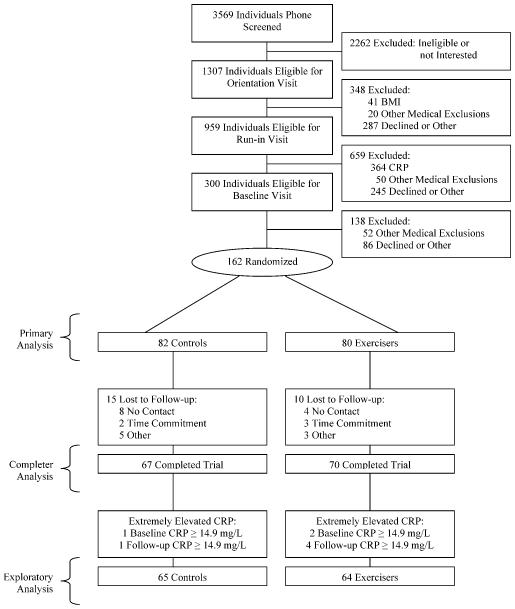
We conducted a total of 3569 telephone screens between March 2005 and August 2006 (Figure 1). After giving written informed consent, 162 men and women between the ages of 30 and 75 who were sedentary (not exercising more than 20 minutes on 3 or more days a week) and who had an elevated plasma CRP concentration (≥2.0 mg/L but <10.0 mg/L) at the initial screening were randomized to one of the groups. Exclusion criteria included smoking and a history of stroke, diabetes, heart attack, body mass index ≤18.5 or ≥ 40.0 kg/m2, or any serious medical condition that prevented participants from adhering to the protocol or exercising safely. Persons on beta-blockers or systemic steroids and women on hormone therapy were also excluded. We chose to exclude these women because hormone therapy substantially raises CRP, and there is little evidence that exercise can counter these hormone-induced CRP elevations. With regard to medications that have been demonstrated to substantially reduce CRP, such as statin mediations, we decided that use of these medications was not cause for exclusion as long as the minimum CRP level was met and the participant was on a stable dose for a minimum of two consecutive months prior to screening. It is worth noting that only 3 individuals in the control group and 2 individuals in the exercise group were taking statins at baseline, and removing them from the analysis had not effect on the outcomes.
Figure 1.
Flow of participants through THE INFLAME trial.
The screening CRP range was chosen based on information from previous studies. The minimal screening CRP for inclusion was 2.0 mg/L because it is prevalent in sedentary populations and also associated with increased CVD risk.(11) The maximum CRP cutoff point was set at 10.0 mg/L because less than 5% of the general population has a higher CRP level, and such values are often the result of an acute infection or other inflammatory condition.(10) The CRP value used to assess eligibility was measured as part of the prescreening process and was not used in outcome assessment. Further the CRP measure at the baseline assessment played no role in participant eligibility. For example, if an individual had a screening value less than 10.0 mg/L but a baseline value greater than 10.0 mg/L, the participant was still eligible and the data was still included in the primary analysis (n=8). Similarly if an individual had a screening value greater than 2.0 mg/L but a baseline value less than 2.0 mg/L, they remained still eligible for the study (12 controls and 12 exercisers). Removing participants with baseline CRP value less than 2.0 mg/L from the analysis had not effect on the outcomes.
Participants were recruited using a wide variety of techniques including newspaper, radio, television, mailers, community events, and email distributions.
Non-Exercise Control Group
Participants in the non-exercise control group were asked to maintain their current level of activity during the 4-month study period. Participants in the control groups were asked to submit step counter tracking and health status forms on a monthly basis.
