Abstract
A variety of medications have been assessed for their potential efficacy for the treatment of methamphetamine dependence. We conducted this study in an attempt to evaluate the potential of a novel class of medications, angiotensin converting enzyme inhibitors, as treatments for methamphetamine dependence. All participants met DSM-IV-TR criteria for methamphetamine abuse or dependence and were not seeking treatment at the time of study entry. The study was conducted using a double-blind design. Subjects received a baseline series of IV doses of methamphetamine (15mg and 30mg) and placebo. Subjects received a second identical series of methamphetamine doses 3 and 5 days after initiation of once-daily oral placebo or perindopril treatment. The dose of perindopril was 2mg, 4mg, or 8mg administered in the morning. Perindopril treatment was tolerated well. There were no main effects of perindopril on methamphetamine-induced changes in cardiovascular or subjective effects. There were significant perindopril * methamphetamine interactions for diastolic blood pressure and for ratings of “Any Drug Effect”, indicating inverted U dose-effect functions for these indices.
Keywords: Methamphetamine, Angiotensin, Perindopril, Subjective Effects
1. Introduction
A variety of medications have been assessed for their potential efficacy for the treatment of methamphetamine dependence. We recently reported that bupropion, an antidepressant binding to dopamine (DA) and norepinephrine (NE) transporters, with additional activity at nicotinic acetylcholine receptors, reduced the euphoria associated with experimental administration of methamphetamine, and cue-induced craving for MA, in human volunteers (Newton et al., 2006). In a subsequent randomized clinical trial, bupropion treatment was more effective than placebo for reducing methamphetamine use in patients with modest pre-randomization use frequency (Elkashef et al., 2007; Shoptaw et al., 2008). Taken together, the available data suggest that medications that reduce the positive subjective effects of MA may be useful therapeutic agents in the treatment of methamphetamine dependence.
We conducted this study in an attempt to evaluate the potential of a novel class of medications, angiotensin converting enzyme (ACE) inhibitors, as treatments for methamphetamine dependence. Preclinical data indicates that treatment with ACE inhibitors increases striatal DA content (Jenkins et al., 1997) and hastens recovery from MPTP-induced neurotoxicity (Kurosaki et al., 2005), suggesting previously unexpected links between the renin-angiotensin system and the striatal DA system. Clinical experience suggests that treatment with ACE inhibitors may improve functioning of patients with Parkinson’s disease who are receiving L-DOPA (Reardon et al., 2000), leading others to suggest that ACE inhibitors may be beneficial treatments for stimulant use disorders (Margolin et al., 2000).
It is becoming increasingly clear that angiotensin II, binding at central AT-1 receptors, alters drug-seeking behavior in preclinical models, though as yet studies have been limited to ethanol consumption in rats and mice (Grupp 1992; Lingham et al., 1990). Transgenic mice expressing a rat angiotensinogen gene (thereby over-expressing angiotensinogen) demonstrated enhanced ethanol self-administration, and this is blocked in mice expressing an antisense angiotensinogen gene (Maul et al., 2001). Mice that over-expressed angiotensinogen, and therefore had increased levels of angiotensin II, consumed substantially more alcohol than wild-type mice. Treatment with the ACE inhibitor spirapril reduced alcohol consumption in both groups of mice. In a subsequent study that used transgenic rats expressing an antisense RNA against angiotensinogen (Maul et al., 2005), expression of the antisense RNA resulted in reduced angiotensin II levels exclusively in the central nervous system. Transgenic rats with reduced CNS angiotensin II consumed markedly less alcohol (but not sucrose solution) compared to their wild-type controls. Additional experiments in AT-1, angiotensin II, and bradykinin receptor knockout mice confirmed that the central effect of angiotensin II on alcohol consumption was mediated exclusively by the angiotensin II receptor AT-1.
