Abstract
Anaplasma phagocytophilum infects neutrophils and myeloid, endothelial, and tick cell lines to reside within a host cell-derived vacuole that is indispensible for its survival. Here, we identify APH_0032 as an Anaplasma-derived protein that associates with the A. phagocytophilum-occupied vacuolar membrane (AVM). APH_0032 is a 66.1 kDa acidic protein that electrophoretically migrates with an apparent molecular weight of 130 kDa. It contains a predicted transmembrane domain and tandemly arranged direct repeats that comprise 46% of the protein. APH_0032 is undetectable on Anaplasma organisms bound to the surfaces of HL-60 cells, but is detected on the AVM and surfaces of intravacuolar bacteria beginning 24 h post-infection. APH_0032 localizes to the AVM in HL-60, THP-1, HMEC-1, and ISE6 cells. APH_0032, along with APH_1387, which encodes a confirmed AVM protein, is transcribed during A. phagocytophilum infection of tick salivary glands and murine neutrophils. APH_0032 localizes to the AVM in neutrophils recovered from infected mice. The Legionella pneumophila Dot/IcM type IV secretion system (T4SS) can heterologously secrete a CyaA-tagged version of the A. phagocytophilum VirB/D T4SS effector, AnkA, but fails to secrete CyaA-tagged APH_0032 or -APH_1387. These data confirm APH_0032 as an Anaplasma-derived AVM protein and hint that neither it nor APH_1387 are T4SS effectors.
Keywords: Pathogenesis, Anaplasma, Ehrlichia, pathogen-occupied vacuole, intravacuolar pathogen
1. Introduction
Anaplasma phagocytophilum is a tick-transmitted obligate intracellular bacterium that infects granulocytes to cause the emerging and potentially fatal disease, human granulocytic anaplasmosis (HGA) [1, 2]. HGA is the second most common tick-transmitted disease of humans in the United States. A. phagocytophilum is a member of the family Anaplasmataceae, which contains other tick-transmissable pathogens that infect peripheral white and red blood cells [2]. A. phagocytophilum successfully establishes a vacuolar protective niche within neutrophils as well as mammalian myeloid and endothelial cell lines and tick embryonic cell lines [2–5]. The A. phagocytophilum-occupied vacuole (ApV) is altered in its fusogenicity. It lacks all and most markers for early and late endosomes, respectively. It does not acidify and prevents fusion with lysosomes and NADPH oxidase- and proteolytic enzyme-carrying specific granules and secretory vesicles [6–9]. Though the exact mechanisms by which A. phagocytophilum converts its host cell-derived vacuole into a safe haven are not fully understood, this strategy is accomplished through the actions of bacterial proteins. Indeed, tetracycline treatment results in acidification and fusion of the ApV with lysosomes [10, 11].
Intravacuolar pathogen-derived proteins that localize to the pathogen-occupied vacuolar membrane (PVM) play crucial pathobiological roles, including intercepting host cell vesicular trafficking and signaling pathways as well as providing structural integrity to the PVM [12]. As such, the identification and characterization of bacterial PVM proteins is an intense area of investigation. We recently demonstrated APH_1387 as the first bacterial-derived A. phagocytophilum vacuolar membrane (AVM) protein [13]. APH_1387 is an acidic, tandem repeat-containing protein (TRP) that carries hydrophobic segments that presumably traverse the AVM [13]. Ehrlichia chaffeensis is an Anaplasmataceae pathogen that invades monocytic cells to reside within a PVM [2]. Acidic TRPs encoded by E. chaffeensis include effectors that localize to the PVM and/or interact with host proteins [14, 15]. It has been demonstrated that expression of chlamydial inclusion membrane proteins (Incs) is induced following infection of host cells [16–18]. APH_0032 is another A. phagocytophilum acidic TRP that, along with APH_1387, is induced during A. phagocytophilum infection of dogs and humans [19]. In their abstract presented at the American Society for Rickettsiology-Bartonella as an Emerging Pathogen Group 2001 Joint Conference, Nelson, Ahn, Herron, and Goodman reported that antisera against APH_0032 recognized the AVM, which implicates APH_0032 as a bacterial-encoded AVM protein. Because of the relevance of bacterial PVM proteins to intravacuolar pathogens and to gain further insight into how A. phagocytophilum modifies its host cell-derived vacuole, we set out to confirm whether APH_0032 is a bona fide AVM protein, characterize its expression pattern in a variety of host cells, and determine whether it and APH_1387 can be translocated by a heterologous type IV secretion system (T4SS).
2. Materials and methods
2.1. Cell lines and in vitro cultivation of A. phagocytophilum
The human promyelocytic cell lines, HL-60 (American Type Culture Collection [ATCC]; Manassas, VA; ATCC code CCL-240) and THP-1 (ATCC code TIB-202) and A. phagocytophilum (NCH-1 strain) infected HL-60 and THP-1 cells were cultivated as described [13]. The human microvascular endothelial cell line, HMEC-1 [20] was obtained from the Centers for Disease Control (Atlanta, GA) and was maintained as described [13].
2.2. In silico sequence analyses
The full APH_0032 sequence was analyzed using algorithms to help predict its secondary structure and its ability to associate with the AVM. TMPred (www.ch.embnet.org/software/TMPRED_form.html) was used to determine if APH_0032 carries a transmembrane domain. Protean, which is part of the Lasergene software package (version 8.02; DNASTAR, Madison, WI), was used to assess APH_0032 for regions of hydrophobicity using the Kyte-Doolittle algorithm [21]. BlastP (blast.ncbi.nlm.nih.gov/Blast.cgi) was used to identify protein sequences to which APH_0032 exhibits homology. The SWISS-MODEL (swissmodel.expasy.org), 3Djigsaw (bmm.cancerresearchuk.org/~3djigsaw), ESyPred3D (www.fundp.ac.be/sciences/biologie/urbm/bioinfo/esypred), and Geno3d (geno3d-pbil.ibcp.fr/cgi-bin/geno3d_automat.pl?page=/GENO3D/geno3d_home.html) algorithms were used in attempts to predict a tertiary structure for APH_0032.
2.3. Generation of maltose binding protein (MBP)-APH_0032 and anti-APH_0032 polyclonal antiserum
The construction of pMal-c2x/DEST has been described [13]. APH_0032 was amplified from A. phagocytophilum genomic DNA using primers APH_0032-001F-ENTR and APH_0032-1860R (Table 1). The amplicon was purified and ligation-independently cloned into pENTR/D-Topo (Invitrogen) as described [13] to yield the entry plasmid, pENTR- APH_0032. One hundred fifty ng of pENTR- APH_0032 was incubated with 150 ng of pMal-c2x/DEST and LR Clonase II (Invitrogen) at 25 C for 1 h to facilitate recombination of the APH_0032 insert downstream of the gene encoding maltose-binding protein of pMal-c2x/DEST to yield pMal-c2x-APH_0032. Proteinase K (0.18 μg/ μl) was added and incubation was continued at 37 C for 10 min after which the LR recombination reactions were cloned into E. coli DH5α cells (Novagen, Madison, WI). Induction and purification of MBP-APH_0032 were performed as described [13]. MBP-APH_0032 was submitted to Animal Pharm Services (Healdsburg, CA) for production of rabbit polyclonal antiserum. Immunoreactivity against MBP-APH_0032 and native A. phagocytophilum APH_0032 was confirmed via western blot, as was the lack of recognition of APH_0032 by preimmune serum (data not shown). Anti-MBP-APH_0032 will be referred to as anti-APH_0032 for the remainder of this paper.