Exercise-Training Group
In order to maintain the clinical and public health relevance of the intervention, the exercise dose chosen could be easily prescribed by professionals and was expected to have reasonable adherence in sedentary but otherwise healthy adults. Specifically, the exercise dose was 16 KKW divided into three to five sessions per week in a supervised exercise laboratory. This dose falls within the consensus public health recommendation for moderate- to vigorous-intensity physical activity of 30 minutes or more on most days of the week, or approximately 150 to 210 minutes of moderate-intensity activity. For the purposes of this study, moderate- to vigorous-intensity exercise was defined as 60 to 80% VO2max. (9;19;22)
All exercise sessions were performed under observation and supervision in an exercise laboratory, with strict monitoring of the amount of exercise completed in each session. Participants were weighed each week, and their weight was multiplied by 16 to determine the number of calories to be expended for the week. Exercise intensity was quantified using data from a Polar XL heart rate monitor worn by participants. The appropriate heart rate range for the prescribed intensity (60–80% VO2max) was calculated by the study’s exercise physiologist from the baseline maximal exercise test. If a participant’s heart rate fell or rose out of range during the intervention, the speed and/or grade of the treadmill or watts on the cycle ergometer were adjusted to maintain the prescribed intensity. Adherence to exercise training over the entire 4-month period was calculated for each individual by dividing the kilocalories expended during the exercise training by the kilocalories prescribed for the training period and multiplying by 100%.
Outcomes
The primary outcome was change in CRP level. Prior to all blood draws, participants fasted for 10–12 hours and refrained from consuming alcohol or exercising for 24 hours. Participants also refrained from acutely using aspirin or anti-inflammatory medications for 48 hours since these types of medications may modify markers of inflammation. In addition, for premenopausal women, blood draws occurred within 8 days of menstrual cessation because changes in ovarian hormones can influence markers of inflammation.(16)
Baseline and follow-up plasma, serum, and RBC with buffy coat samples were stored in a −80°C freezer. Serum CRP was measured by a solid-phase, chemiluminescent immunometric assay (Immulite 2000 High-Sensitivity CRP, Diagnostic Products Corporation, Los Angeles, CA) after baseline and follow-up samples were available in order for each individual’s set of samples to be measured using the same assay kit. The coefficient of variation for CRP is 6.5% in the Pennington Biomedical Research Center clinical laboratory for CRP levels similar to this study group.
Fitness Testing
Fitness testing was conducted using a Lode Excalibur Sport cycle ergometer (Groningen, Netherlands), an electronic, rate-independent ergometer. Participants cycled at 30 watts (W) for 2 minutes and 50 W for 4 minutes, followed by increases of 20 W every 2 minutes until they could no longer maintain a pedal cadence of 50 rpm. Respiratory gases were measured using a Parvomedics True Max 2400 Metabolic Measurement Cart. Volume and gas calibrations were conducted before each test. Gas-exchange variables (VO2, CO2 production, ventilation, and respiratory exchange ratio [RER]) were recorded every 15 seconds. Heart rate was measured directly from the ECG monitoring system. Ratings of perceived exertion (RPE) were obtained using the 20-point Borg scale. Fitness assessment staff members were blinded to the participant’s randomization assignment.
Anthropometry and Body Composition
Weight was measured on an electronic scale (Siemens Medical Solutions, Malvern, PA), and height was measured using a standard stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Body composition was measured with dual-energy X-ray absorptiometry (DEXA) scans using a Hologic Bone Densitometer (Hologic Inc., Bedford, MA). Axial images of the abdomen were obtained using an electron beam CT (Imatron, General Electric, Milwaukee, WI). Abdominal visceral adiposity and subcutaneous fat were measured as described by Ross et al.(15)
Daily Physical Activity and Other Measures
To assess potential changes in non-supervised physical activity, all randomized participants wore a step counter (Accusplit Eagle, Japan) and recorded their daily steps. Diet was assessed by the Food Intake and Analysis System semiquantitative food frequency questionnaire (The University of Texas HSoPH. Food intake analysis system. 1996. Report No.: Version 3.0.) Participants were asked not to change their diet during the study period.
Randomization
Eligible participants were randomized after completing run-in and baseline assessments. The randomization sequence was computer generated. The sequence was determined from randomly permuted blocks of equal length with fixed numbers of treatment allotments to balance treatment enrollment over time. Randomization was implemented with treatment assignment letters placed into sequentially numbered, opaque envelopes sealed by the statistician.(21)
Statistical Analysis
Statistical power considerations were previously reported.(21) For all power calculations, the exercise versus control group comparison was based on the two-sample t-test for change-score differences, with equal group size and 5% significance (two-sided). For CRP, average change values and variability were based on observations from published reports.(7;18) The correlation between pre- and post-trial CRP measures was estimated from a previous trial. Power was calculated for 30% and 40% changes in CRP (compared to no change in the control group), which were derived from previous reports of CRP being reduced 31% and 35% in response to exercise training in uncontrolled studies.(7;18). Assuming 77 participants in each group, we calculated power to be 0.94 and 0.99 to detect differences in CRP between the experimental and control groups of 30% and 40%, respectively. Thus enrolling 82 controls and 80 exercisers into the study provided more than adequate power to test the hypothesized changes in CRP.
