Abstract
PURPOSE
To validate a two-regression model for predicting energy expenditure (EE) from ActiGraph GT1M accelerometer generated activity counts using a whole-room indirect calorimeter and the doubly-labeled water (DLW) technique. We also investigated if a low-pass filter (LPF) approach would improve the model’s accuracy in the minute-to-minute EE prediction.
METHODS
Thirty-four healthy volunteers (age 20-67 yrs, BMI-19.3-52.1 kg/m2) spent ~24-h in a room calorimeter while wearing a GT1M monitor and performed structured and self-selected activities followed by overnight sleep. The EE predicted by the models and expressed in metabolic equivalents (MET-min) during waking times was compared to the room calorimeter measured EE. A subset of volunteers (n=22) completed a 14-day DLW protocol in free-living while wearing an ActiGraph. The average daily EE predicted by the models (MET-min) was compared to the DLW.
RESULTS
Compared to the room calorimeter, the two-regression model over-predicted EE by 10.2±11.4% (1,282±125 and 1,174 ± 152 MET-min, p<0.001) and time spent in moderate physical activity (PA) by 36.9 ± 46.0 min while underestimating the time spent in light PA by - 48.3 ± 55.0 min (p<0.05). The LPF reduced the squared and mean absolute error in the EE prediction (p<0.05), but not the prediction error in time spent in moderate or light PA (both p>0.05). The EE measured by DLW (2,108±358 MET-min/day) and predicted by both filtered and unfiltered models (2,104 ± 218 and 2,192 ± 228 MET-min/day, respectively) were similar (p>0.05).
CONCLUSIONS
The two-regression model with LPF showed good agreement with total EE measured using room calorimeter and DLW. However, the individual variability in assessing time spent in sedentary, low, and moderate PA intensities and related EE remains significant.
Keywords: Accelerometry, indirect calorimetry, doubly labeled water, low-pass filter
Introduction
Accelerometers are a common research tool used to assess physical activity (PA) in various populations. The accurate characterization of the relationship between accelerometer output, reflecting body movements over selected time intervals, and the energy expenditure (EE) related to these movements has been a challenge (12). Early efforts focused on developing a single linear relationship between EE and activity recorded in arbitrary units (counts) (6, 10, 23), which has been shown to be challenging (19). Recently, several alternative approaches such as multiple linear regressions (9), power models (3), polynomial fits (20, 28), and machine learning (16, 18, 22, 29) have been developed. These models have utilized more information about PA, such as one-second averaged or raw activity data (5, 16, 18, 22, 29) and have the potentials to generate a more accurate prediction of EE associated with PA.
One such method is the two-regression model developed for estimating EE of PA using GT1M ActiGraph accelerometer (ActiGraph, Pensacola, FL) recordings measured in one second epochs (5). The two-regression method used the coefficient of variation (CV = standard deviation / mean * 100) and the sum of six 10 sec epochs to categorize each minute of accelerometer data into resting, walking and jogging, and lifestyle activities. Separate regression equations for walking/jogging and lifestyle activities were then used to estimate minute-to-minute EE. In a study conducted using a portable indirect calorimeter (K4b2 by Cosmed, Rome, Italy) to measure the EE, this model showed significant improvement in the estimation of EE in several lifestyle and sports-related activities relative to previously published regression equations for the ActiGraph in adults. Although the model development procedure included common lifestyle, leisure-time, and sports-related activities all performed at self-selected rates, these activities were completed over relatively short time intervals (5-10 min) and, in many cases, in an unfamiliar environment while wearing a device that includes a facemask and analyzer system that weighs approximately 2 kg (5). We believed that the two-regression model could benefit from an independent validation test which includes a free-living component. Consequently, we examined its prediction ability and accuracy using a whole-room indirect calorimeter (12) and the doubly-labeled water (DLW) technique (21). We also investigated if applying a low pass filter to the predicted EE would improve the two-regression model’s estimation of the minute-to-minute EE without compromising its predictive accuracy for total EE.
Methods
Overview
To rigorously validate the two-regression model, we examined its prediction ability and accuracy using a whole-room indirect calorimeter (12) and the doubly-labeled water (DLW) technique (21). The whole-room indirect calorimeter allows for accurate minute-to-minute EE measurement over a longer time period than portable calorimeters (usually 24-h) and does not require participants to wear a face mask or a mouthpiece during the measurement (3, 18). The DLW technique measures total EE over a 10-14 day period in a free-living environment but it does not provide data about PA performed during this period. Therefore, a combination of these two techniques offers a robust validation of models developed to estimate EE from data generated by PA monitors (15).