Table 1.
Oligonucleotide primers used in this study.
| Designation | Sequence (5’ to 3’) | Targeted nucleotides |
|---|---|---|
| APH_0032-001F | ATGTTTGAACACAATATTCCTGATAC | Bases 1–26 (+ strand) |
| APH_0032-001F-ENTR | CACCATGTTTGAACACAATATTCCTGATACa | Bases 1–26 (+ strand) |
| APH_0032-1860R | TCACAACGCGAGCACGTC | Bases 1843–1860 (− strand) |
| APH_0032-876R | TGGAGGGCACTCAAGAGC | Bases 858–876 (− strand) |
| APH_1387-001F | ATGTATGGTATAGATATAGAGCTAAG | Bases 1–26 (+ strand) |
| APH_1387-533R | CCGTTGTGGAGGCATTATCGC | Bases 513–533 (− strand) |
| APH_0032-001F-pJB2581 | TTCCGGCTATGGATCCATGTTTGAACACAATATTCCTGATACb | Bases 1–26 (+ strand) |
| APH_0032-1860R-pJB2581 | ACAAGCTTGCATGCCTGCAGTCACAACGCGAGCACGTCAc | Bases 1844–1860 (− strand) |
| aph0740-3391F-pJB2581 | TTCCGGCTATGGATCCGGAACTAGTAGCTCTTTTGCAGCT | Bases 3391–3414 (+ strand) |
| aph0740-3699R-pJB2581 | ACAAGCTTGCATGCCTGCAGCTACCTACCGCGACCTCCTT | Bases 3680–3699 (− strand) |
| APH_1387-691F-pJB2581 | TTCCGGCTATGGATCCGTGGTAGTTGCTCCAGAAGCGC | Bases 691–712 (+ strand) |
| APH_1387- 1737R-pJB2581 | ACAAGCTTGCATGCCTGCAGCTAATAACTTAGAACATCTTCATCGTC | Bases 1711–1737 (− strand) |
Underlined nucleotides correspond to a Gateway entry vector-compatible sequence.
Nucleotides in underlined italics correspond to pJB2581 compatible sequences.
2.4. Preparation of host cell-free A. phagocytophilum populations containing dense core (DC) and reticulate cell (RC) organisms or DC organisms only
To isolate host cell-free A. phagocytophilum DC and RC, infected (≥90%) HL-60 cells were broken by repeated passage through a 27-guage needle. To isolate DC organisms, infected HL-60 cells were subjected to eight 8-sec bursts on ice interspersed with 8-sec rest periods using a Misonix S4000 ultrasonic processor (Farmingdale, NY) on an amplitude setting of 30, which destroys host cells and RC but not DC, as confirmed by electron microscopy (data not shown). Bacteria were separated from unbroken host cells and debris by differential centrifugation as described [22].
2.5. Western blot
Whole cell lysates, MBP, and MBP-APH_0032 were resolved, transferred to nitrocellulose, and screened using anti-APH_0032 followed by horseradish peroxidase-conjugated goat anti-rabbit IgG as described [23]. Actin and A. phagocytophilum Msp2 (P44) were detected using anti-human actin mAb (Sigma; St. Louis, MO) and anti-Msp2 (P44) mAb 20B4 (a gift from J. Stephen Dumler of Johns Hopkins University, Baltimore, MD) [24, 25], respectively. Some blots were screened using HGA patient antiserum or normal human serum (generously provided by Erol Fikrig of Yale University). Densitometric analyses were performed using ImageJ (National Institutes of Health, USA; rsb.info.nih.gov/ij) to quantitatively assess the relative amounts of APH_0032 in whole cell lysates of DC organisms versus DC and RC organisms.
2.6. Infection time-courses
Host cell-free A. phagocytophilum bacteria were obtained and synchronous infections were initiated as described [22]. At the appropriate post-bacterial addition time-point, aliquots were removed and processed for western blot analyses as described above or immunofluorescence or immunoelectron microscopy as described below.
2.7. Laser-scanning confocal microscopy (LSCM)
C3H/HeNscid mice were infected with A. phagocytophilum (NCH-1 strain) as described [26]. On day 8 post-infection, whole blood was collected and buffy coat slides were prepared as described [13]. Ulrike Munderloh (University of Minnesota, Minneapolis) kindly provided slides of paraformaldehyde-fixed A. phagocytophilum infected ISE6 cells. A. phagocytophilum infected HL-60, THP-1, or HMEC-1 cells were prepared and all samples were screened with rabbit anti-APH_0032 and mouse anti-Msp2 (P44) mAb 20B4 as described [13] Samples were examined for A. phagocytophilum inclusions and surface bound bacteria using a TCS-SP2 AOBS confocal laser-scanning microscope (Leica Microsystems, Bannockburn, IL), which is housed in the Virginia Commonwealth University Department of Neurobiology and Anatomy Microscopy Core Facility.
2.8. Immunoelectron microscopy
HL-60 cells were incubated with host cell-free A. phagocytophilum for 40 min at 37°C. The cells were washed three times with phosphate buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4) and centrifuged at 300 g for 5 min to remove unbound bacteria. At each appropriate time point 9 x 106 cells were processed, stained sequentially for 1 h each with (1:200) anti-APH_0032 and (1:10) goat anti-rabbit IgG conjugated to 6 nm gold particles (Electron Microscopy Sciences, Hatfield, PA), and examined by transmission electron microscopy using a JEM-1230 transmission electron microscope (Joel, Tokyo, Japan) equipped with a Gatan UltraScan 4000SP 4K x 4K CCD camera as described [13]. Because these samples were fixed with gluteraldehyde, which is necessary for performing immunoelectron microscopy, the differences in the electron densities between DC and RC organisms was not discernible. DC organisms, which are spheroid and have ruffled outer membranes could be discerned from RC organisms, which are pleomorphic, have smooth outer membranes, and are generally more elongated than DC organisms.
2.9. RT-PCR
Sukanya Narasimhan (Yale University) kindly provided total RNA isolated from A. phagocytophilum infected Ixodes scapularis nymph salivary glands. Total RNA isolated from neutrophils (Gr-1-positive splenocytes) recovered from A. phagocytophilum infected C3H/HeN mice on day 8 post-infection has been described previously [27]. cDNA stocks and PCR amplicons were generated as described [27] using APH_0032-001F and APH_0032-1860R (Table 1) to target APH_0032 transcript and APH_1387-001F and APH_1387-533R to target APH_1387 transcript.