Descriptive baseline characteristics of groups were tabulated as means and standard deviations (SDs) or as percentages. Continuous variables were compared using Student’s t-tests, and categorical variables were compared using Chi-square tests. Mean step data was calculated per month for each randomization group. Between-group differences in mean monthly steps were tested using ANOVA without adjustment, and within-group differences were tested using t-tests.
The primary analysis was conducted using the intent-to-treat principle; if the outcome value was missing for the participant, we inserted the baseline value for that outcome (i.e., last observation carried forward). Median CRP change values were compared between groups using the Wilcoxon Signed-Rank test. Due to baseline differences in gender distribution and baseline anthropometric measures, CRP change values among groups were tested by ANOVA with adjustment for gender and baseline weight. Results are presented as adjusted least-squares means with confidence intervals. We repeated the primary CRP analysis limiting the data set to participants with baseline and follow-up data. In addition, we conducted a third analysis of the primary outcome in which we eliminated individuals with baseline or follow-up CRP values more than three SDs (≥14.9 mg/L) from the mean.
Differences in secondary outcomes among the randomization groups were tested using the intent-to-treat principal by ANOVA with adjustment for gender and the baseline value of the tested variable. Results are presented as adjusted least-squares means with confidence intervals.
The association between change in CRP and changes in adiposity and fitness was examined using Spearman correlations. As an exploratory analysis, we tested the change in CRP across tertiles of changes in weight and DEXA-measured body fat in the exercise group. Results are presented as adjusted least-squares means with 95% confidence intervals. All associations and exploratory analyses were performed with completers only and the outliers removed.
All reported p-values are two-sided. All analyses were performed using SAS version 9.1 (Cary, NC).
RESULTS
The study population had a mean (SD) age of 49.7 (10.9) years, a mean BMI of 31.8 (4.0) kg/m2, and a mean daily steps of 5792 (2689), and it was 35% non-Caucasian and 72.8% female. The median and mean CRP baseline levels were 4.1 (2.5, 6.1) and 4.8 (3.4) mg/L, respectively. The mean VO2abs and VO2rel values were low at 1.69 (0.61) L · min−1 and 18.9 (5.6) mL · kg−1 · min−1, respectively. As summarized in Table 1, descriptive data was similar across groups except for gender distribution and for some of the anthropometric data and high-density lipoprotein (HDL) cholesterol. As detailed in Figure 1, follow-up data was available for 67 of 82 (82%) individuals in the control groups and 70 of the 80 (88%) individuals in the exercise group for a total of 137 (85%) of the participants with follow-up data. For exercisers who completed the study, the mean and median exercise compliance was 91% and 99.9%, respectively. The mean exercise-training intensity was 75.3% (6.5) of maximal heart rate, and the mean number of minutes per week spent exercising was 204 (45) (excluding the ramp-up period).
Table 1. Baseline Participant Characteristics*.