Of the available ACE inhibitors we selected perindopril because it is known that this drug alters central dopamine turnover when administered peripherally (Jenkins et al., 1997). Other ACE inhibitors (e.g. captopril) have more modest effects on brain ACE activity (Cushman et al., 1989).
2. Methods
2.1 Subjects
Participants were recruited through advertisements and were paid for their participation. All met DSM-IV-TR criteria for methamphetamine abuse or dependence and were not seeking treatment at the time of study entry. Other inclusion criteria included being between 18 and 45 years of age, a history of using methamphetamine by the smoked or intravenous (IV) route, and normal laboratory assessment and vital signs. Exclusion criteria included a history of seizure disorder, head trauma, dependence on other drugs aside from nicotine, prior adverse reaction to methamphetamine, or the presence of any other axis I psychiatric disorder. Serious medical conditions such as heart disease, AIDS, asthma, Parkinson’s disease and other serious medical conditions were also exclusionary. Concomitant use of psychotropic medications was not allowed. Thirty participants completed the study. This study was approved by the institutional review board at UCLA, and all participants gave informed consent after being fully appraised of potential risks of participation.
2.2 Study Design
The study was conducted using a double-blind design. Following admission to the clinical research center (CRC), subjects received IV doses of methamphetamine (15mg and 30mg, in that order) on two subsequent days. The investigators (but not the participants) were unblended as to the order of the methamphetamine doses. Each methamphetamine infusion was preceded or followed by an IV infusion of saline in a random order in order to maintain the blind. Subjects received a second identical series of methamphetamine/saline doses beginning 3 days after initiation of once-daily oral placebo or perindopril treatment. Methamphetamine and saline were administered using a syringe pump, ensuring that dosing procedures were consistent across doses of methamphetamine and across subjects. The dose of perindopril was 2mg, 4mg, or 8mg administered in the morning. Dose assignment was random.
2.3 Data Analysis
Analysis of variance (ANOVA) was used to determine effects of perindopril dose, methamphetamine dose, and the interaction term (perindopril * methamphetamine). Significant perindopril * methamphetamine interactions were followed with Bonferonni-Dunn post-hoc tests to evaluate differences between perindopril dosages.
4. Results
4.1 Participants
Thirty participants completed the study (Table 1). The majority of participants were Caucasian and male, with an approximate age of 34 years, had received 13 years of education on average, had used methamphetamine for about 10 years, and used methamphetamine for 17 of the past 30 days. The majority of participants used MA by the smoked route, and also smoked cigarettes, used alcohol, and a majority also used marijuana on occasion.
Table 1.
Participant Characteristics
| Perindopril Groups | ||||
|---|---|---|---|---|
| 0 mg (N=5) |
2 mg (N=8) |
4 mg (N=8) |
8 mg (N=9) |
|
| Gender | ||||
| Male | 5 | 8 | 8 | 7 |
| Female | 0 | 0 | 0 | 2 |
| Ethnicity | ||||
| White (Not Hispanic) | 4 | 4 | 5 | 4 |
| Hispanic or Latino | 0 | 3 | 1 | 4 |
| African American | 1 | 1 | 1 | 0 |
| Native American | 0 | 0 | 1 | 1 |
| Age | 30.4±3.6 | 34.6±2.4 | 37.5±2.7 | 34.7±3.9 |
| Education | 14.4±0.7 | 12.6±0.4 | 12.9±0.7 | 13.2±0.5 |
| Substance Use | ||||
| Years of MA use | 4.0±1.5 | 10.0±2.5 | 13.1±2.9 | 12.4±2.9 |
| MA use last 30 Days | 15.6±2.2 | 21.0±2.7 | 14.9±2.9 | 21.9±3.5 |
| Nicotine Use in last 30 days (%) | 60 | 88 | 100 | 78 |
| Route of MA Use | 60% Smoke 20% IV 20% Oral |
63% Smoke 37% Multiple |
25% Smoke 25% Nasal 25% IV 25% Multiple |
63% Smoke 13% Oral 13% IV 26% Multiple |
| Alcohol Use in last 30 days (%) | 100 | 83 | 88 | 56 |
| Marijuana Use in last 30 days (%) | 80 | 57 | 75 | 67 |
| Cocaine Use in last 30 days (%) | 60 | 0 | 38 | 11 |
| Heroin Use in last 30 days (%) | 0 | 0 | 0 | 0 |
| Sedatives Use in last 30 days (%) | 20 | 0 | 13 | 0 |
| PCP Use in last 30 days (%) | 20 | 14 | 0 | 0 |
4.2 Tolerability
Methamphetamine-dependent volunteers generally tolerated perindopril treatment well. There were no differences across the treatment groups for total number of adverse events (reported complaints or abnormal laboratory findings) (F3,26=1.15, p=0.35). Adverse events were relatively uncommon, averaging less than 1 event per participant. The most common adverse events were sleep disturbance, headache, and gastrointestinal distress.