2.10. CyaA plasmid construction and clone verification
The complete open reading frame of APH_0032 was amplified from A. phagocytophilum DNA by PCR using AccuPrime Pfx polymerase (Invitrogen) and APH_0032-001F-pJB2581 and APH_0032-1860R-pJB2581 (Table 1). Difficulty in cloning and expressing the entire open reading frames of APH_0740 (ankA) and APH_1387 necessitated cloning the C-terminal 100 residues of these proteins using APH_0740-3391F-pJB2581, APH_0740-3699R-pJB2581, APH_1387-691F-pJB2581, and APH_1387-1737R-pJB2581 (Table 1). It is important to note that the translocation signal of T4SS substrates is located in the C-terminus and the final 100 aa of T4SS substrates are sufficient for translocation. To allow high-efficiency recombinational cloning, the 5′ ends of forward and reverse primers contain 15 bases that are homologous with the sequence at the ends of the CyaA fusion vector pJB2581 [28] linearized with BamHI and PstI. PCR products were cloned into pJB2581 linearized with BamHI/PstI using the In-Fusion Advantage PCR Cloning Kit (BD Clontech, Mountain View, CA) to generate gene fusions encoding A. phagocytophilum proteins N-terminally fused to the C-terminus of CyaA. L. pneumophila was transformed with plasmid constructs by electroporation as previously described [29]. Plasmids were isolated from colonies, and BamHI/PstI restriction enzyme digests were performed to confirm the presence of the expected restriction fragment. Expression of CyaA fusion proteins by wild type and DotA− L. pneumophila was verified by immunoblotting [29].
2.11. Legionella pneumophila
L. pneumophila JR32 (wild type) and LELA3118 (DotA−) strains were generously provided by Howard Shuman (Columbia University) and were cultured on charcoal yeast extract (CYE) agar plates containing streptomycin (50 μg/ml) and kanamycin (25 g/ml), respectively. For plasmid selection, L. pneumophila transformants were additionally grown in the presence of chloramphenicol (10 μg/ml).
2.12. CyaA translocation assay
L. pneumophila transformants were incubated overnight in AYE broth containing chloramphenicol, diluted and then cultured until organisms displayed obvious motility by phase-contrast light microscopy. Prior to infection, THP-1 cells were treated with 200 nM phorbol 12-myristate 13-acetate (PMA; EMD Biosciences, San Diego, CA) to promote differentiation to an adherent, macrophage-like cell as previously described [30]. Prior to infection, isopropyl-beta-D-thiogalactopyranoside (IPTG) was added to L. pneumophila cultures at a final concentration of 1 mM for 2 h to induce expression of CyaA fusion proteins. PMA-differentiated THP-1 cells were plated in 24-well plates (1 X 106 cells/well) and infected with L. pneumophila expressing CyaA fusion proteins at a multiplicity of infection of approximately 20. Bacteria were added to wells and the plate centrifuged for 5 min at 180 g to initiate contact between organisms and THP-1 cells. After a 30 min incubation at 37 C in the presence of 1 mM IPTG, cells were washed three times in PBS and lysed in 500 μl of a solution containing 50 mM HCl and 0.1% Triton X-100. Samples were boiled for 5 min followed by the addition of 30 μl of 0.5 M NaOH to neutralize the acid. One ml of 95% ethanol was added to samples, which were incubated on ice for 5 min, dried under a vacuum, and resuspended in assay buffer [0.05 M sodium acetate (pH 5.8), 0.02% bovine serum albumin]. The level of adenosine 3′,5′-monophosphate (cAMP) in dried samples was determined using the cAMP Enzymeimmunoassay (GE Healthcare, Piscataway, NJ) according to the manufacturer’s protocol. CyaA fusion proteins were considered positive for translocation when the increase in cAMP over CyaA alone (negative control) was at least 2.5-fold and this increase was abolished following expression of fusion proteins in the L. pneumophila DotA− mutant (LELA3118). A CyaA fusion to RalF, a well-characterized L. pneumophila Dot/Icm effector [31] was used as a positive control. cAMP levels resulting from translocation of CyaA-RalF were similar to those reported by Bardill et al. [28].
2.13. Statistical analyses
Statistical analysis was performed using the Prism 4.0 software package (Graphpad; San Diego, CA). If Analysis of Variance (ANOVA) indicated a group difference (p < 0.05) then Tukey’s test was used to test for a significant difference among groups.
3. Results
3.1. APH_0032 displays limited similarity to other known proteins but exhibits predicted secondary structural characteristics that are suggestive of AVM localization
APH_0032 is a 619 aa acidic protein (pI = 3.6) with a predicted molecular weight of 66.1 kDa. Amino acids 313–597 (46.0% of the protein’s sequence) encode a tandem repeat region (Figure 1, A and C). Eight direct repeats, which are nearly identical and range from 33 to 35 amino acids long, are preceded by a truncated segment that is homologous to the last 10 amino acids of each repeat and followed by a truncated segment that is homologous to the first 3 amino acids of each repeat (Figure 1A). BLASTP searches using the entire APH_0032 sequence or the repeat region revealed that amino acids 341–609 exhibit 30% identity to PY03917, which is a hypothetical protein of Plasmodium falciparum. A BLASTP search using the N-terminal non-repeat region (amino acids 1–312) yielded hits to hypothetical proteins of Microcoleus chthonoplastes and Entamoeba dispar and a putative RNA methylase from Methanococcus voltae that ranged from 22 to 30% identity. APH_0032 is predicted to be largely hydrophilic (Figure 1B). Chlamydial Inc proteins possess transmembrane domains (TMD) that facilitate insertion into the PVM and often carry hydrophilic domains that extend into the host cell cytoplasm to interact with host proteins [16, 32, 33]. TMPred analysis predicts APH_0032 to carry a TMD that spans amino acids 144–162 (Figure 1B) [34]. Attempts to predict a tertiary structure for APH_0032 using SWISS-MODEL, ESyPred3D, 3Djigsaw, and GENO3D were unsuccessful because none of these programs were able to identify similar sequences of known structure on which to model APH_0032. Sequencing of PCR products of the APH_0032 coding sequence amplified from A. phagocytophilum strains NCH-1, HZ, and HGE-1 revealed that this gene’s sequence is identical among the 3 strains, as well as the USG3 strain for which it was originally sequenced [19] (data not shown).
Figure 1. Schematic representation of A. phagocytophilum APH_0032, sequence, and secondary structure analyses.

(A) The amino (N)-terminal region (amino acids 1–312) precedes the repeat region (amino acids 313–597), which precedes a short carboxy (C)-terminal region (amino acids 598–619). The repeat region consists of 8 imperfect direct repeats (denoted by grey arrows) of 34, 34, 34, 34, 33, 34, 34, and 35 amino acids that are arranged in tandem. The 8 direct repeats are preceded by a truncated segment that is homologous to the last 10 amino acids of each repeat and followed by a truncated segment that is homologous to the first 3 amino acids of each repeat. The black bar and “TMD” designation denote the predicted transmembrane domain encoded by amino acids 144–162, as determined by the TMPRED algorithm. (B) Hydrophobicity analysis of APH_0032. The numerical scale demarcates the protein sequence at 50-amino acid intervals. The Kyte-Doolittle algorithm was used to denote hydrophobic (black filled histogram above the x-axis) and hydrophilic (black filled histogram below the axis) regions. (C) APH_0032 tandem repeat sequence alignment. The amino acid (aa) positions and sequence of each repeat are provided. Dashes denote gaps in the alignment. Grey highlighting denotes identical amino acids. Bolded amino acid segments are those that are homologous to a segment that occurs 4 times in the E. chaffeensis ECH0039 (P120) tandem repeat region [19, 57].