| Randomization Groups | |||
|---|---|---|---|
| Control | Exercise | P-value | |
| Characteristics | (n=82) | (n=80) | |
| Demographics | |||
| Age, y | 49.4 (11.3) | 50.0 (10.4) | 0.71 |
| Female, n (%) | 54 (65.9) | 64 (80.0) | 0.04 |
| Ethnicity, n (%) | |||
| Caucasian | 49 (59.8) | 56 (70.0) | 0.5 |
| African-American | 22 (26.8) | 15 (18.8) | |
| Hispanic/Other | 11 (13.4) | 9 (11.2) | |
| Alcoholic Drinks per Week | 1.1 (2.0) | 1.4 (2.6) | 0.50 |
| C-Reactive Protein, mg/L | |||
| Mean | 4.6 (3.0) | 4.9 (3.7) | 0.62 |
| Median (IQR) | 3.9 (2.4, 6.3) | 4.1 (2.6, 5.7) | |
| Exercise Test Variables | |||
| Maximal Heart Rate, beats/min | 156.5 (22.8) | 158.5 (21.8) | 0.57 |
| Respiratory Exchange Ratio | 1.13 (0.09) | 1.15 (0.09) | 0.37 |
| Peak Absolute VO2, L/min | 1.74 (0.64) | 1.66 (0.57) | 0.40 |
| Peak Relative VO2, mL/kg/min | 18.8 (5.6) | 19.1 (5.6) | 0.76 |
| Anthropometrics | |||
| Weight, kg | 91.6 (15.3) | 87.1 (15.1) | 0.06 |
| Body Mass Index, kg/m2δ | 32.3 (3.7) | 31.2 (4.2) | 0.09 |
| Body Fat Percent, % | 39.1 (6.9) | 40.6 (6.4) | 0.16 |
| Fat Body Mass, DEXA | 35.6 (7.8) | 35.6 (9.1) | 0.98 |
| Lean Body Mass, DEXA | 56.0 (12.6) | 51.6 (9.9) | 0.01 |
| Abdominal Visceral Fat, kg | 5.4 (2.8) | 4.6 (2.6) | 0.06 |
| Abdominal Subcutaneous Fat, kg | 13.3 (4.1) | 11.8 (4.3) | 0.02 |
| Cardiovascular Disease Factors | |||
| LDL-C, mg/dL | 115.2 (24.0) | 116.2 (25.0) | 0.80 |
| HDL-C, mg/dL | 51.9 (12.6) | 57.3 (14.7) | 0.01 |
| Triglycerides, mg/dL | 115.5 (48.6) | 113.6 (48.3) | 0.80 |
| Fasting Glucose, mg/dL | 96.3 (11.0) | 93.8 (10.5) | 0.14 |
| Fasting Insulin, μU/mL | 13.1 (7.2) | 11.8 (5.9) | 0.19 |
| Systolic Blood Pressure, mm Hg | 131.8 (19.3) | 130.3 (20.3) | 0.63 |
| Diastolic Blood Pressure, mm Hg | 82.5 (10.6) | 81.2 (11.1) | 0.42 |
Abbreviations: IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VO2, volume of oxygen consumed
SI conversions: To convert LDL-C and HDL-C to mmol/L, multiply by 0.0259. To convert triglycerides to mmol/L, multiply by 0.0113. To convert glucose to mmol/L, multiply by 0.0555.
All continuous variables presented as means (SD), except C-reactive protein which is also presented as median (IQR). Dichotomous variables presented as percentages.
Calculated as weight in kilograms divided by height in meters squared.
During the intervention, the mean steps per day did not change in either group, nor were there any statistically significant differences between groups at any time point. Specifically, the mean (SD) steps per day for the control group were 6649 (2876), 6559 (2990), 6434 (2913), and 6800 (2799) across months 1 through 4. For the intervention group, the mean (SD) steps per day were 5447 (2881), 6298 (3369), 6314 (3295), and 6414 (3327) across months 1 though 4. There was no change in daily caloric intake between baseline and follow-up for either group, and there were no between-group differences at either baseline or follow-up. For the control group, the mean (SD) daily caloric intake was 1920 (887) and 1755 (699) at baseline and follow-up, and in the exercise group, it was 1793 (700) and 1664 (667), respectively. Given the large measurement error associated with this type of dietary assessment, this data should be interpreted with caution.
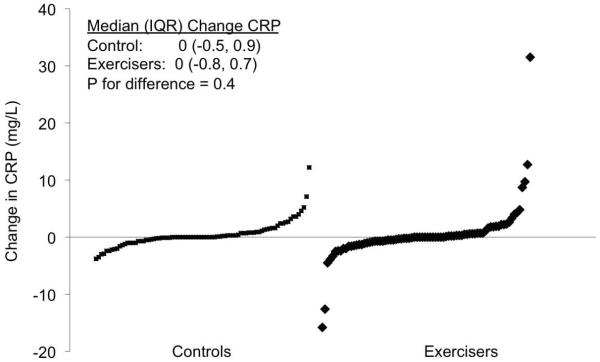
Figure 2 depicts the distribution of the CRP change values in the control and exercise groups. The shape of the distribution of change values was similar between groups, and there were no differences in median (IQR) CRP change between the control and exercise groups (0.0 [−0.5, 0.9] versus 0.0 [−0.8, 0.7] mg/L, p=0.4).