In the laboratory validation phase, minute-by-minute and total EE predicted with the two-regression model was compared with EE measured simultaneously by the room calorimeter during the waking period of a 24-h stay. In the second phase conducted for 14 days in free-living conditions, average daily EE predicted by the model was compared with the DLW measured EE. Our null hypothesis was that individual EE predicted by the models will not be different from EE measured by both room calorimeter and DLW techniques. We also investigated if applying a low pass filter to the predicted EE would improve the two-regression model’s estimation of the minute-to-minute EE without compromising its predictive accuracy for total EE.
Participants
Thirty-four adult volunteers (11 males, 23 females) with a wide range of age (20 - 67 yrs) and BMI (19.3 - 52.1 kg/m2) were recruited from the middle Tennessee area using flyers and email distribution lists. Before participation, all volunteers signed an informed consent document approved by the Vanderbilt University Committee for the Protection of Human Subjects. All 34 subjects completed the room calorimeter study. A subsample of 7 males (age 47 ± 19 yrs, BMI 25.2 ± 4.5 kg/m2) and 15 females (age 39 ± 11 yrs, BMI 30.8 ± 9.8 kg/m2) completed the DLW phase of the study. Participants were free of diseases and medications known to alter metabolic rate, major orthopedic limitations, and were non-smokers. The characteristics of the participants are shown in Table 1.
TABLE 1.
Characteristics of participants in whole-room indirect calorimeter (IC) and doubly labeled water (DLW) phases of the validation study. Participants of the DLW phase are a subsample of participants who completed the room calorimeter phase of the study. Data are presented in mean ± standard deviation (Range)
| Room Calorimeter Study | DLW Study | |
|---|---|---|
| N | 34 | 22 |
| Age (years) | 40.1 ± 12.2 (20 – 67) | 41.8 ± 13.9 (20 – 67) |
| Height (cm) | 166.0 ± 8.7 (154.0 – 184.0) | 165.1 ± 8.6 (154.0 – 184.0) |
| Weight (kg) | 84.2 ± 22.3 (47.8 – 134.5) | 79.1 ± 21.0 (47.8 – 123.5) |
| BMI (kg/m2) | 30.7 ± 8.8 (19.3 – 52.1) | 29.3 ± 8.8 (19.3 – 52.1) |
Experimental Procedures
Anthropometric measurements
Body weight was measured to the nearest 0.05 kg with a digital scale (Detecto-Medic, Detecto Scales, Inc, Northbrook, IL) with the participants wearing light clothing and no shoes. Height was measured using a wall-mounted stadiometer (Perspective Enterprises, Portage, MI).
Whole-room indirect calorimetry protocol
Each participant was asked to stay in the whole room indirect calorimeter for approximately 24-hours while second-by-second activity movement in the vertical direction was recorded using ActiGraph GT1M worn at the hip, wrist, and ankle. The room calorimeter is an airtight room measuring 2.5 × 3.4 × 2.4 m capable of measuring energy expenditure (EE) on a minute-by-minute basis using room O2 and CO2 concentrations. Housed within the Vanderbilt Clinical Research Center, it is equipped with a toilet and sink, desk, chair, telephone, television, DVD player, stereo system, bed, treadmill, and exercise bike. A computerized system with a touch monitor and an audible signal prompted participants to start and finish each scheduled activity. Technical details of the room calorimeter have been reported previously (24).
Each participant was asked to engage in two sessions of structured activities. The morning session included several self-paced walking and jogging bouts performed on the room floor and on the treadmill. The afternoon session contained several sedentary activities, such as deskwork, along with stationary biking, and stepping onto a block. Each activity was performed for 10-min followed by a 10-min rest to allow the metabolic rate to return to baseline and help with post hoc discrimination between activity types. During periods when no specific activity was scheduled, participants were encouraged to engage in their normal daily PA routine as much as possible. Details of the exercise protocols conducted in the room calorimeter have been previously described (18).
Doubly-labeled water
Following the room calorimeter stay, participants received DLW sample (0.15 g of 18O2 and 0.09 g of 2H2 per kg of body mass). Urine samples were collected at baseline pre-dose, 4-7 hours post-dose, and on days 7 and 14 post-dose. Analysis was conducted at the USDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine, Houston, TX. The details of the technique have been described previously (17, 21).
Physical Activity Monitoring
Movement was measured using the ActiGraph GT1M monitor, a small device (5.3 × 5.1 × 2.2 cm) that is usually worn at the hip, wrist, or ankle (14) and measures acceleration in the vertical plane using a uniaxial piezoelectric accelerometer. Detailed specifications of the hardware and a full description of how the monitor acquires and filters activity counts have been described previously (27). Data output from the ActiGraph is reported as activity counts using the manufacturer’s Actilife v.4.3.0 software (1). Only the hip data was used for this analysis.