3.2. APH_0032 is present in higher abundance in A. phagocytophilum RC organisms than in DC organisms
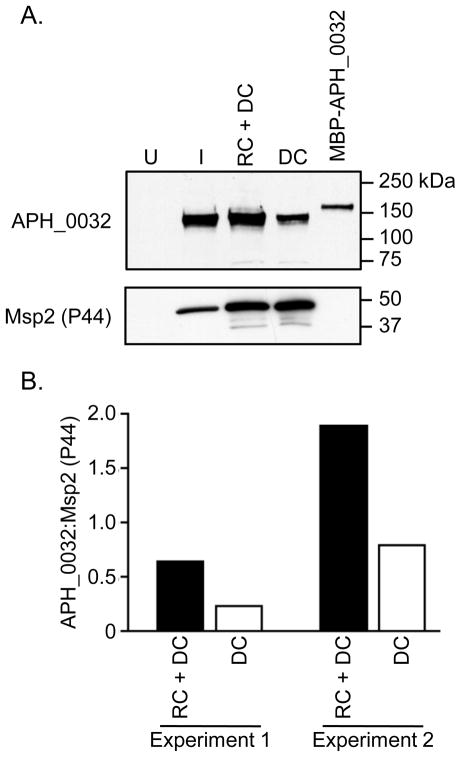
We performed Western blot analysis to determine if APH_0032 is expressed during A. phagocytophilum infection of promyelocytic HL-60 cells. Anti-APH_0032 recognized APH_0032 as a single band of ~130 kDa in an A. phagocytophilum infected, but not an uninfected HL-60 cell lysate (Figure 2). APH_0032’s apparent MW is considerably larger than its predicted size of 66.1 kDa. Likewise, MBP-APH_0032 (pI = 4.1) migrates with an apparent MW of ~160 kDa, which is larger than its predicted MW of 109.3 kDa. A. phagocytophilum undergoes a biphasic developmental cycle in which an infectious DC organism binds, invades, and develops into a replicative RC organism that divides by binary fission [22]. The numerous RC bacteria, which are non-infectious, revert to DC organisms before being released to infect naïve host cells. We have determined that a host cell-free population of A. phagocytophilum DC and RC organisms can be recovered following syringe lysis, which does not damage the fragile RC, while a pure DC population can be recovered following sonication, which destroys the RC (data not shown). To assess whether DC or RC organisms express more APH_0032, we screened Western-blotted lysates of host cell-free A. phagocytophilum organisms recovered following syringe lysis (RC and DC) or sonication (DC only) that were normalized according to the levels of Msp2 (P44), which is a constitutively expressed outer membrane protein [35]. APH_0032 was detected in lysates derived from RC and DC organisms and from DC organisms alone (Figure 2A). Densitometric analyses of two separate experiments revealed that there is 2.37 to 2.74 times more APH_0032 in lysates derived from RC and DC organisms as compared to lysates derived from DC organisms (Figure 2B).
Figure 2. APH_0032 is present in greater abundance in lysates of A. phagocytophilum RC organisms as compared to DC organisms.
(A) Western-blotted lysates of uninfected (U) and A. phagocytophilum infected HL-60 cells (I) as well as host cell-free A. phagocytophilum populations consisting of RC and DC organisms (RC + DC) or only DC organisms (DC) was screened with anti-APH_0032. MBP-APH_0032 served as a positive control. The blot was stripped and screened with anti-Msp2 (P44) to confirm that equivalent amounts of lysates derived from RC and DC organisms and only DC organisms were used. The results are representative of two separate experiments. (B) Densitometry to quantify the intensities of APH_0032 and Msp2 (P44) bands. The ratio of the APH_0032 densitometric value to the Msp2 (P44) densitometric value is shown for RC + DC and DC lysates for two separate experiments.
3.3. APH_0032 is not detected until 24 h post-infection and localizes to the AVM
Next, we screened A. phagocytophilum infected HL-60 cells with anti-APH_0032 in conjunction with a mAb against Msp2 (P44) [24, 25] at 0.7, 8.5, and 24 h post-infection and examined the cells by LSCM. At 0.7 h post-infection, essentially no APH_0032 was detected on Msp2 (P44)-positive organisms that were bound to the HL-60 cell surface (Figure 3, A–D). By 8.5 h, some A. phagocytophilum organisms that were positive for both Msp2 (P44) and APH_0032 were detectable (Figure 3, E–H). At 24 h, the AVM was strikingly distinguishable from enclosed bacteria by exclusive staining for APH_0032 (Figure 3, I–L). A. phagocytophilum organisms within inclusions that were positive for both Msp2 (P44) and APH_0032 appeared as green (corresponding to Msp2 [P44] staining) spheroid organisms, each of which was surrounded by a red ring (corresponding to APH_0032 staining; Figure 3, J–L ) or as yellow organisms. At 24 h, 56.6 ± 3.1% of A. phagocytophilum inclusions were positive for APH_0032 (Figure 3L). The AVM was negative for Msp2 (P44) at all time points.
Figure 3. A. phagocytophilum APH_0032 is detectable on the surfaces of intravacuolar bacteria and localizes to the AVM in infected host cells.
