Abstract
Objective
To evaluate the pathophysiology of chronic nitric oxide synthase (NOS) inhibition-induced fetal growth restriction (FGR) in the rat.
Methods
Timed-pregnant rats received L-NAME (2.5 mg/kg/h) with or without endothelin (ET-1) receptor A (ETA) antagonist from day 14 to 21 of gestation. In separate groups, ETA antagonist and/or L-NAME were discontinued on day 18. On day 21 fetal and placental weights, and maternal and fetal plasma nitrate/nitrite (NOx) were determined.
Results
L-NAME led to FGR, and decreased maternal and fetal NOx. Maternal NOx was further decreased when ETA antagonist was co-administered with L-NAME. ETA antagonism along with L-NAME did not impact fetal growth. Discontinuation of L-NAME on day 18 resulted in normal fetal and placental growth at day 21 and an increase of maternal NOx. Simultaneous cessation of both NOS inhibition and ETA antagonism on day 18 produced FGR at day 21, whereas continuation of ETA antagonism after discontinuation of L-NAME resulted in normal fetal growth.
Conclusions
NOS inhibition in the pregnant rat leads to decreased maternal and fetal nitric oxide (NO) production and FGR. The effects of NOS inhibition on fetal growth are reversible, and are mediated at least in part by ET-1. With chronic NOS inhibition, ETA antagonism improves but does not normalize fetal growth, and may allow increased access of L-NAME to the fetal compartment. Continued access of L-NAME to the fetal compartment may limit the effect on fetal growth of any therapeutic intervention in this model of FGR.
Keywords: Endothelin antagonism, Fetal growth restriction, Nitric oxide synthase inhibition, Rat
INTRODUCTION
Nitric oxide synthase (NOS) inhibition in the pregnant rat has been used as a model for the study of fetal growth restriction (FGR) (1–3). NOS inhibition causes a decrease in the production of nitric oxide (NO), a vasodilator. During pregnancy, administration of the NOS inhibitor L-NAME results in decreased uterine and placental perfusion and ultimately leads to fetal and placental growth restriction (1,2,4,5). Fetal growth is compromised as early as day four, and placental growth as early as day one, of a seven day infusion of L-NAME (days 15 through 21 of gestation) (5). We and others have demonstrated that L-NAME administration also results in increased maternal circulating endothelin-1 (ET-1), a potent vasoconstrictor (6,7). We have established that this increased ET-1 is of primary importance in the pathophysiology of the decreased uterine and placental perfusion observed in this model. ET receptor antagonism in the presence of continuing NOS inhibition normalizes placental perfusion (and fetal growth) early in the treatment period, but this improvement in perfusion and growth is not sustained (5). This finding led us to investigate other mechanisms by which chronic NOS inhibition may impact perfusion and growth. These could include irreversible effects of L-NAME on vascular integrity or fetal growth and augmented transport of L-NAME to the fetus. If early L-NAME exposure led to irreversible effects on fetal growth in spite of L-NAME being discontinued, it would suggest an underlying vascular insult that may not be responsive to therapeutic interventions intended to improve perfusion. Similarly, if therapeutic interventions led to increased placental perfusion and consequently to increased transport of L-NAME to the fetal compartment, fetal growth may paradoxically be adversely affected. These factors would need to be taken into account when evaluating the effects of therapeutic interventions.
The purpose of this study was to determine whether the effects of chronic L-NAME administration are reversible and whether the maternal administration of L-NAME has an impact on fetal NO production. By answering these questions, we can correctly evaluate the impact of therapeutic interventions in this model of FGR.
MATERIALS AND METHODS
Nitric oxide synthase inhibitor and endothelin antagonist
The NOS inhibitor, nitro-L-arginine methyl ester (L-NAME), was obtained from Sigma (St. Louis, MO). The ETA antagonist ABT-546, a nonpeptide ETA receptor antagonist with 28,000-fold selectivity for ETA over ETB (8), was provided by Abbott Laboratories, Abbott Park, IL.