4.3 Cardiovascular Effects
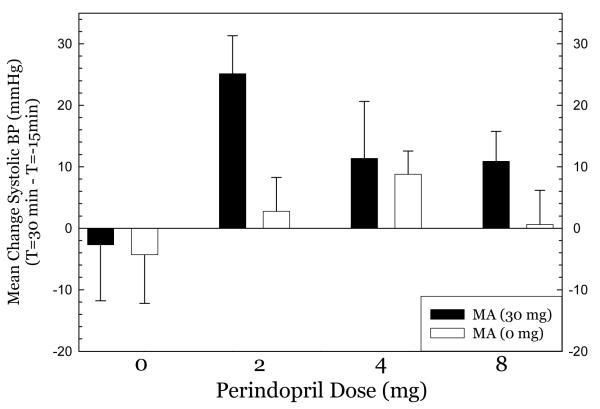
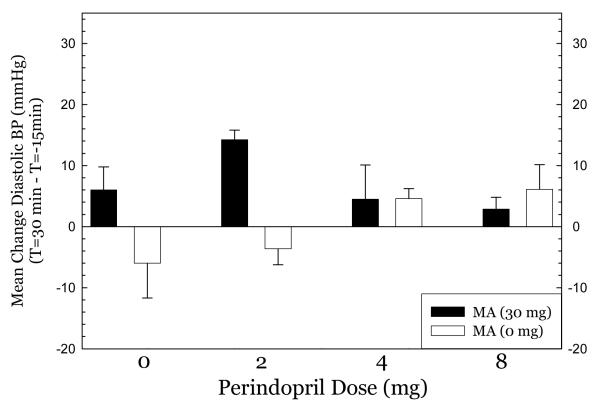
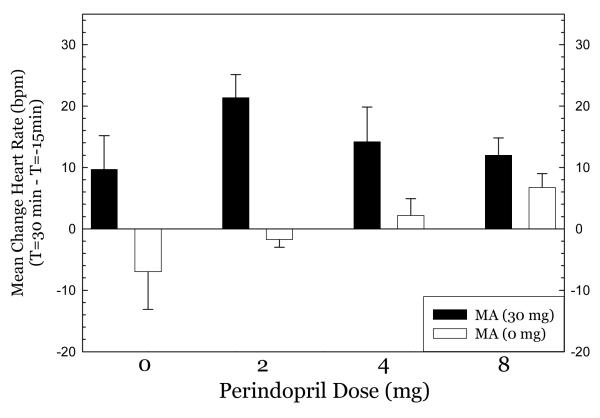
Perindopril treatment did not produce hypotension at any dose tested. For systolic blood pressure (Figure 1), methamphetamine had very modest effects in the 0mg perindopril group, greatest effects in the 2mg group, and smaller effects in the 4mg and 8mg groups. ANOVA indicated no significant effect of perindopril dose (F3,50=2.11, p=0.11), a significant effect of methamphetamine dose (F1,50=3.88, p=0.05), and no significant interaction of perindopril * methamphetamine (F3,50=1.14, p=0.34). For diastolic blood pressure (Figure 2), there was a similar pattern, with modest effects in the 0mg group, greatest effects in the 2mg group, and smaller effects in the 4mg and 8mg groups. ANOVA indicated no significant effect of perindopril dose (F3,50= 0.78, p=0.51), a significant effect of methamphetamine dose (F1,50= 7.98, p=0.007), and a significant interaction of perindopril * methamphetamine (F3,50= 5.38, p=0.003). For heart rate (Figure 3), nearly identical patterns were seen. ANOVA indicated no significant effect of perindopril dose (F3,50=1.63, p=0.19), a significant effect of methamphetamine dose (F1,50= 28.9, p<0.001), and no significant interaction of perindopril * methamphetamine (F3,50= 2.55, p=0.07). Post-hoc tests did not indicate significant differences between perindopril 0 mg vs. 2, 4, or 8 mg for any of the cardiovascular indices.