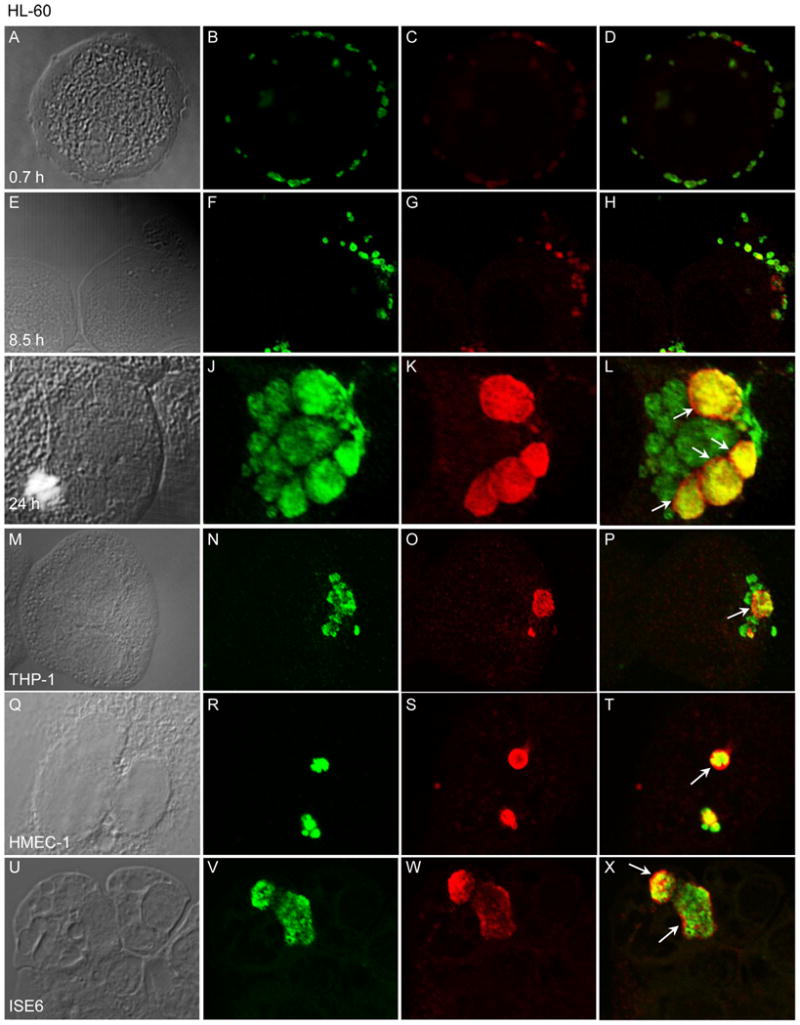
(A–L) HL-60 cells were synchronously infected with A. phagocytophilum. At 0.7 (A–D), 8.5 h (E–H), and 24 h post-infection (I–L), the cells were fixed and viewed by LSCM for immunoreactivity with antibodies against Msp2 (P44) (major bacterial surface protein; used to denote bacteria in B, F, and J) and APH_0032 (C, G, and K). DIC images are presented in panels A, E, and I. Merged fluorescent images are presented in D, H, and L. (M–X) A. phagocytophilum infected THP-1 (M–P), HMEC-1 (Q–T), and ISE6 (U–X) cells were fixed and viewed by LSCM for immunoreactivity with antibodies against Msp2 (P44) (N, R, and V) and APH_0032 (O, S, and W). DIC images are presented in panels M, Q, and U. Merged fluorescent images are presented in panels P, T, and X. (L, P, T, and X) Arrows denote representative AVMs that exhibit exclusive staining for APH_0032 surrounding Msp2 (P44)-positive or Msp2 (P44)- and APH_0032-dual-positive intravacuolar bacteria. The results are representative of three separate experiments.
3.4. APH_0032 localizes to the AVM during A. phagocytophilum infection of human myeloid and endothelial cell lines and tick embryonic cell lines
In addition to HL-60 cells, A. phagocytophilum infects and resides within host cell-derived inclusions in the human monocytic cell line, THP-1, the human microvascular endothelial cell line, HMEC-1, and the Ixodes scapularis embryonic cell line, ISE6 [2–5]. To confirm whether APH_0032 is expressed and localizes to the AVM within each of these cell lines, A. phagocytophilum infected THP-1, HMEC-1, and ISE6 cells were examined by LSCM at 24 h post-infection. APH_0032-positive inclusions were detected within each cell line (Figure 3, M–X). As observed for HL-60 cells, a notable number of ApVs were negative for APH_0032 staining (Figure 3, panels P, T, and X). Indeed, at 24 h post-infection only 54% of ApVs were positive for APH_0032 in THP-1 cells (data not shown).
3.5. APH_0032 is not amply expressed by A. phagocytophilum and does not localize to the AVM until 24 h post infection of HL-60 cells
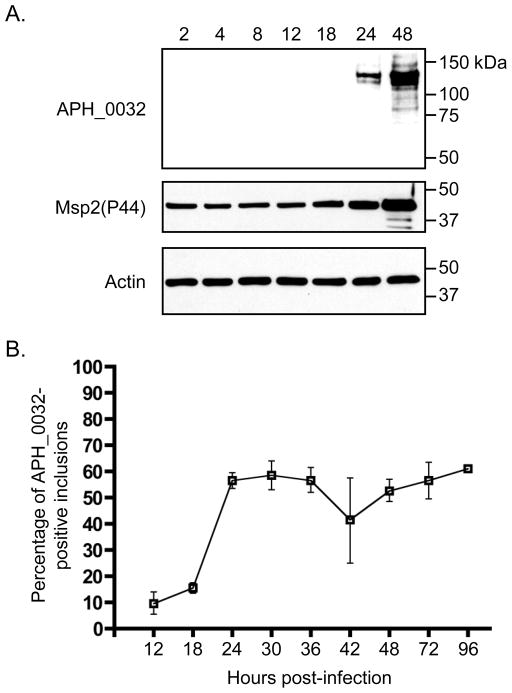
We next monitored the temporal expression and AVM localization kinetics of APH_0032 over the course of a synchronous A. phagocytophilum infection of HL-60 cells. Host cell-free organisms were added to HL-60 cells and allowed to bind for 40 min after which unbound bacteria were removed. Our and other laboratories have shown that it takes approximately 4 h for bound A. phagocytophilum DC organisms to enter into nascent vacuoles [6, 7, 36]. APH_0032 is not detectable by western blot analysis until 24 h post-bacterial addition, which corresponds to ~20 h post-entry (Figure 4A). At 48 h, the intensity of the APH_0032 130-kDa band is considerably stronger and additional less intense bands of higher and lower apparent molecular weights are also detected. One of the lower molecular weight bands is ~66 kDa, which is in agreement with the predicted size for APH_0032. Consistent with what we have shown previously [27], the intensity of the Msp2 (P44) band, which serves as an infection control, increases throughout the course of infection. APH_0032 is detectable on 9.8 ± 4.3% and 15.5 ± 2.0% of ApVs at 12 and 18 h, respectively (Figure 4B). By 24 h, the number of APH_0032-positive ApVs had increased to 56.6 ± 3.1%. The percentage of APH_0032-positive ApVs fluctuated between 41.4 ± 16.2 and 61.1 ± 1.4% for the remainder of the time course. The decrease in the percentage of APH_0032-positive ApVs that occurs at 42 h is likely due to reinfection, which we have shown occurs by or shortly after 36 h [22].
Figure 4. Kinetics of APH_0032 expression and AVM localization in A. phagocytophilum infected HL-60 cells.
HL-60 cells were synchronously infected with A. phagocytophilum. At the indicated post-infection time points, aliquots were processed and analyzed by western blot analysis (A) or immunofluorescence microscopy (B). (A) Western blots of A. phagocytophilum infected HL-60 whole cell lysates were screened with antibodies against APH_0032, Msp2 (P44) (infection control), and actin (loading control). (B) Percentages of ApVs [based on the presence of Msp2 (P44)-positive A. phagocytophilum organisms] that are also positive for APH_0032 exclusive staining of the AVM. Results are the mean (±SD) of 3 separate experiments. 828-1471 Msp2 (P44)-positive inclusions were scored for APH_0032 staining per time point.