Animals
Female and male Sprague-Dawley rats (Harlan Sprague Dawley, Madison, WI) received a standard laboratory rodent diet (PMI Feeds, St. Louis, MO), water ad libitum, and were kept on a 12-hour light/12-hour dark cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee. Rats were bred at an age of 11–20 weeks and a weight of 225–250 g. Date of sperm positivity was designated day 0 of a 22-day gestation.
Nitric oxide synthase inhibition and ET receptor antagonism
On day 14 of gestation, two Alzet osmotic pumps (model 2ML1, output=10 μl/h, Durect Corp., Palo Alto, CA) were placed subcutaneously on the back of the rat between the scapulae. One pump was used to infuse either L-NAME (2.5 mg/kg/h) or normal saline vehicle. L-NAME infusion continued through gestation day 21 (7 days) in rats used for perfusion and fetal growth studies (n=6 per group). For studies of fetal growth and NOx recovery after NOS inhibition, the L-NAME infusion continued through day 18 (4 days), at which time the pump was removed under anesthesia and the rats continued their gestation until day 21 (n=6 per group).
The second pump was used to infuse ABT-546 (20 mg/kg/day), or vehicle (20% ethyl alcohol, 40% propylene glycol, and 0.04 M NaOH in H2O). This dose of the ETA antagonist has proven effective in in vivo pseudoefficacy studies to block ET-1-induced increases in mean arterial pressure in rats (9). ABT-546 administration was continued for 7 days (to determine the impact of ETA antagonism on L-NAME-induced FGR), or for 4 days (to determine the impact of simultaneous cessation of NOS inhibition and ETA antagonism).
Nitrate/nitrite assay in maternal and fetal plasma
Blood for nitrate/nitrite assay was collected on gestation day 21. Maternal blood was drawn from the abdominal vena cava into a heparin-treated syringe at laparotomy and fetal blood was collected by heparin-treated capillary tube after incision across the carotid and jugular vessels in anesthetized pups. Fetal blood from each litter was pooled.
For the study of NOx recovery after NOS inhibition, maternal rats were infused with L-NAME or vehicle for 4 days. Maternal blood was collected from a tail vein on gestation day 14 (before L-NAME pump insertion), day 18 (before pump removal), and from the vena cava on day 21 at laparotomy.
Plasma was prepared from the blood samples and was frozen at −80°C until assayed. Residual L-NAME, which interferes with NO analysis on the NO analytic instrument, was removed from the plasma on a Dowex 50WX8-400 ion exchange column (Sigma, St Louis, MO). Plasma nitrate/nitrite (NOx) was evaluated using a Sievers 280i NO analyzer (GE Analytical Instruments, Boulder, CO). After conversion of nitrate to nitrite, total NOx was quantified using a dilution series of nitrate standards. Results were expressed as nmol NOx/ml plasma.
Statistical analyses
Results are presented as mean ± standard error of the mean (SE). Statistical comparisons were made using an analysis of variance (ANOVA) with post hoc Newman-Keuls test (Figures 1 and 2), a repeated measures ANOVA (Figure 3, intragroup), or a Mann-Whitney U test (Figure 3, between groups on specific days). All statistical tests were two-tailed and results were considered statistically significant at P<0.05.
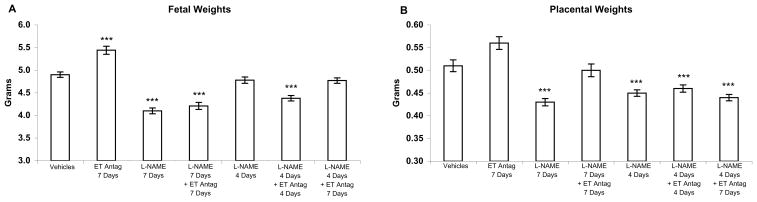
Figure 1.