Figure 1.
Effect of methamphetamine on systolic blood pressure.
Figure 2.
Effect of methamphetamine on diastolic blood pressure
Figure 3.
Effect of methamphetamine on heart rate.
4.4 Subjective Effects
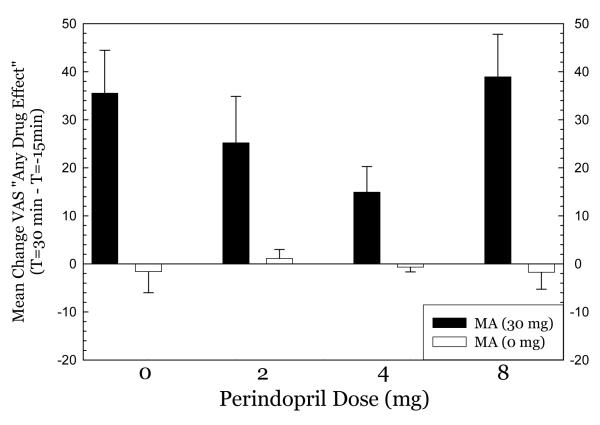
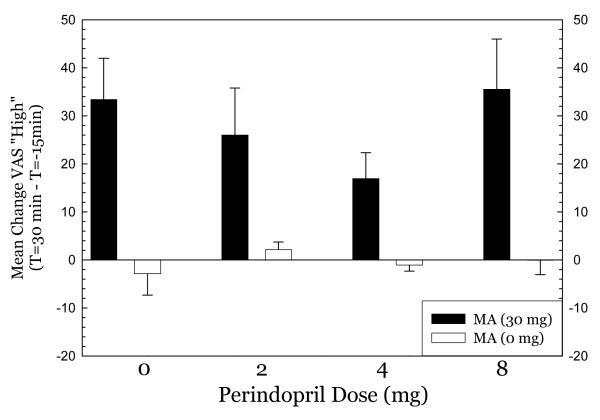
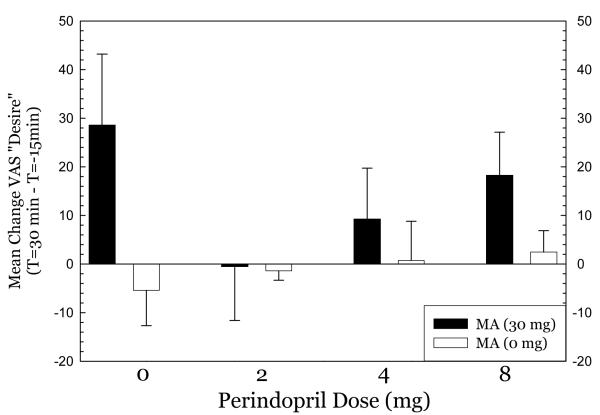
For Any Drug Effect (Figure 4), methamphetamine produced substantial effects in the 0mg perindopril group which progressively declined in the 2mg and 4mg perindopril groups. Methamphetamine produced substantial effects in the 8mg perindopril group, however. ANOVA indicated no significant effect of perindopril dose (F3,79=2.26, p=0.09), a significant effect of methamphetamine dose (F1,79= 67.4, p<0.001), and a significant interaction for perindopril * methamphetamine (F3,79= 2.89, p=0.04). For High (Figure 5), a nearly identical pattern emerged. ANOVA indicated no significant effect of perindopril dose (F3,79=1.52, p=0.22), a significant effect of methamphetamine dose (F1,79= 61.7, p<0.001), and no significant interaction for perindopril * methamphetamine (F3,79= 1.62, p=0.19). For Desire (Figure 6), methamphetamine produced substantial increases in desire in the 0mg perindopril group, but this increase was not seen in the 2mg and 4mg perindopril groups. As with the other subjective effects measures, the effects of methamphetamine were substantial in the 8mg perindopril group. ANOVA indicated no significant effect of perindopril dose (F3,79=1.05, p=0.35), a significant effect of methamphetamine dose (F1,79= 6.97, p=0.01), and no significant interaction for perindopril * methamphetamine (F3,79= 1.54, p=0.26). Post-hoc tests did not indicate significant differences between perindopril 0 mg vs. 2, 4, or 8 mg for any of the subjective effects measures.
Figure 4.
effect of metamphetamine on any drug effect.
Figure 5.
effect of metasmphetamine on “high” drug effect.
Figure 6.
effect of metamphetamine on “desire”.
5. Discussion
Perindopril treatment was well tolerated, with minimal adverse reactions and no dose-dependent adverse events or complaints. Unlike other antihypertensive medications (e.g. adrenergic α-1 antagonists such as prazosin), perindopril was not associated with hypotension at any dose tested.