Figure 2.
Distribution of change in C-reactive protein for each study group
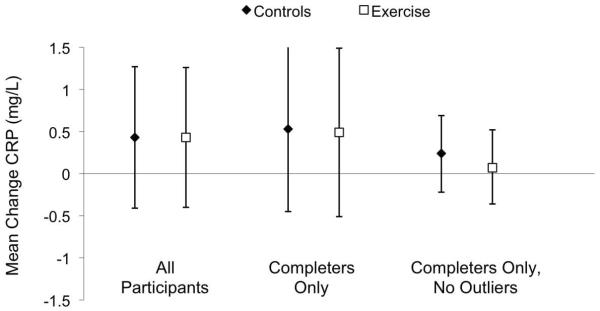
Figure 3 depicts the change in CRP adjusted for gender and baseline weight in the control and exercise groups. The adjusted mean CRP change was similar in the control and exercise groups with no significant difference between the two groups (0.5 [−0.4, 1.3] versus 0.4 [−0.5, 1.2] mg/L, p=0.9). Figure 3 also shows analyses limited to individuals with follow-up data and with CRP outliers removed. Further, removing the data of individuals in the exercise group with compliance less than 90% (n=11) also had no effect on change in CRP. Gender was not a significant covariate in any of the above models and all analyses were repeated within each gender group with similar results. In addition the gender X group interaction term was not significant for any of the above analyses.
Figure 3.
Change in C-reactive protein for each study group. The data represent the the least-squares means adjusted for gender and baseline weight. The left set of data represents all participants with baseline values carried forward for participants without follow-up values. The middle set of data represents only participants with follow-up data and the far right set of data represents participants with follow-up data with the outliers removed (baseline or follow-up CRP ≥14.9 mg/L). Error bars indicate 95% confidence intervals.
Table 2 presents the changes in secondary outcome measures. All values are least-squares mean changes adjusted for baseline value and gender. The exercise group had a significant improvement in VO2abs and percent change in VO2 (12%) compared to the control group. There was no difference in change in energy intake between groups. While there were no significant between-group differences for changes in weight, lean mass, or abdominal visceral (p= 0.07) or subcutaneous fat, the exercise group did have a significant reduction in DEXA-measured body fat compared to the control group (−0.4 [−0.7, −0.1] versus 0.2 [−0.3, 0.6], p=0.02). For individuals in the exercise group, the caloric expenditure from supervised exercise (total) was divided by 7700 kcal to create a variable of predicted weight change. This was based on the assumption that 1 kg of weight represented 7700 kcal in energy.(3) The predicted mean weight loss in the exercise group was −2.4 (0.7) kg, which was significantly (p<0.001) less than the observed weight loss of −0.7 (3.4) kg. Thus only 29% of expected weight loss was achieved. There were no significant between-group differences for changes in any of the CVD risk factors which not unexpected give the relatively healthy baseline risk factor profiles of the study participants in both groups.
Table 2. Change in Secondary Outcome Measures After Intervention.