ActiGraph two-regression model
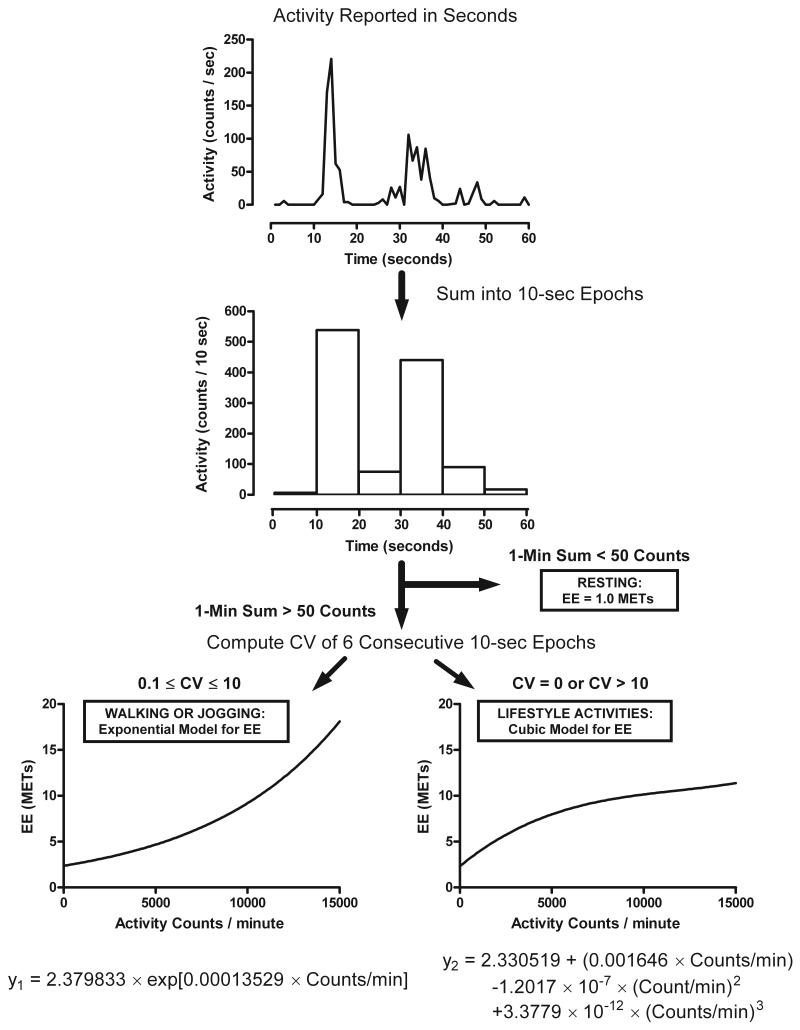
The two-regression model proposed by Crouter et al (5) requires the collection of ActiGraph data in 1-sec epochs. Data from each of the 10-sec segments within each minute are summed to generate six 10-sec epochs. The coefficient of variation (CV = standard deviation / mean * 100) and the sum (1-min count value) of the six 10-sec epochs are then used to categorize each minute of accelerometer data into resting (< 50 counts/min), walking and jogging (>50 counts/min and CV between 0.1 and 10), and lifestyle activities (50 counts/min and CV equal to 0 or >10). The 1-min count value is used to compute a predicted EE in units of metabolic equivalents (MET = total EE/resting EE) using either an exponential model, for the lifestyle activities, or a cubic model for walking and jogging. All resting minutes are assigned an EE value of 1 MET. A graphical representation of the two-regression model is shown in Figure 1.
FIGURE 1.
Schematic illustration of the two-regression model for ActiGraph GTM1 accelerometer, as described by Crouter, et al. (5).
Data Analysis
The two-regression ActiGraph model was developed to estimate EE associated with PA. Therefore, to assure best criteria for unbiased comparison we selected 780-min of each participant’s waking (non-sleeping) period measured in room calorimeter for the analysis. For the DLW study, accelerometer wear time was determined for each day using the criteria specified by Troiano et al (26), where non-wear time is defined as a minimum of 60-min of consecutive zero activity counts, allowing for 1-2 min of non-zero counts less than 100 counts/min. Valid days for the free living study were defined as consisting of at least 600-min of wear time. During the 14-day monitoring period, participants had average 11.9 ± 2.1 valid days.