In subjects receiving intravenous methamphetamine, pretreatment with perindopril produced no significant effects on heart rate, but did modify methamphetamine-induced increases in systolic and diastolic blood pressure. Although comparisons between patients receiving placebo and different doses of perindopril did not reach significance, the pattern observed is consistent with greater methamphetamine-induced changes systolic and diastolic blood pressure in subjects receiving the lowest (2mg) dose of perindopril. This is likely due to modest cardiovascular responses to methamphetamine in the group that received placebo perindopril, as in a sample of 67 participants who received methamphetamine alone, 30mg produced increases in heart rate of about 15 BPM and increases in blood pressure of 15 mmHg systolic and 8 mmHg diastolic (Fleury et al., 2008). The increases we observed in the 0mg perindopril group were much smaller than these, suggesting that the small sample size allowed the chance inclusion of several low responding individuals to influence the group mean. For subjects receiving perindopril 2mg to 8mg, cardiovascular indices tended to moderate as the perindopril dose increased, though due to the small sample size this did not reach statistical significance.
Pretreatment with perindopril produced significant effects on ratings of Any Drug Effect, without modifying other VAS measures in methamphetamine-treated subjects. While comparisons between patients receiving placebo and different doses of perindopril again did not reach significance, the pattern observed is consistent with a diminished subjective effect of methamphetamine in subjects receiving the lowest (2mg) and intermediate (4mg) doses of perindopril. Relative to placebo treatment, values for responding on the Any Drug scale were approximately 30% less in subjects receiving low-dose perindopril, and approximately 60% less in subjects receiving intermediate-dose perindopril (see Figure 4). In contrast, values for responding to this scale were similar for subjects receiving placebo and the highest (8 mg) dose of perindopril, indicating a U-shaped dose-effect curve of oral perindopril on nonspecific subjective effects of intravenous methamphetamine. While this may be due to effects of perindopril on enzyme systems that mediate opposing actions, it more likely is due to the small sample size and resultant type 1 errors.