| Randomization Groups | |||
|---|---|---|---|
| Control | Exercise | P-value† | |
| Characteristics | (n=82) | (n=80) | |
| Fitness and Diet Variables * | |||
| Peak Absolute VO2, L/min | −0.01 (−0.06, 0.03) | 0.17 (0.13, 0.22) | <0.001 |
| Percent Change Peak VO2, % | 0.5 (−1.93, 2.9) | 12.0 (9.5, 14.5) | <0.0001 |
| Energy Intake, kcal/day | −113 (−238, 11) | −138 (−264, −11) | 0.78 |
| Anthropometrics | |||
| Weight, kg | 0.1 (−0.5, 0.7) | −0.6 (−1.2, 0.0) | 0.10 |
| Body Fat Percent, % | 0.1 (−0.2, 0.4) | −0.4 (−0.7, −0.1) | 0.03 |
| Fat Body Mass, DEXA | 0.2 (−0.3, 0.6) | −0.6 (−1.0, −0.1) | 0.02 |
| Lean Body Mass, DEXA | −0.04 (−0.4, 0.3) | −0.06 (−0.4, 0.3) | 0.91 |
| Abdominal Visceral Fat, kg | 0.00 (−0.1, 0.1) | −0.16 (−0.3, −0.1) | 0.07 |
| Abdominal Subcutaneous Fat, kg | 0.00 (−0.3, 0.2) | −0.3 (−0.5, −0.1) | 0.16 |
| Cardiovascular Disease Risk Factors | |||
| LDL-C, mg/dL | 1.0 (−2.5, 4.5) | 3.0 (−0.5, 6.5) | 0.42 |
| HDL-C, mg/dL | −2.4 (−3.6, −1.2) | −1.4 (−2.6, −0.2) | 0.25 |
| Triglycerides, mg/dL | 0.5 (−7.5, 8.6) | 1.1 (−7.0, 9.2) | 0.92 |
| Fasting Glucose, mg/dL | 0.8 (−0.6, 2.3) | 0.9 (−0.6, 2.4) | 0.92 |
| Insulin, μU/mL | −0.2 (−1.0, 0.6) | −0.8 (−1.6, 0.03) | 0.34 |
| Blood Pressure, mm Hg | |||
| Systolic | −1.9 (−4.4, −0.7) | −4.1 (−6.7, −1.5) | 0.22 |
| Diastolic | −2.1 (−3.7, −0.5) | −1.8 (−3.4, −0.2) | 0.78 |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VO2, volume of oxygen consumed
SI conversions: To convert LDL-C and HDL-C to mmol/L, multiply by 0.0259. To convert triglycerides to mmol/L, multiply by 0.0113. To convert glucose to mmol/L, multiply by 0.0555.
Values are expressed as fitted mean (95% CI) and are adjusted for baseline value and gender.
P-values for differences between groups determined by analysis of variance adjusted for baseline value and gender.
Table 3 presents the Spearman associations for change in CRP with changes in measures of fitness and body composition. Overall, changes in weight, DEXA-measured body fat, and abdominal body fat were associated with change in CRP. When stratified by randomization group, CRP change was associated with changes in weight and DEXA body fat in the exercise group but not in the control group.
Table 3. Spearman Correlations Between Changes in C-Reactive Protein and Changes in Secondary Outcomes.
| Change C-Reactive Protein | |||
|---|---|---|---|
| All (n=129) |
Control (n=65) |
Exercise (n=64) |
|
| Change Peak Absolute VO2, L/min | −0.13 | −0.19 | −0.01 |
| Change Weight, kg | 0.18* | 0.05 | 0.34† |
| Change Fat Body Mass, DEXA | 0.24† | 0.13 | 0.35† |
| Change Lean Body Mass, DEXA | −0.01 | −0.15 | 0.15 |
| Change Abdominal Visceral Fat, kg | 0.17* | 0.18 | 0.16 |
| Change Abdominal Subcutaneous Fat, kg | −0.02 | −0.17 | 0.07 |
Data limited to participants with baseline and follow-up C-reactive protein values < 14.9 mg/L.
p≤0.05
p<0.01
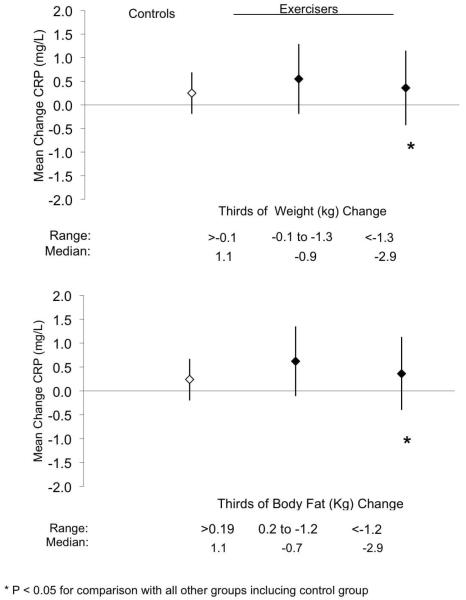
Figure 4 graphically depicts the change in CRP across tertiles of changes in weight and DEXA-measured body fat in the exercise group compared to the control group. The range for each tertile and median value of each tertile are provided in the lower portion of each graph. For both weight loss and body fat loss, the exercise group tertile with the largest reduction in these variables had significant reductions in CRP compared to all groups. Further adjusting for gender had no meaningful effect on either of the above analyses and in both instances the gender X group interaction term was not significant.