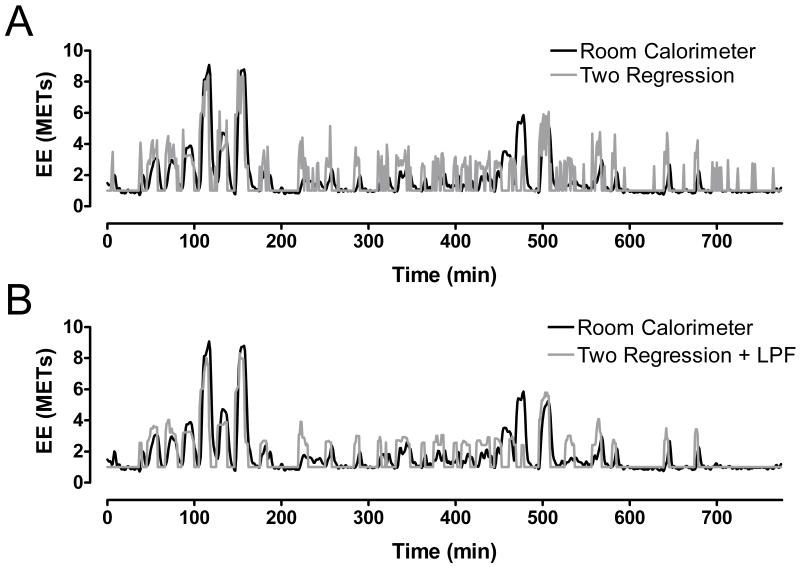
The individual room calorimeter data was converted to METs by dividing the total EE by his/her measured resting EE. We observed high variability (noisy) data in the predicted minute-to-minute EE (Figure 2A). Therefore, a low pass five point median filter (LPF), a commonly used method to remove transient artifacts (7), was applied to the EE estimation (Figure 2B).
FIGURE 2.
Representative plot of energy expenditure (EE) measured during waking (8:30 am – 9:30pm) by the whole-room indirect calorimeter (black line) and estimated with the two-regression model (gray line –Panel A) and the low-pass filtered (LPF) model (gray line – Panel B). The plots represent data for a 45-year-old African-American female (height = 160 cm; body mass = 118 kg, BMI = 46 kg/m2).
The average individual daily EE computed using the two-regression model both with and without the LPF were then compared to the EE measured during free-living with DLW. The DLW energy expenditure was converted to METs by dividing average daily EE by resting EE measured in the room calorimeter.
Statistical Analysis
Data are presented as mean, standard deviation and total range unless otherwise indicated. The total EE, absolute percent difference in total EE, the minute-by-minute mean absolute error, mean squared error, and the number of minutes spent in sedentary (1.0 – 1.5 METs), light (1.5 – 3.0 METs), moderate (3.0 – 6.0 METs), and vigorous (> 6.0 METs) PA were compared between the two-regression model and the room calorimeter data using paired t-tests, p<0.05 was considered significant. In the DLW study, the average daily EE was compared between the two-regression model and the DLW measurements using paired t-tests. Bland-Altman plots (2) were used to assess magnitude bias in average daily EE and the average daily EE. All analyses were conducted using MatLab 2008a (Mathworks, Natick MA).
Results
Phase 1: Whole Room Indirect Calorimeter Study
The two-regression model significantly overestimated the total METs measured by the room calorimeter during waking (non-sleeping) time (1,282 ± 125 vs. 1,174 ± 152 MET-min, p < 0.001). The LPF decreased the two-regression model estimation to 1,195 ± 119 MET-min and the difference with room calorimeter was not significant (p = 0.419).
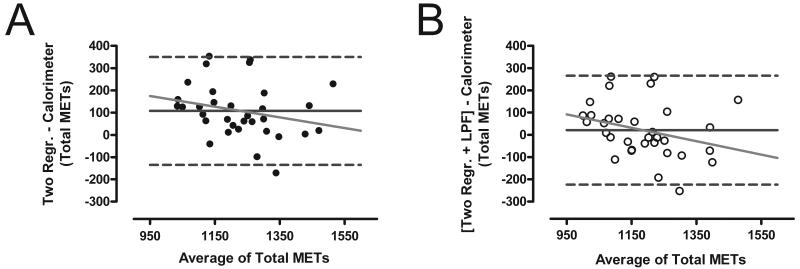
The individual variability and magnitude bias in the total MET-min measured during the non-sleeping minutes was assessed using Bland-Altman plots (Figure 3). The mean difference between the room calorimeter and the two-regression model was 108 MET-min, indicating an overestimation of 10.15 ± 11.42 %. The LPF significantly reduced the mean difference to 21 MET-min, or 2.72 ± 10.9 % (p<0.001). Neither method demonstrated a significant magnitude bias in the estimation of total MET-min (all p> 0.05).