Perindopril is an ACE inhibitor which acts to reduce blood pressure by blocking the conversion of angiotensin I (AT-1) into angiotensin II. Angiotensin II binds AT-1 receptors within the CNS, potently increasing blood pressure though a variety of mechanisms that are not entirely understood. These include enhancing release of norepinephrine (Wang et al., 2001), resulting in centrally and peripherally mediated constriction of arterioles (Armando et al., 2004). Peripheral angiotensin II also acts on AT-1 receptors in the kidney, resulting in secretion aldosterone and retention of sodium (Allen et al., 2000; Macfadyen et al., 1990). There are AT-1 receptors within brain regions unrelated to blood pressure regulation (MacGregor et al., 1995; von Bohlen Und Halbach and Albrecht 2006), and these mediate, at least in part, other effects of perindopril.
ACE is a dipepdidyl peptidase that cleaves two-amino acid fragments from short peptides. Known substrates include AT-1, substance P, and neurotensin (Skidgel et al., 1987; Skidgel and Erdos 1987; 2004). Other brain peptides may be substrates as well, though little research has focused on this. This multiplicity of activities may account in part for the complex dose-effect relationship that was observed in the present study. Central administration of antagonists for angiotensin II can attenuate psychostimulant-induced activation of the sympathetic nervous system in rats (Knuepfer et al., 2001). In the present study, low doses of perindopril may have also attenuated the ability of angiotensin II to produce increases in sympathetic tone, resulting in a diminished subjective effect of methamphetamine. In contrast, higher doses of perindopril may have increased levels of substance P, enhancing the subjective effects of methamphetamine (Hanson and Lovenberg 1980). Though speculative given the small magnitude of the increase in substance P observed in the preclinical study, this interpretation could be tested using available NK-1 (substance P receptor) antagonists such as aprepitant. Neurotensin is also inactivated by ACE, and enhanced activity of neurotensin subsequent to ACE inhibition could contribute to some of the effects of ACE inhibition, including the increases in striatal DA (Binder et al., 2001) and enhanced recovery following MPTP lesioning (Georgiev and Kambourova 1991; Jenkins et al., 1999; Kurosaki et al., 2005; Munoz et al., 2006). The role of neurotensin in mediating these neuroprotective effects could be explored through the use of AT-1 receptor antagonists, which would not alter neurotensin activity.
Although used as a treatment for hypertension because of its ability to decrease blood pressure through selective inhibition of ACE (Brugts et al., 2009), low-dose perindopril unexpectedly augmented methamphetamine-induced increases in blood pressure in the current study. Relatively few previous reports have examined the effects of ACE inhibitors on increases in blood pressure produced by methamphetamine or other psychostimulants. Cocaine-induced increases in blood pressure in animals were recently shown to be mediated by central AT-1 receptors, with this effect attenuated by pretreatment with an ACE inhibitor (Rowe et al., 2006). The mechanisms through which perindopril increased methamphetamine-induced blood pressure in the present study are unknown, but appear to involve different effects of ACE inhibition on blood pressure under basal conditions and after treatment with methamphetamine.
The main limitation of this study was the small sample size. Effects of treatment with each dose of perindopril or placebo were assessed in less than 10 volunteers, increasing the likelihood of error. ACE is known to have a common polymorphism, the deletion/insertion (D/I) polymorphism (Rigat et al., 1990). The resulting three phenotypes were likely distributed unevenly across our study groups, possibly increasing variability. In addition, we did not measure plasma renin activity (renin mediates the synthesis of AT-1 from angiotensinogen), which has been associated to varying degrees with responsiveness to ACE inhibitors (Seki et al., 2006; Tang et al., 2004). Finally, we did not measure effects of perindopril on methamphetamine self-administration; behavioral effects of drugs frequently parallel subjective effects produced by drugs, but they need not (Dews 1977; Haney and Spealman 2008). Subsequent studies should assess effects of perindopril treatment on drug-seeking or taking behaviors. Until these studies have been completed it is premature to impute therapeutic efficacy for perindopril as a treatment for methamphetamine dependence.