We utilized immunoelectron microscopy to screen synchronously infected HL-60 cells over a 48 h period to further examine when APH_0032 is expressed and when it localizes to the AVM during A. phagocytophilum infection. APH_0032-negative DC organisms were bound to the HL-60 cell surface at 0.7 h (Figure 5A) and had internalized into nascent vacuoles by 4 h (Figure 5B). By 8 h, which corresponds to ~4 h post-entry, internalized bacteria had transitioned into elongated, pleomorphic RC and initiated replication (Figure 5C). Consistent with our observations obtained using western blot analysis and LSCM, A. phagocytophilum organisms exhibited very little anti-APH_0032 reactivity and the AVM was negative for APH_0032 at 8, 12, and 18 h (Figure 5, C–E). APH_0032 was not convincingly detected at the AVM of any inclusion through 18 h. At 24 h, individual HL-60 cells contained numerous ApVs that harbored several APH_0032-positive A. phagocytophilum organisms; and the AVM was heavily decorated by APH_0032 (Figure 5F). We have previously demonstrated that an A. phagocytophilum infection of HL-60 cells that was synchronously initiated becomes asynchronous after 24 h due to reinfection by DC organisms [22]. Figure 5G presents a representative example of an asynchronously infected HL-60 cell at 48 h that harbors numerous ApVs differing in their maturities. Immature inclusions are those that contain either an individual DC organism or those that contain few RC organisms. A mature inclusion containing several DC organisms having rough outer membranes is also present. APH_0032 heavily labels the AVM of the mature ApV, while little to no APH_0032 is detected on the AVMs of less-mature inclusions (Figure 5, G and H).
Figure 5. Assessment of A. phagocytophilum APH_0032 expression and localization to the AVM by immunoelectron microscopy.
HL-60 cells were synchronously infected with A. phagocytophilum. At 0.7 (A), 4 (B), 8 (C), 12 (D), 18 (E), 24 (F), and 48 h (G) post-bacterial addition, samples were fixed and screened with anti-APH_0032 followed by goat anti-rabbit IgG conjugated to 6nm gold particles and examined by transmission electron microscopy. Representative results of two separate experiments are shown. (A and B) Asterisks denote bound or newly internalized A. phagocytophilum DC organisms. (F to H) Arrowheads denote representative portions of the AVM that are labeled with gold particles. (H) Magnified view of the region in panel G that is demarcated by a hatched box. Scale bars, 0.5 m.
3.6. APH_0032 is expressed and localizes to the AVM during in vivo A. phagocytophilum infection and APH_0032 and APH_1387 are transcribed during A. phagocytophilum residence in tick salivary glands
APH_0032 is expressed and elicits a humoral immune response during HGA as MBP-APH_0032, but not MBP alone is recognized by antiserum from an HGA patient (Figure 6A). This is in agreement with our results obtained using another HGA patient’s antiserum (data not shown) and with a report by Storey and colleagues [19]. Seronegative normal human serum recognizes neither MBP-APH_0032 nor MBP alone (data not shown). We next investigated transcription of APH_0032 and APH_1387, which encodes the only other known bacterial AVM protein [13], during murine infection. Both transcripts are detected in total RNA recovered from GR-1-positive neutrophils isolated from A. phagocytophilum infected mice on day 8 post-infection [27] (Figure 6B). Moreover, Msp2 (P44)- and APH_0032-positive inclusions are detectable by LSCM in murine buffy coats obtained from A. phagocytophilum infected mice at 8 days post-infection (Figure 6, C–F). Consistent with our observations of A. phagocytophilum infected mammalian cell lines, multiple APH_0032-negative inclusions are also present within murine buffy coat cells (Figure 6F). Because APH_0032 (Figure 3) and APH_1387 [13] are expressed in ISE6 cells, we investigated whether APH_0032 and APH_1387 are transcribed by A. phagocytophilum during its residence in Ixodes scapularis nymphal salivary glands. RT-PCR analysis revealed that both genes are expressed in tick salivary glands (Figure 6G).
Figure 6. A. phagocytophilum APH_0032 is expressed and modifies the AVM during in vivo infection.
(A) Antiserum from an HGA patient recognizes MBP-APH_0032, but not MBP alone. (B) RT-PCR utilizing primers targeting APH_1387 (1387), APH_0032 (0032), murine hprt (loading control), and the A. phagocytophilum 16S rRNA gene (infection control) and total RNA isolated from neutrophils recovered from A. phagocytophilum infected mice on day 8 was performed in the presence (+RT) and absence (-RT) of reverse transcriptase. A. phagocytophilum DNA and water served as positive and negative controls, respectively. (C–F) Buffy coats isolated from an A. phagocytophilum-infected mouse on day 8 post-infection were fixed and viewed by LSCM for immunoreactivity with antibodies against Msp2 (P44) (D) and APH_0032 (E). DIC images are presented in panel C. A merged fluorescent image is presented in F. The arrow denotes exclusive APH_0032 staining of the AVM. (G) RT-PCR targeting APH_1387, APH_0032, msp2 (p44) (infection control), and human actin (loading control) was performed on total RNA isolated from uninfected (U) and A. phagocytophilum infected (I) Ixodes scapularis nymph salivary glands. Reactions performed in the absence of reverse transcriptase (−) served as negative controls. The results are representative of two to three separate experiments for each analysis.
3.7. A. phagocytophilum AnkA, but not APH_0032 or APH_1387 can be heterologously secreted by the L. pneumophila Dot/Icm T4SS secretion system
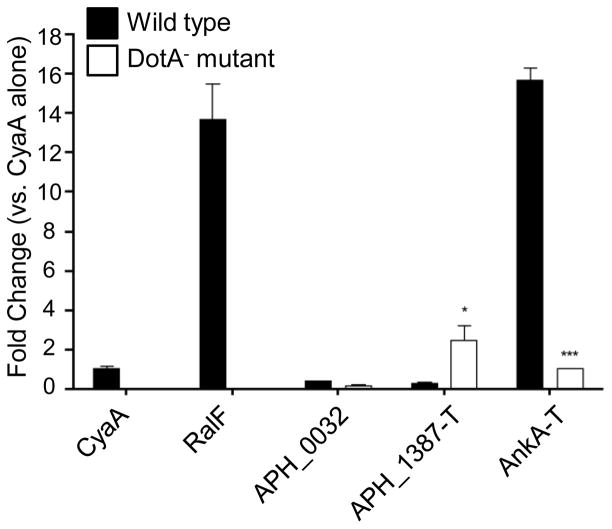
The presence of APH_0032 and APH_1387 [13] at the AVM indicates that both proteins are secreted. A. phagocytophilum encodes a VirB/D T4SS that is similar to that of Agrobacterium tumefaciens [37]. Indeed, a GFP-tagged version of the A. phagocytophilum T4SS effector, AnkA (APH0740), can be secreted by A. tumefaciens [38]. To determine whether APH_0032 or APH_1387 are T4SS substrates, each was N-terminally fused to the enzymatic reporter, Bordetella pertussis CyaA, and expressed in the surrogate host, L. pneumophila, which utilizes its Dot/Icm secretory apparatus to export T4SS substrates [39]. The ability of a surrogate host that uses the Dot/Icm system to secrete VirB/D substrates has been previously reported [40]. THP-1 cells were infected with L. pneumophila transformants expressing CyaA-fusions of APH_0032, APH_1387, A. phagocytophilum AnkA, RalF (L. pneumophila Dot/Icm effector; positive control), or CyaA alone (negative control) and changes in host cAMP levels were measured. Expression of each CyaA-tagged protein in infected THP-1 cells was confirmed by western blot analysis (data not shown). As presented in Figure 7, cAMP levels were substantially elevated relative to that in L. pneumophila expressing CyaA alone (negative control) when cells were infected with bacteria producing CyaA-RalF or CyaA-AnkA, but not CyaA-APH_0032 or CyaA-APH_1387. As anticipated, cAMP levels were low for host cells infected with transformants of a L. pneumophila DotA mutant, which is T4SS-defective.