FIGURE 3.
Bland-Altman plots comparing the total METs measured by the whole-room indirect calorimeter and predicted using the unfiltered two-regression model (Panel A; Two Regr.) and the low-pass filtered model (Panel B; Two Regr. + LPF).
Compared to the unfiltered two-regression model, the LPF model significantly decreased the minute-to-minute mean absolute error from 0.54 ± 0.11 to 0.44 ± 0.09 MET-min and mean squared error from 0.73 ± 0.25 to 0.49 ± 0.20 (MET-min)2 (both p< 0.01).
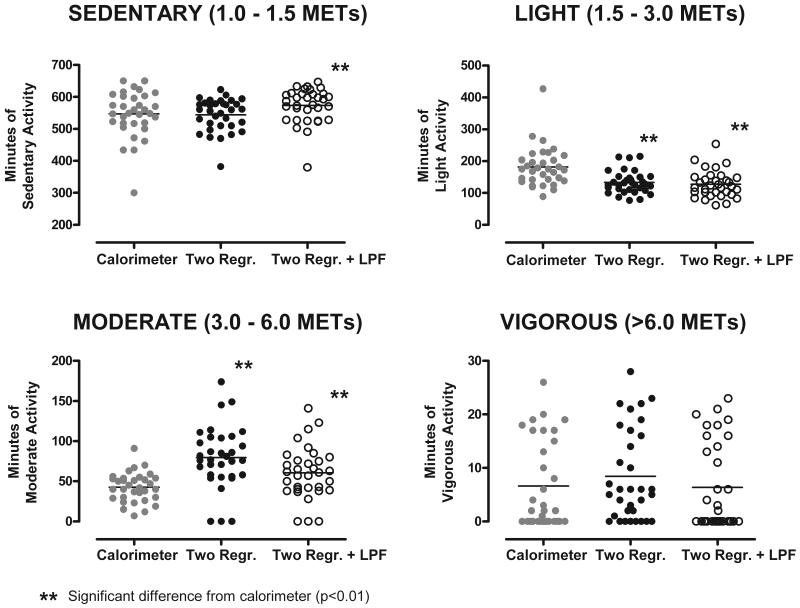
A comparison of time spent in sedentary (1.0 – 1.5 MET), light (1.5 – 3.0 MET), moderate (3.0 – 6.0 MET), and vigorous (> 6.0 MET) PA intensity categories computed using the unfiltered and LPF models and measured by the room calorimeter is presented in Figure 4. Time spent in the sedentary PA overestimated by the LPF model (26.3 ± 54.7 min, p = 0.008). Both models significantly underestimated time spent in light PA (−48.3 ± 55.0 and −54.6 ± 50.0 min) and overestimated time spent in moderate PA (36.9 ± 46.0 and 17.8±39.5 min; p<0.05 for all comparisons). No differences were detected between the models and time spent in the vigorous PA intensity (p>0.05).
FIGURE 4.
Time spent in (1.0 – 1.5 METs), light (1.5 – 3.0 METs), moderate (3.0 – 6.0 METs), and vigorous (> 6.0 METs) physical activity (PA) intensity categories during waking period during ~24-h stay in a whole-room indirect calorimeter.
Stage 2: Free-living DLW Study
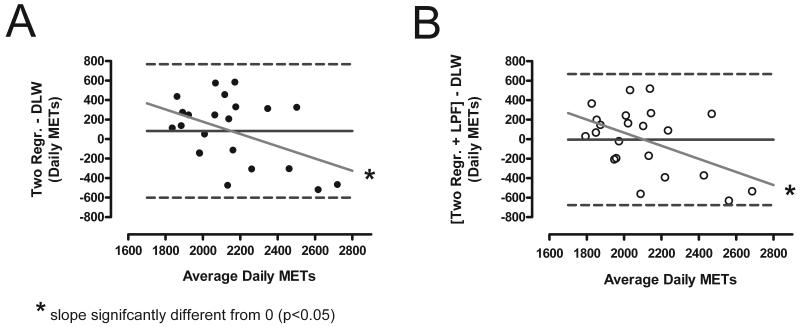
Both the two-regression and LPF were used to estimate the energy expenditure in free-living and compared to the DLW-measured EE. There was no significant difference between the group-averaged daily EE measured with the DLW (2,108 ± 358 MET-min/day) and the EE predicted using either the unfiltered or LPF two-regression models (2,192 ± 228 and 2,104 ± 218 MET-min/day), as demonstrated in Figure 5. However, compared to the EE predicted with unfiltered two-regression model, the EE predicted using the LPF model had a significantly lower mean difference (84 ± 342 vs. −4 ± 336 MET-min/day, p<0.001) and percent difference (5.97±16.0 % vs. −1.71±15.3 %, p<0.001) to the EE measured with the DLW.