Acknowledgement
We would like to acknowledge support by grants DA017182, DA17754, RR00865 from the NIH. We would also like to acknowledge the medical assistance of Dr. Roger Donovick
6. References
- Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. American Journal on Hypertenssion. 2000;13:31S–38S. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- Armando I, Jezova M, Bregonzio C, Baiardi G, Saavedra JM. Angiotensin II AT1 and AT2 receptor types regulate basal and stress-induced adrenomedullary catecholamine production through transcriptional regulation of tyrosine hydroxylase. Annals of the New York Acadamy of Science. 2004;1018:302–9. doi: 10.1196/annals.1296.036. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacology Review. 2001;53:453–86. [PubMed] [Google Scholar]
- Brugts JJ, Ferrari R, Simoons ML. Angiotensin-converting enzyme inhibition by perindopril in the treatment of cardiovascular disease. Expert Reviews of Cardiovascular Therapy. 2009;7:345–60. doi: 10.1586/erc.09.2. [DOI] [PubMed] [Google Scholar]
- Cushman DW, Wang FL, Fung WC, Harvey CM, DeForrest JM. Differentiation of angiotensin-converting enzyme (ACE) inhibitors by their selective inhibition of ACE in physiologically important target organs. American Journal on Hypertension. 1989;2:294–306. doi: 10.1093/ajh/2.4.294. [DOI] [PubMed] [Google Scholar]
- Dews P. Remarks. In: Thompson T, Unna K, R., editors. Predicting Dependence Liability of Stimulant and Depressant Drugs. University Park Press; Baltimore: 1977. pp. 75–79. [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2008;33:1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fleury G, De La Garza R, 2nd, Mahoney JJ, 3rd, Evans SE, Newton TF. Predictors of cardiovascular response to methamphetamine administration in methamphetamine-dependent individuals. American Journal on Addiction. 2008;17:103–10. doi: 10.1080/10550490701861078. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Kambourova T. Behavioural effects of angiotensin II in the mouse following MPTP administration. General Pharmacology. 1991;22:625–30. doi: 10.1016/0306-3623(91)90067-g. [DOI] [PubMed] [Google Scholar]
- Grupp LA. Effects of angiotensin II and an angiotensin converting enzyme inhibitor on alcohol intake in P and NP rats. Pharmacology, Biochemistry, and Behavior. 1992;41:105–8. doi: 10.1016/0091-3057(92)90067-p. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Lovenberg W. Elevation of substance P-like immunoreactivity in rat central nervous system by protease inhibitors. Journal of Neurochemistry. 1980;35:1370–4. doi: 10.1111/j.1471-4159.1980.tb09011.x. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Mendelsohn FA, Chai SY. Angiotensin-converting enzyme modulates dopamine turnover in the striatum. Journal of Neurochemistry. 1997;68:1304–11. doi: 10.1046/j.1471-4159.1997.68031304.x. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Wong JY, Howells DW, Mendelsohn FA, Chai SY. Effect of chronic angiotensin-converting enzyme inhibition on striatal dopamine content in the MPTP-treated mouse. Journal of Neurochemistry. 1999;73:214–9. doi: 10.1046/j.1471-4159.1999.0730214.x. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM, Purcell RM, Gan Q, Le KM. Hemodynamic response patterns to acute behavioral stressors resemble those to cocaine. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001;281:R1778–86. doi: 10.1152/ajpregu.2001.281.6.R1778. [DOI] [PubMed] [Google Scholar]