Figure 4.
Change in C-reactive protein for control group (open diamond), and the exercise group with categorization by thirds of change weight and DEXA body fat (closed diamonds). The data represent the least-squares means. Error bars indicate 95% confidence intervals. * P < 0.05 versus all other groups including the control group
DISCUSSION
The primary finding from this prospective, randomized, controlled exercise trial in sedentary individuals with elevated levels of CRP is that exercise training without weight loss was not associated with a reduction in CRP. Our participant retention was high (85%), and exercise adherence was excellent (>90%). Overall, we observed a 12% increase in fitness in the exercise group, and the study was adequately powered to observe change in CRP. Thus it seems highly unlikely that failure to see a decrease in CRP was due to methodological issues surrounding the exercise intervention or other design issues. Despite an exercise energy expenditure of 16 KKW (approximately 1500 kcal/wk per participant), we failed to observe a significant decrease in body weight and had only a small decrease in total body fat. Numerous exercise trials have failed to produce substantial weight loss despite delivering a large dose of exercise.(14) It has been demonstrated that exercise training can result in increased caloric intake, which subsequently reduces or negates the exercise-induced negative caloric balance.(6) (1) We hypothesize that the small percentage of expected weight loss (29%) achieved in the exercise group accounts for our failure to see a significant mean reduction in CRP. This hypothesis is supported by our observation that there was a strong association between fat loss and change in CRP and that the individuals in the exercise group who lost the most weight (and fat) had a significant decrease in CRP. Our data suggests that exercise training-induced reductions in CRP are due primarily to fat loss, as we did not observe any weight-independent benefits of exercise to CRP. These findings support the observation that fatness may mediate the previously observed associations between physical activity and CRP.
There are several potential explanations as to why many cross-sectional studies have reported exercise to be inversely associated with CRP while exercise-training studies have not confirmed this observation. There may be a “publication bias” in which studies showing a positive association are more likely to be submitted and published. Further, measures of fitness or self-reported activity may reflect long-term patterns of activity as opposed to recent changes. Thus exercise may be effective in preventing the development of elevated CRP and less effective in reducing an established elevated CRP level. In addition there may be confounding by uncontrolled factors. Individuals who report higher levels of activity may also have healthier diets such as a Mediterranean-style diet, which is associated with lower CRP.(2) One could propose similar hypotheses for stress, subclinical chronic conditions, or general well-being. There are likely many variables, other than just physical activity and weight, that influence CRP, and these variables are likely to interact with each other. An example of the complexities of studying CRP and exercise is illustrated by Milani et al in a study of CRP and cardiac rehabilitation. This study is frequently cited as evidence that exercise reduces CRP because participation in a high-quality cardiac rehabilitation program was associated with a 36% reduction in CRP despite no change in weight.(8) However, it is important to point out that this study did not have a randomized control group, the participants all had coronary heart disease, and the cardiac rehabilitation consisted of dietary counseling with emphasis on the Mediterranean diet, smoking cessation, stress management, hypertension and diabetes management, and exercise training. While the cardiac rehabilitation program appears to be beneficial to CRP, it is difficult to dissect which element(s) of the rehabilitation program were responsible for the observed reduction in CRP.
Our findings do confirm numerous reports that weight loss is an effective means to reduce CRP.(17) However, there is a need for a tightly controlled, lifestyle-based study examining the dose-response relation between weight loss and change in CRP in individuals with elevated CRP at baseline. This type of trial could help inform future clinical guidelines examining nonpharmacological methods to reduce CRP.
Strengths and Limitations
The primary strength of the INFLAME study is that it is an efficacy study specifically designed to examine the effect of an exercise intervention on CRP. The exercise intervention was a tightly controlled exercise dose, with all exercise taken in the laboratory and extensive monitoring of energy expenditure. We obtained excellent exercise adherence and had a low dropout rate.