FIGURE 5.
Bland-Altman plots of the average daily EE measured during 14-day free living period using doubly labeled water (DLW) technique and estimated using the unfiltered two-regression model (Panel A; Two Regr.) and the low-pass filtered model (Panel B; Two Regr. + LPF).
Bland-Altman plots (Figure 5) revealed that while the mean difference between the EE predicted by the models and the average DLW-measured EE is relatively low, there is a high inter-individual variation measured by the 95% confidence intervals (CI 670 and 659 MET-min/day for the unfiltered and LPF models). A significant negative magnitude bias was detected for both models (p<0.05).
Discussion
In this study we determined the validity the two-regression model (5) to predict EE of PA from body movements recorded by the ActiGraph GTM1 accelerometer. We found good agreement between the average daily EE predicted by the filtered two-regression model and EE measured using both a whole-room indirect calorimeter and free-living DLW in a group of adults with wide range of age (20–67 yrs) and BMI (19–52 kg/m2). However, the accuracy of the model was shown to have a large inter-individual variability.
To our knowledge this is the first reported study that independently validated the two-regression model proposed by Crouter et al (5) using whole-room indirect calorimetry and DLW techniques. The model over-estimated the group average total daily EE by approximately 10.15 ± 11.42% when compared to EE measured by the room calorimeter and by 5.97 ± 16.0% when measured by the DLW. Our data also showed that the model underestimated the time spent in the light (1.5-3.0 MET) PA intensity and overestimated time spent in the moderate (3.0-6.0 MET) PA intensity observed in 29 of 33 our study participants. This suggests that the model may consistently overestimate EE prediction in light PA intensity category triggering misclassification of time intervals (min) spent in light into the moderate intensity PA category. This finding could be important in free-living studies using the two-regression model and carried out in populations spending significant amount of time in light PA intensity.
Using minute-to-minute simultaneous ActiGraph and EE measurements in the room calorimeter, we found that transient increases in EE predicted by the two-regression model do not always correspond to changes in measured EE. We also noticed that during activities characterized by constant intensity, such as running on the treadmill with a steady speed, the predicted minute-to-minute EE changed more than EE measured by the room calorimeter. This discrepancy could be considered a limitation of the model since a small change in the number of activity counts and/or their variability can lead to much larger changes in the predicted EE. To mitigate this effect, we applied the LPF to smooth the rapid minute-to-minute changes in the predicted EE. This approach led to a significant reduction in the mean absolute and mean squared error terms of the EE prediction reflecting physiological response to the movement. The LPF also significantly reduced the average total daily error from approximately 10% to 3% during the room calorimeter and from 6% to −2% during the DLW free-living phases of the study. However, the significant magnitude bias identified in both the filtered and unfiltered models during the DLW study indicate that both methods tend to over-predict EE in individuals with lower daily EE and under-predict EE in those with higher daily EE. The LPF model also improved the agreement between measured and predicted time spent in moderate and vigorous PA categories. However, the LPF technique reduced the ability of the model to accurately determine time spent in sedentary PA. This may be an important limitation of the LPF, since there is growing interest in characterizing the duration and intensity of sedentary PA behaviors (8, 13). Nevertheless, the LPF method may be useful because it can be applied to existing ActiGraph data sets to recalculate and perhaps correct predicted time spent in moderate and vigorous PA intensities and improve accuracy of the minute-to-minute predictions of EE. Future work should determine if the threshold used for activity count variability (CV) and/or the length of the LPF (5 minutes) used in this study should be modified to minimize the significant interindividual variability between measured and predicted EE in the free-living found in our study.