- Kurosaki R, Muramatsu Y, Kato H, Watanabe Y, Imai Y, Itoyama Y, Araki T. Effect of angiotensin-converting enzyme inhibitor perindopril on interneurons in MPTP-treated mice. European Neuropsychopharmacology. 2005;15:57–67. doi: 10.1016/j.euroneuro.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lingham T, Perlanski E, Grupp LA. Angiotensin converting enzyme inhibitors reduce alcohol consumption: some possible mechanisms and important conditions for its therapeutic use. Alcoholism: Clinical and Experimental Research. 1990;14:92–9. doi: 10.1111/j.1530-0277.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Macfadyen RJ, Lees KR, Reid JL. Perindopril. A review of its pharmacokinetics and clinical pharmacology. Drugs. 1990;39(Suppl 1):49–63. doi: 10.2165/00003495-199000391-00009. [DOI] [PubMed] [Google Scholar]
- MacGregor DP, Murone C, Song K, Allen AM, Paxinos G, Mendelsohn FA. Angiotensin II receptor subtypes in the human central nervous system. Brain Research. 1995;675:231–40. doi: 10.1016/0006-8993(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Setaro JF, Rinder HM, Grupp L. Cocaine, HIV, and their cardiovascular effects: is there a role for ACE-inhibitor therapy? Drug and Alcohol Dependence. 2000;61:35–45. doi: 10.1016/s0376-8716(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Maul B, Krause W, Pankow K, Becker M, Gembardt F, Alenina N, Walther T, Bader M, Siems WE. Central angiotensin II controls alcohol consumption via its AT1 receptor. Faseb Journal. 2005;19:1474–81. doi: 10.1096/fj.05-3742com. [DOI] [PubMed] [Google Scholar]
- Maul B, Siems WE, Hoehe MR, Grecksch G, Bader M, Walther T. Alcohol consumption is controlled by angiotensin II. Faseb Journal. 2001;15:1640–2. doi: 10.1096/fj.00-0797fje. [DOI] [PubMed] [Google Scholar]
- Munoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, Labandeira-Garcia JL. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51:112–20. doi: 10.1016/j.neuropharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:537–44. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Reardon KA, Mendelsohn FA, Chai SY, Horne MK. The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson’s disease. Australia New Zealand Journal of Mededicine. 2000;30:48–53. doi: 10.1111/j.1445-5994.2000.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. Journal of Clinical Investigation. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe KD, Schwartz JA, Lomax LL, Knuepfer MM. Central angiotensin II receptors mediate hemodynamic response variability to stressors. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2006;291:R719–27. doi: 10.1152/ajpregu.00825.2005. [DOI] [PubMed] [Google Scholar]
- Seki N, Hashimoto N, Suzuki Y, Yagui K, Saito Y. Differential effects of RAS inhibitors associated with ACE gene polymorphisms in type 2 diabetic nephropathy. Diabetes Research and Clinical Practice. 2006;72:135–41. doi: 10.1016/j.diabres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2008;96:222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel RA, Defendini R, Erdos EG. Angiotensin I converting enzyme and its role in neuropeptide metabolism. In: Turner AJ, editor. Neuropeptides and their peptidases. Ellis Horwood; New York: 1987. pp. 165–182. [Google Scholar]
- Skidgel RA, Erdos EG. The broad substrate specificity of human angiotensin I converting enzyme. Clinical and Experimental Hypertension A. 1987;9:243–59. doi: 10.3109/10641968709164184. [DOI] [PubMed] [Google Scholar]
- Skidgel RA, Erdos EG. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25:521–5. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Tang WH, Vagelo s R.H., Yee YG, Fowler MB. Impact of angiotensin-converting enzyme gene polymorphism on neurohormonal responses to high- versus low-dose enalapril in advanced heart failure. Americal Heart Journal. 2004;148:889–94. doi: 10.1016/j.ahj.2004.05.020. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell and Tissue Research. 2006;326:599–616. doi: 10.1007/s00441-006-0190-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang H, Raizada MK. Angiotensin II increases vesicular trafficking in brain neurons. Hypertension. 2001;37:677–82. doi: 10.1161/01.hyp.37.2.677. [DOI] [PubMed] [Google Scholar]