Fetal and placental weights from pregnant rats in response to L-NAME and an ETA antagonist. Infusion of the NOS inhibitor L-NAME (2.5 mg/kg/h), and the ETA antagonist ABT-546 (20 mg/kg/day) was for 4 or 7 days (as indicated on the chart), gestation days 14–18 or 14–21, and weights were recorded on day 21. (A) Fetal weights were decreased by L-NAME and were not improved by ETA antagonist in combination with L-NAME when infusion of both agents was for 7 days. Discontinuation of L-NAME at day 18 resulted in normal fetal growth. When L-NAME and ABT-546 were infused together, simultaneous discontinuation of both L- NAME and ETA antagonist at day 18 produced growth restriction at day 21 whereas continuation of ETA antagonism after discontinuation of NOS inhibition resulted in normal growth. (B) Placental weights were also decreased by 7 days of L-NAME but were significantly improved by the ETA antagonist. Neither discontinuation of L-NAME nor continuation of ETA antagonism after cessation of NOS inhibition improved placental weights. Results are presented as mean weight in grams ± SE (n=6 maternal rats per group; litter sizes (range = 10.7–14.3 fetuses/litter) did not differ significantly among groups). ***P<0.001 by ANOVA compared to vehicle-treated rats.
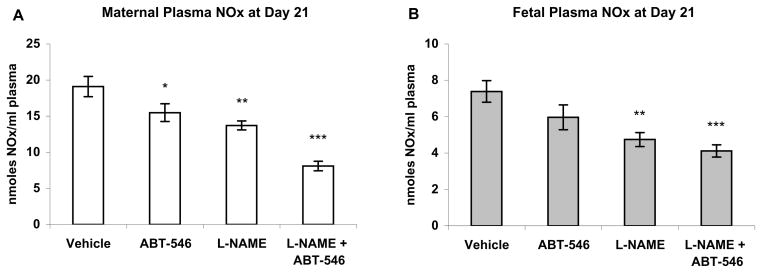
Figure 2.
Maternal (A) and fetal (B) plasma nitrate/nitrite (NOx) at gestation day 21 in rats treated for 7 days (days 14–21) with the NOS inhibitor L-NAME (2.5 mg/kg/h), and the ETA antagonist ABT-546 (20 mg/kg/day). L-NAME significantly lowered both maternal and fetal NOx. ABT-546 in combination with L-NAME produced a further decrease in both maternal and fetal NOx. A slight lowering of maternal NOx was produced by the antagonist alone. Results are presented as mean nmoles NOx/ml plasma ± SE (n=6 maternal rats per group). *P<0.05, ** P<0.01, ***P<0.001, by ANOVA compared to vehicle-treated rats.
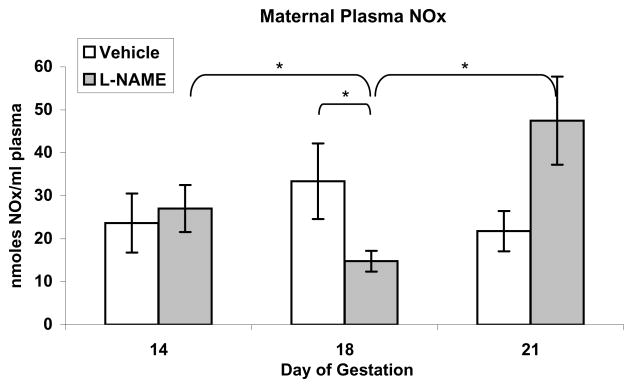
Figure 3.
Maternal plasma nitrate/nitrite (NOx) in pregnant rats treated with the NOS inhibitor L-NAME (2.5 mg/kg/h) for 4 days, (gestation days 14–18), followed by 3 days without L-NAME. Plasma was collected on gestation day 14 just before L-NAME treatment was begun (baseline), on day 18 just before L-NAME pump removal, and on day 21 at laparotomy. Maternal plasma NOx did not change over time in vehicle-treated rats. L-NAME significantly reduced maternal plasma NOx. Cessation of NOS inhibition at day 18 resulted in a significant increase in maternal plasma NOx at day 21 that was higher than the baseline value at day 14. Results are presented as mean nmoles NOx/ml plasma ± SE (n=6 maternal rats per group). *P<0.05, by repeated measures ANOVA for comparisons between times within each group and by Mann-Whitney U for comparison between groups at each time.