Figure 7. A. phagocytophilum AnkA, but not APH_0032 or APH_1387 can be secreted by the L. pneumophila Dot/Icm secretion system.
PMA-stimulated THP-1 cells were infected with L. pneumophila transformants expressing CyaA-tagged RalF, APH_0032, or N-terminally truncated APH_1387 (APH_1387-T), and N-terminally truncated AnkA (AnkA-T). Black bars present the fold change in intracellular [cAMP] for THP-1 cells infected with wild-type L. pneumophila transformants expressing CyaA-fusions relative to control cells infected with transformants expressing CyaA alone. White bars correspond to the fold change in intracellular [cAMP] measured for THP-1 cells infected with T4SS-defective L. pneumophila DotA− transformants. Results are representative of three independent experiments. Statistically significant (*P<0.05; ***P<0.001) values are indicated.
4. Discussion and conclusion
We have confirmed APH_0032 as an A. phagocytophilum-derived protein that localizes to the AVM during infection of human myeloid, human microvascular endothelial, and I. scapularis embryonic cell lines and murine neutrophils. A recent whole genome transcription profiling study that demonstrated APH_0032 transcription in HL-60, HMEC-1, and ISE6 cells [41] corroborates these results. APH_0032, along with APH_1387, is transcribed in I. scapularis salivary glands. APH_0032 exhibits a high degree of nucleotide sequence conservation among at least 4 geographically diverse A. phagocytophilum strains. APH_0032 may exert a function that is unique to A. phagocytophilum, as it exhibits very little to no sequence similarity to any other known protein. APH_0032 is expressed in high abundance beginning ~20 h post-invasion and is retained on the AVM for the remainder of the infection. A plausible speculation is that it may play an important role during late-stage infection of mammalian and tick host cells, such as preparing the ApV for eventual release of infectious DC organisms, and is less likely important for preventing early host cell-mediated killing. APH_0032 can serve as a marker for distinguishing more mature ApVs that contain DC organisms and/or RC organisms that are transitioning to DC organisms (APH_0032-positive) from less mature ApVs that contain RC organisms (APH_0032-negative). It is important to note that not all AVMs are positive for APH_0032 at any given time point, which may be directly related to the maturity of the ApVs. For instance, the majority of the APH_0032-negative ApVs in Figure 3L are smaller and presumably contain fewer and less mature organisms than those ApVs that are APH_0032-positive. Likewise, the AVM that is heavily decorated by APH_0032 in Figure 5G encloses the most bacteria of any ApV in that cell. The bacteria within that strongly APH_0032-positive vacuole display ruffled outer membranes and are ~0.5 m in diameter, both characteristics of which are consistent with DC organisms and late-stage RC organisms [22]. An alternative explanation is that APH_0032 may be dispensable for A. phagocytophilum intracellular survival, perhaps because it shares a redundant function with another Anaplasma AVM protein.
APH_0032 presumably traverses the AVM by means of its predicted TMD. The region of the protein that is C-terminal to the TMD likely projects into the host cell cytoplasm since it is highly hydrophilic and lacks additional TMDs or hydrophobic stretches that are of requisite length (≥20 aa) to traverse the AVM [42]. This is in agreement with a theme among bacterial-encoded PVM proteins, the cytoplasmic domains of which serve as platforms for interacting with host cell signaling cascades and vesicular traffic.
Acidic tandem repeat proteins are emerging as key effectors and AVM proteins of Anaplasmataceae pathogens. Indeed, like APH_0032, APH_1387 is an acidic AVM protein that is expressed in mammalian and tick host cell environments [13]. Unlike APH_0032, it does not carry a traditional TMD, but does carry 3 bi-lobed hydrophobic regions analogous to those carried by chlamydial Incs that conceivably facilitate its insertion into the AVM. Also unlike APH_0032, APH_1387 is expressed throughout the course of A. phagocytophilum infection. Identified TRP E. chaffeensis proteins are P47, P120, and variable-length PCR target protein [14, 19, 43, 44]. A protein alignment of APH_0032 and P120 (ECH0039 in the annotated E. chafeensis genome) [45] revealed that a short amino acid segment that occurs in each of the full length tandem repeats of APH_0032 (Figure 1C) shares sequence similarity to a segment that occurs 4 times in the tandem repeat region of P120 [19]. P47 interacts with host molecules involved in signal transduction, transcriptional regulation, and vesicle trafficking [46]. Tandem repeat proteins of other bacterial pathogens play roles in a variety of host-pathogen interactions, including adhesion, immune evasion, and hijacking host cell signaling pathways [38, 46–52].
APH_0032 migrates predominantly as a 130-kDa band in SDS-polyacrylamide gels, which is considerably larger than its predicted MW of 66.1 kDa. Others have proposed that this phenomenon is due to APH_0032 being a glycoprotein [34]. However, we find no evidence of glycosylation of APH_0032 (data not shown). The anomalous migration of APH_0032 is likely due to its acidic nature, which conceivably prevents SDS from amply coating the protein and thereby inhibits its proper electrophoretic mobility. This phenomenon has been demonstrated for other acidic proteins in SDS-polyacrylamide gels, including E. chaffeensis P47 [53]. Indeed, Wakeel and colleagues showed that recombinant forms of the full-length and C-terminal region of P47, both of which have overall acidic pIs, but not the N-terminal region, which has a near neutral pI, exhibit slower than predicted electrophoretic mobilities upon SDS-PAGE [53].
Even though APH_0032 is detected on the surfaces of intravacuolar A. phagocytophilum organisms and is detected in great abundance at the AVM, it is not detected on bacteria that were bound at the HL-60 cell surface by indirect immunofluorescence. A similar trend was observed for APH_1387 [13]. Because these bacteria had been recovered following disruption of infected HL-60 cells, washed with PBS, and added to naïve HL-60 cells, we propose that APH_0032 and APH_1387 are not integral membrane proteins, but are instead loosely associated with the Anaplasma outer membrane while being secreted and are easily washed away during bacterial isolation. APH_0032 is more abundantly detected in an A. phagocytophilum lysate of RC and DC organisms as compared to a lysate derived from only DC organisms. RC organisms are metabolically active and therefore actively express APH_0032, while metabolically inert DC will presumably have less APH_0032 protein.