The inclusion/exclusion criteria were designed to identify individuals with chronically elevated CRP and to exclude persons with acute inflammatory conditions. We controlled for potential confounding in several ways. Confounding by medication usage was limited by restricting entry into the study and excluding individuals taking medications that might prevent reductions in CRP. However, we did not assess changes in medications. Confounding by additional daily physical activity was accounted for by monitoring daily steps taken outside the exercise sessions. Similarly, we monitored energy (dietary) intake. We observed no differences among groups for either of these factors. To our knowledge, INFLAME is the first randomized, controlled trial designed and conducted to specifically examine the benefit of exercise training on CRP. The intervention was limited to aerobic exercise training, and there is preliminary evidence that other types of training such as resistance training might reduce CRP.(20) Similarly, we chose not to provide dietary counseling to allow us to test the independent effect of exercise on CRP. An intervention that includes dietary counseling to promote weight loss could test the relationship between weight loss and CRP. INFLAME was only four months in duration and it is conceivable a longer exercise intervention may have produced reductions in CRP.
Conclusions
The primary finding from this prospective, randomized, controlled exercise trial in sedentary individuals with elevated levels of CRP is that exercise training without weight loss is not associated with a reduction in CRP. However, in the exercise group, changes in weight and fat were associated with changes in CRP, suggesting that exercise-induced reductions in CRP are primarily due to reductions in weight and body fat.
Acknowledgements
This work was supported by NIH grant #HL66262. We thank Life Fitness for providing exercise equipment. This work was performed at The Cooper Institute, and the staff is especially commended for its efforts. We thank the INFLAME participants. The of this study do not constitute endorsement by ACSM.
This work was supported by NIH grant #HL66262. We thank Life Fitness for providing exercise equipment.
Footnotes
Conflicts of Interest
None
Trial Registration: clinicaltrials.gov Identifier NCT00113061
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS.ONE. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito K, Marfella R, Ciotola M, Di PC, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004 Sep 22;292(12):1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 3.Forbes GB, Kreipe RE, Lipinski B. Body composition and the energy cost of weight gain. Hum Nutr Clin Nutr. 1982;36(6):485–7. [PubMed] [Google Scholar]
- 4.Hamer M. The relative influences of fitness and fatness on inflammatory factors. Prev.Med. 2007 Jan;44(1):3–11. doi: 10.1016/j.ypmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll.Cardiol. 2005 May 17;45(10):1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 6.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008 Jan;32(1):177–84. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 7.Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000 Jan;21(1):21–4. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 8.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll.Cardiol. 2004 Mar 17;43(6):1056–61. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 9.NIH Consensus Development Panel on Physical Activity and Cardiovascular Health Physical activity and cardiovascular health. JAMA. 1996;276(3):241–6. [PubMed] [Google Scholar]
- 10.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001 Apr 3;103(13):1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N.Engl.J Med. 2008 Nov 9;:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N.Engl.J Med. 2002 Nov 14;347(20):1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001 Jun;33(6 Suppl):S521–S527. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992 Feb 1;72(2):787–95. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Miyake S, Akatsu J, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosom Med. 1995 Jul;57(4):331–5. doi: 10.1097/00006842-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Selvin E, Paynter NP, Erlinger TP. The Effect of Weight Loss on C-Reactive Protein: A Systematic Review. Arch Intern Med. 2007 Jan 8;167(1):31–9. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999 May 12;281(18):1722–7. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 19.Snyder KA, Donnelly JE, Jacobsen DJ, Hertner G, Jakicic JM. The effects of long-term, moderate intensity, intermittent exercise on aerobic capacity, body composition, blood lipids, insulin and glucose in overweight females. Int J Obes Relat Metab Disord. 1997;21:1180–9. doi: 10.1038/sj.ijo.0800533. [DOI] [PubMed] [Google Scholar]
- 20.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, McFarlin BK, Coen PM, Talbert E. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007 Oct;39(10):1714–9. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 21.Thompson AM, Mikus CR, Rodarte RQ, Distefano B, Priest EL, Sinclair E, Earnest CP, Blair SN, Church TS. Inflammation and exercise (INFLAME): Study rationale, design, and methods. Contemp.Clin Trials. 2008 May;:418–27. doi: 10.1016/j.cct.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S.Department of Health and Human Services . Physical activity and health: A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. pp. 1–300. [Google Scholar]