RESULTS
Fetal and placental weights
Administration of L-NAME for 7 days resulted in significant reduction of fetal and placental weights. Co-administration of ABT-546 had no impact on fetal growth, although placental growth was normalized (Figure 1). ETA antagonist administration without L-NAME resulted in significantly increased fetal weights.
In rats treated with L-NAME alone, when this NOS inhibitor was discontinued after 4 days (gestation day 18), fetal weights from L-NAME-treated rats did not differ significantly from control fetal weights at day 21 (Figure 1). In contrast, placental weights remained significantly reduced after the cessation of NOS inhibition. In rats treated with both L-NAME and ABT-546, neither fetal nor placental weights improved when ETA antagonism was discontinued at the same time as the discontinuation of NOS inhibition (gestation day 18). However, when ETA antagonism was continued through day 21, after discontinuation of NOS inhibition at day 18, fetal weights, but not placental weights, were the same as normal control fetal weights.
Maternal and fetal NOx
Maternal and fetal NOx on day 21 were both reduced in response to NOS inhibition (Figure 2). Maternal NOx was further decreased when the highly ETA-selective antagonist, ABT-546, was present with L-NAME, but fetal NOx was not. The antagonist administered alone caused a slight reduction in maternal but not fetal NOx.
Maternal NOx did not change significantly over time throughout pregnancy in control rats (Figure 3). In rats treated with L-NAME for 4 days only (days 14–18), maternal NOx was significantly reduced on day 18, but recovered and was not significantly different from control values by the end of pregnancy
DISCUSSION
NOS inhibition in the pregnant rat is a model that has been used to study both FGR and preeclampsia (1,2,4,5,10,11). Nitric oxide is a vasodilator that plays an important role in the regulation of vascular tone in normal and pathologic circumstances. A deficiency of NO in the uteroplacental circulation may contribute to the pathophysiology of FGR and preeclampsia in humans. Therefore, understanding the physiologic response to NOS inhibition becomes central to understanding the model, particularly when evaluating potential therapeutic interventions in this model.
As expected, NOS inhibition leads to decreased maternal NO production. We confirmed decreased NO production by demonstrating decreased maternal NOx in this model. It had not previously been determined whether the effects of NOS inhibition on fetal growth during pregnancy were reversible. If the effects were irreversible, this would indicate that the vasculature was no longer capable of responding to vasoactive mediators and would explain the inefficacy of ETA antagonism. If irreversibility had been demonstrated, this would need to be taken into account when evaluating the effect of a therapeutic intervention. To investigate this possibility, we administered L-NAME only for days 14–18 and then evaluated NO production and fetal growth on day 21. We found that fetal growth returned to normal by the end of gestation (day 21). Therefore, the effects of L-NAME administration on fetal growth do in fact appear to be reversible. Of note, placental weights remained decreased despite discontinuation of L-NAME. However placental function was apparently adequate in these pups, as evidenced by normal fetal weights. Maternal NO production after discontinuation of L-NAME was increased. This may be a compensatory response contributing to the improvement in fetal growth after discontinuation of L-NAME.