Our results are similar to previous ActiGraph DLW free-living validation studies that used linear regression equations to predict EE from the ActiGraph data (10-13, 15). The confidence intervals (CIs) calculated for the DLW in our study are also comparable to those reported previously. In a similar DLW validation study reported by Leenders, et al (11) involving 13 healthy females wearing ActiGraph and Tritrac (Reining International, Inc., Madison, WI, USA) accelerometers for 7 days, the authors found that the EE predictions using the ActiGraph equations developed by Hendelman, et al (10) and Swartz, et al (25) based on lifestyle activities had the best agreement with EE measured by DLW, with differences (expressed as mean ± SD [range]) of −2 ± 16.6 % [−24 to 23%] and −4 ± 16.6 % [−29 to 24%], respectively. In the current study, we observed similar differences of −1.71 ± 15.3 % [−23.7 to 28.3%] and 5.97 ± 16.0% [−20.0 to 32.3%] for the filtered and unfiltered model, respectively. This may suggest that the two-regression model is not providing additional significant improvements in individual estimation of total EE when compared to equations reported earlier. Potential contributors to the variability between measured and predicted EE include individual variation in sleeping EE and sleep patterns, variability in accelerometer wear-time during waking hours, and variable amounts of activities which are difficult to measure using current accelerometer technology (e.g. biking, weight training, climbing stairs, etc.). These and other factors should be considered in designing and conducting studies in which energy expenditure and activity patterns are major outcome variables. Using similar validation approaches, future studies should also assess the accuracy and variability of more recently published approaches and equations (4, 16, 18, 28) and accelerometer placement. Similarly, many generalized prediction equation, such as two-regression model tested in this study, have not been rigorously validated for use in specialized populations with more unique PA patterns, including children, morbidly obese, older adults, and subjects experiencing significant weight changes. Furthermore, the limitations and variability of these generalized prediction equations should be taken into consideration in designing research protocols or clinical applications in which the amount and pattern of free-living PA or PA related EE are important outcome measures. For example, while we found the two-regression model with LPF might be sufficient to monitor average daily EE as a group, the original model would be better suited to identify sedentary activities.
Although using both the room calorimeter and free-living DLW assessment as calibration tools is a robust way to validate EE prediction equations from accelerometer data, we recognize that these methods have some limitations. First, the effect of diet-induced thermogenesis, which typically accounts for approximately 10% of the total daily EE, was not specifically measured or taken into account in either validation method. Second, while the standardization of activity bouts performed in the room calorimeter were advantageous for the consistency of the results, they may have directed the activity profile toward certain PA intensity categories which may not accurately represent the subjects’ normal daily PA patterns. Third, the relatively small size of the room calorimeter (20.4 m3) limited the equipment used and types of activities that were performed during the study. Finally, variations in the sleeping EE and sleep patterns during the free-living phase of the study were not accounted for and may have contributed to the variation in differences between measured and predicted EE observed in our study.
In conclusion, the EE predicted by a low-pass filtered version of the two-regression model proposed by Crouter et. al. (5) showed good agreement with total EE measured in laboratory condition and in free-living using reference standards. The LPF approach offered improved total EE prediction and minute-to-minute accuracy compared to the original model. Despite the improvements in total EE prediction, the individual variability in assessing time spent in sedentary, low, and moderate PA intensities and related EE remained significant.
Acknowledgements
We acknowledge the contribution of Elizabeth Booth, Stephane Daphnis, Cindy Dorminy, Kristen Jevsevar, Liz Provenzano, Jason Rapaport, and Lauren Whitaker from the Energy Balance Laboratory as well as Clinical Research Center at Vanderbilt University Medical Center staff for help with conducting the study. This study was supported in part by R01 DK69465 from NIH/NIDDK, the Vanderbilt Institute for Clinical and Translational Research (VICTR) grant 1UL1 RR024975 from NCRR/NIH, Vanderbilt Diabetes Research and Training Center grant DK20593. MSB was also supported by R01 HL082988 from NIH/NHLB. MPR, RJB, and KYC were supported by Intramural Research Program of the NIDDK/NIH (Z01-DK071044).
This study was supported in part by R01 DK69465 from NIH/NIDDK, the Vanderbilt Institute for Clinical and Translational Research (VICTR) grant 1UL1 RR024975 from NCRR/NIH, Vanderbilt Diabetes Research and Training Center grant DK20593. NM, and MSB were also supported by R01 HL082988 from NIH/NHLB. KYC, MR, and RJB were supported by Intramural Research Program of the NIDDK/NIH (Z01-DK071044) at the time of this study.