NOS inhibition in the pregnant rat leads to decreased uterine and placental perfusion (5) as well as increased circulating ET-1 levels (6,7). ET-1, acting via the ETA receptor may further decrease uteroplacental perfusion beyond that caused by decreased NO production. L-NAME has the capacity to cross the placenta from the maternal to the fetal vasculature and may have an adverse impact on fetal growth independent of the known adverse impact of L-NAME on maternal uteroplacental perfusion. Therefore, any intervention that increases uteroplacental perfusion (e.g. ETA antagonism) and/or the ability of L-NAME to access the fetal compartment could paradoxically have an adverse impact on fetal growth. To evaluate this possibility, we measured NO metabolites in the maternal and fetal vasculature. Administration of the ETA antagonist alone resulted in slightly decreased maternal NOx. This is expected, given that NO balances the physiologic effects of ET-1 and inhibition of ET-1 activity by ETA receptor blockade would reduce the necessity for NO production. We did not observe this decrease in our previous study with A-127722, another ETA antagonist (5). This may be a result of lower selectivity of this agent. Administration of L-NAME alone resulted in decreased maternal and fetal NOx. In our prior study, we reported that fetal NOx was not significantly decreased with L-NAME administration. This is likely because the variation in our measurements was higher in that study than in this study. Administration of an ETA antagonist in the setting of L-NAME administration did not have a significant impact on fetal NOx production compared to administration of L-NAME alone. We have shown previously that ETA antagonism in the setting of NOS inhibition normalizes uterine and placental perfusion through day 4 of antagonist infusion (5). Increased tissue perfusion may allow persistent access of L-NAME into the fetus, allowing for continued suppression of fetal NOx. NOS inhibition in the fetal compartment likely leads both to systemic fetal effects and to reduced fetal-placental perfusion that result in growth restriction. L-NAME also leads to increased uterine and placental apoptosis (12) which may contribute to the observed FGR. The decreased fetal NOx does not appear to be an effect of the ETA antagonist per se, given that fetal NOx was not impacted by administration of the ETA antagonist alone. In this model, the potentially beneficial vasodilatory effects of ETA antagonism on placental perfusion paradoxically allow continued, and possibly increased access of L-NAME to the fetal compartment. This limitation should be taken into consideration when evaluating the effects of any therapeutic intervention that is intended to increase placental perfusion in this model of FGR.
This study provides further evidence that ET-1 has an important role in NOS inhibition-induced FGR. When NOS inhibition and ETA antagonism were both stopped at 4 days, fetal growth was restricted. However, when ETA antagonism was continued after NOS inhibition was discontinued, fetal weights were normal. Reduced fetal weights when NOS inhibition and ETA antagonism are discontinued simultaneously are evidence of the importance of ET-1 in the pathophysiology of NOS inhibition-induced FGR. ET receptor antagonism results in increased ET-1 production both in humans (13–15) and in rats (16). NOS inhibition also leads to increased ET-1 production (6,7). When both ETA antagonism and L-NAME are discontinued, the upregulated ET-1 is unopposed, and fetal growth is restricted. The observed full recovery of normal fetal weights when ETA antagonism is continued beyond the cessation of NOS inhibition is further evidence for the involvement of ET-1. In spite of the increased production of ET-1, continued presence of the antagonist blocks the action of ET-1 and prevents it from affecting fetal growth.
NOS inhibition in the pregnant rat is an established model for the study of FGR. In this model, decreased maternal NO production leads to decreased uteroplacental perfusion and ultimately to FGR. The effects of L-NAME on fetal growth are reversible. L-NAME can cross the placenta and may independently contribute to FGR. We have shown previously that ET-1, through its vasoconstrictive activity, is of primary importance in the pathophysiology of this model (5). Any intervention to circumvent the vasoconstriction and improve uteroplacental perfusion in this model has the potential to increase access of L-NAME to the fetal compartment, thus confounding the evaluation of the impact of that intervention on fetal growth.
Acknowledgments
This work was supported by National Institutes of Health grant HD046968.
References
- 1.Wight E, Küng CF, Moreau P, Takase H, Lüscher TF. Chronic blockade of nitric oxide-synthase and endothelin receptors during pregnancy in the rat: effect on pregnancy outcome. J Soc Gynecol Investig. 1998;5:132–139. doi: 10.1016/s1071-5576(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 2.Thaete LG, Neerhof MG, Silver RK. Differential effects of endothelin A and B receptor antagonism on fetal growth in normal and nitric oxide-deficient rats. J Soc Gynecol Investig. 2001;8:18–23. [PubMed] [Google Scholar]
- 3.Helmbrecht GD, Farhat MY, Lochbaum L, Brown HE, Yadgarova KT, Eglington GS, Ramwell PW. L-arginine reverses the adverse pregnancy changes induced by nitric oxide synthase inhibition in the rat. Am J Obstet Gynecol. 1996;175:800–805. doi: 10.1016/s0002-9378(96)80002-0. [DOI] [PubMed] [Google Scholar]
- 4.Diket AL, Pierce MR, Munshi UK, Voelker CA, Eloby-Childress S, Greenberg SS, Zhang X-J, Clark DA, Miller MJS. Nitric oxide inhibition causes intrauterine growth retardation and hind-limb disruptions in rats. Am J Obstet Gynecol. 1994;171:1243–1250. doi: 10.1016/0002-9378(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 5.Thaete LG, Kushner DM, Dewey ER, Neerhof MG. Endothelin and the regulation of uteroplacental perfusion in nitric oxide synthase inhibition-induced fetal growth restriction. Placenta. 2005;26:242–250. doi: 10.1016/j.placenta.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DL, Arora CP, Bui DT, Castro LC. Long-term nitric oxide blockade in the pregnant rat: Effects on blood pressure and plasma levels of endothelin-1. Am J Obstet Gynecol. 1996;175:484–488. doi: 10.1016/s0002-9378(96)70166-7. [DOI] [PubMed] [Google Scholar]
- 7.Neerhof MG, Thaete LG. Chronic NOS-inhibition-induced IUGR is associated with increased circulating endothelin-1 levels in the rat. Hypertens Pregnancy. 1997;16:319. [Google Scholar]
- 8.Wu-Wong JR, Dixon DB, Chiou WJ, Sorensen BK, Liu G, Jae H-S, Tasker A, von Geldern TW, Winn M, et al. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Clin Sci. 2002;103 (Suppl 48):107S–111S. doi: 10.1042/CS103S107S. [DOI] [PubMed] [Google Scholar]
- 9.Wu-Wong JR, Dixon DB, Chiou WJ, Dayton BD, Novosad EI, Adler AL, Wessale JL, Calzadilla SV, Hernandez L, et al. Pharmacology of A-216546: a highly selective antagonist for endothelin ETA receptor. Eur J Pharmacol. 1999;366:189–201. doi: 10.1016/s0014-2999(98)00891-7. [DOI] [PubMed] [Google Scholar]
- 10.Mayr AJ, Lederer W, Wolf HJ, Dünser M, Pfaller K, Mörtl MG. Morphologic changes of the uteroplacental unit in preeclampsia-like syndrome in rats. Hypertens Pregnancy. 2005;24:29–37. doi: 10.1081/PRG-45770. [DOI] [PubMed] [Google Scholar]
- 11.Bahtiyar MO, Buhimschi CS, Ravishankar V, Julien SD, Copel JA, Norwitz ER, Guller S, Lockwood CJ, Buhimschi IA. Nitric oxide (NO) inhibition and hypoxia significantly alters maternal serum angiogenic factors in a rat model of preeclampsia. J Soc Gynecol Investig. 2005;12(Suppl):269A. (Abstract #580) [Google Scholar]
- 12.Miller MJS, Voelker CA, Olister S, Thompson JH, Zhang X-J, Rivera D, Eloby-Childress S, Liu X, Clark DA, et al. Fetal growth retardation in rats may result from apoptosis: role of peroxynitrite. Free Radical Biol Med. 1996;21:619–629. doi: 10.1016/0891-5849(96)00171-2. [DOI] [PubMed] [Google Scholar]
- 13.Haynes WG, Ferro CJ, O’Kane KPJ, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- 14.Weber C, Schmitt R, Birnboeck H, Hopfgartner G, van Marle SP, Peeters PAM, Jonkman JHG, Jones C-R. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–137. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- 15.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. New Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 16.Opgenorth TJ, Wessale JL, Dixon DB, Adler AL, Calzadilla SV, Padley RJ, Wu-Wong JR. Effects of endothelin receptor antagonists on the plasma immunoreactive endothelin-1 level. J Cardiovasc Pharmacol. 2000;36 (Suppl 1):S292–S296. doi: 10.1097/00005344-200036051-00086. [DOI] [PubMed] [Google Scholar]