Footnotes
The authors do not have a conflict of interest. The results of the present study do not constitute endorsement of the product by the authors or ACSM.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ActiGraph . ActiLife Software User’s Manual Rev. H ed. ActiGraph; Fort Walton Beach, FL: 2009. p. 43p. [Google Scholar]
- 2.Bland JM, Altman DJ. Regression analysis. Lancet. 1986;1(8486):908–9. doi: 10.1016/s0140-6736(86)91008-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen KY, Sun M. Improving energy expenditure estimation by using a triaxial accelerometer. J Appl Physiol. 1997;83(6):2112–22. doi: 10.1152/jappl.1997.83.6.2112. [DOI] [PubMed] [Google Scholar]
- 4.Choi L, Chen KY, Acra SA, et al. Distributed Lag and Spline Modeling for Predicting Energy Expenditure from Accelerometry in Youth. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00374.2009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crouter SE, Clowers KG, Bassett DR., Jr. A novel method for using accelerometer data to predict energy expenditure. J Appl Physiol. 2006;100(4):1324–31. doi: 10.1152/japplphysiol.00818.2005. [DOI] [PubMed] [Google Scholar]
- 6.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Gabbouj M, Coyle EJ, Gallagher NC. An Overview of Median and Stack Filtering. Circuits Systems and Signal Processing. 1992;11(1):7–45. [Google Scholar]
- 8.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–71. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 9.Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77(1):64–80. doi: 10.1080/02701367.2006.10599333. [DOI] [PubMed] [Google Scholar]
- 10.Hendelman D, Miller K, Baggett C, et al. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sports Exerc. 2000;32(9 Suppl):S442–9. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 11.Leenders NY, Sherman WM, Nagaraja HN. Energy expenditure estimated by accelerometry and doubly labeled water: do they agree? Med Sci Sports Exerc. 2006;38(12):2165–72. doi: 10.1249/01.mss.0000235883.94357.95. [DOI] [PubMed] [Google Scholar]
- 12.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–22. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 13.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols JF, Morgan CG, Chabot LE, et al. Assessment of physical activity with the Computer Science and Applications, Inc., accelerometer: laboratory versus field validation. Res Q Exerc Sport. 2000;71(1):36–43. doi: 10.1080/02701367.2000.10608878. [DOI] [PubMed] [Google Scholar]
- 15.Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity. 2007;15(10):2371–9. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- 16.Pober DM, Staudenmayer J, Raphael C, et al. Development of novel techniques to classify physical activity mode using accelerometers. Med Sci Sports Exerc. 2006;38(9):1626–34. doi: 10.1249/01.mss.0000227542.43669.45. [DOI] [PubMed] [Google Scholar]
- 17.Prentice AM. Stable isotopic methods for measuring energy expenditure. Applications of the doubly-labelled-water (2H2(18)O) method in free-living adults. Proc Nutr Soc. 1988;47(3):259–68. doi: 10.1079/pns19880043. [DOI] [PubMed] [Google Scholar]
- 18.Rothney MP, Neumann M, Beziat A, et al. An artificial neural network model of energy expenditure using nonintegrated acceleration signals. J Appl Physiol. 2007;103(4):1419–27. doi: 10.1152/japplphysiol.00429.2007. [DOI] [PubMed] [Google Scholar]
- 19.Rothney MP, Schaefer EV, Neumann MM, et al. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity. 2008;16(8):1946–52. doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz KH, Treuth M, Hannan P, et al. Predicting energy expenditure from accelerometry counts in adolescent girls. Med Sci Sports Exerc. 2005;37(1):155–61. doi: 10.1249/01.MSS.0000150084.97823.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoeller DA, Ravussin E, Schutz Y, et al. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250(5 Pt 2):R823–30. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 22.Staudenmayer J, Pober D, Crouter S, et al. An artificial neural network to estimate physical activity energy expenditure and identify physical activity type from an accelerometer. J Appl Physiol. 2009;107(4):1300–7. doi: 10.1152/japplphysiol.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strath SJ, Bassett DR, Jr., Swartz AM. Comparison of MTI accelerometer cut-points for predicting time spent in physical activity. Int J Sports Med. 2003;24(4):298–303. doi: 10.1055/s-2003-39504. [DOI] [PubMed] [Google Scholar]
- 24.Sun M, Reed GW, Hill JO. Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure. J Appl Physiol. 1994;76(6):2686–91. doi: 10.1152/jappl.1994.76.6.2686. [DOI] [PubMed] [Google Scholar]
- 25.Swartz AM, Strath SJ, Bassett DR, Jr., et al. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450–6. doi: 10.1097/00005768-200009001-00003. [DOI] [PubMed] [Google Scholar]
- 26.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 27.Tryon WW, Williams R. Fully proportional actigraphy: A new instrument. Behavior Research Methods Instruments & Computers. 1996;28(3):392–403. [Google Scholar]
- 28.Zakeri I, Adolph AL, Puyau MR, et al. Application of cross-sectional time series modeling for the prediction of energy expenditure from heart rate and accelerometry. J Appl Physiol. 2008;104(6):1665–73. doi: 10.1152/japplphysiol.01163.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Pi-Sunyer FX, Boozer CN. Improving energy expenditure estimation for physical activity. Med Sci Sports Exerc. 2004;36(5):883–9. doi: 10.1249/01.mss.0000126585.40962.22. [DOI] [PubMed] [Google Scholar]