It is unlikely that APH_0032 and APH_1387 are T4SS substrates. Indeed, the C-termini of both proteins are negatively charged, which is in conflict with T4SS substrates having positively charged C-termini [54]. Also, most intravacuolar A. phagocytophilum organisms are not intimately associated with the inner face of the AVM, which goes against the fact that type IV secretion is contact-dependent [54, 55]. Lastly, the L. pneumophila Dot/Icm system failed to translocate CyaA-tagged versions of APH_0032 and APH_1387. One could argue that this is attributable to the Dot/Icm system being unable to secrete heterologous VirB/D effectors. However, Dot/Icm-mediated delivery of other VirB/D substrates has been reported [40]. Moreover, L. pneumophila could translocate CyaA-AnkA, which is the first demonstration that the Dot/Icm system can secrete a heterologous A. phagocytophilum VirB/D effector. These results warrant cautiously accepting the hypothesis that APH_0032 and APH_1387 are not T4SS effectors.
We speculate that A. phagocytophilum delivers APH_0032 and APH_1387 to the intravacuolar space via type I, or ATP-binding cassette-dependent secretion and that the substrates subsequently localize to the AVM. Type I secretion systems recognize a C-terminal uncleaved secretion signal that is rich in certain amino acids (LDAVTSIF) and poor in others (KHPMWC) [56]. 72.7% of the APH_0032 C-terminal amino acids (APSTGVEIRFMDRDSDDDVLAL) are found in type I secretion signals. 71.4% of the APH_1387 C-terminal amino acids (LVDVPTALPLKDPDDEDVLSY) are found in type I secretion signals. Type I secretory substrates are acidic (pI ~ 4) and contain very few or no cysteines [56]. Only 5 of the 619 and 4 of the 578 amino acids that compose APH_0032 and APH_1387, respectively, are cysteines. Accordingly, we propose that APH_0032 and APH_1387 are delivered to the intravacuolar space via type I secretion and subsequently localize to/integrate into the AVM.
APH_0032 is the second confirmed AVM protein. APH_1387 is the first [13]. Their differential expression patterns during the course of infection suggest that they play distinct pathobiological roles. Elucidating the host cell ligands and/or signaling pathways with which APH_0032 and APH_1387 interact and confirming the mechanism by which they are delivered to the AVM, as well as identifying additional bacterial-derived AVM proteins represent ongoing efforts in our laboratory. Achieving these goals will be critical to understanding how A. phagocytophilum facilitates its intracellular survival.
Acknowledgments
Supported by NIH grants DK065039 and AI072683 and funding from the National Research Fund for Tick-Borne Diseases.
We acknowledge Curtis Nelson for openly discussing his unpublished observations; Erol Fikrig for providing HGA patient antisera and seronegative sera; J. Stephen Dumler for providing mAb 20B4; Sukanya Narasimhan for providing RNA isolated from uninfected and A. phagocytophilum infected tick salivary glands; Ulrike Munderloh for providing A. phagocytophilum infected ISE6 cells; Howard Shuman for providing L. pneumophila JR32 and LELA3118 strains; Jere W. McBride and Richard T. Marconi for helpful discussions; and Naomi Walker, Dexter Reneer, and Aaron Wolen for technical assistance. The Virginia Commonwealth University Department of Neurobiology & Anatomy Microscopy Facility is supported, in part, by funding from NIH-NINDS Center core grant 5P30NS047463.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–95. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther. 2009;7:709–22. doi: 10.1586/eri.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Garcia JC, Barat NC, Trembley SJ, Dumler JS. Epigenetic silencing of host cell defense genes enhances intracellular survival of the rickettsial pathogen Anaplasma phagocytophilum. PLoS Pathog. 2009;5:e1000488. doi: 10.1371/journal.ppat.1000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munderloh UG, Lynch MJ, Herron MJ, Palmer AT, Kurtti TJ, Nelson RD, et al. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet Microbiol. 2004;101:53–64. doi: 10.1016/j.vetmic.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Woldehiwet Z, Horrocks BK, Scaife H, Ross G, Munderloh UG, Bown K, et al. Cultivation of an ovine strain of Ehrlichia phagocytophila in tick cell cultures. J Comp Pathol. 2002;127:142–9. doi: 10.1053/jcpa.2002.0574. [DOI] [PubMed] [Google Scholar]
- 6.Carlyon JA, Abdel-Latif D, Pypaert M, Lacy P, Fikrig E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect Immun. 2004;72:4772–83. doi: 10.1128/IAI.72.8.4772-4783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ijdo JW, Mueller AC. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect Immun. 2004;72:5392–401. doi: 10.1128/IAI.72.9.5392-5401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–78. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster P, Ijdo JW, Chicoine LM, Fikrig E. The agent of Human Granulocytic Ehrlichiosis resides in an endosomal compartment. J Clin Invest. 1998;101:1932–41. doi: 10.1172/JCI1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokce HI, Ross G, Woldehiwet Z. Inhibition of phagosome-lysosome fusion in ovine polymorphonuclear leucocytes by Ehrlichia (Cytoecetes) phagocytophila. J Comp Pathol. 1999;120:369–81. doi: 10.1053/jcpa.1998.0287. [DOI] [PubMed] [Google Scholar]
- 11.Huang B, Hubber A, McDonough JA, Roy CR, Scidmore MA, Carlyon JA. The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5:593–601. doi: 10.1016/j.chom.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B, Troese MJ, Ye S, Sims JT, Galloway NL, Borjesson D, et al. Anaplasma phagocytophilum APH1387 is expressed throughout bacterial intracellular development and localizes to the pathogen-occupied vacuolar membrane. Infect Immun. 2010 doi: 10.1128/IAI.01418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle CK, Nethery KA, Popov VL, McBride JW. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun. 2006;74:711–20. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popov VL, Yu X, Walker DH. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb Pathog. 2000;28:71–80. doi: 10.1006/mpat.1999.0327. [DOI] [PubMed] [Google Scholar]
- 16.Bannantine JP, Stamm WE, Suchland RJ, Rockey DD. Chlamydia trachomatis IncA is localized to the inclusion membrane and is recognized by antisera from infected humans and primates. Infect Immun. 1998;66:6017–21. doi: 10.1128/iai.66.12.6017-6021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockey DD, Heinzen RA, Hackstadt T. Cloning and characterization of a Chlamydia psittaci gene coding for a protein localized in the inclusion membrane of infected cells. Mol Microbiol. 1995;15:617–26. doi: 10.1111/j.1365-2958.1995.tb02371.x. [DOI] [PubMed] [Google Scholar]
- 18.Rockey DD, Rosquist JL. Protein antigens of Chlamydia psittaci present in infected cells but not detected in the infectious elementary body. Infect Immun. 1994;62:106–12. doi: 10.1128/iai.62.1.106-112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storey JR, Doros-Richert LA, Gingrich-Baker C, Munroe K, Mather TN, Coughlin RT, et al. Molecular cloning and sequencing of three granulocytic Ehrlichia genes encoding high-molecular-weight immunoreactive proteins. Infect Immun. 1998;66:1356–63. doi: 10.1128/iai.66.4.1356-1363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–90. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 21.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–32. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 22.Troese MJ, Carlyon JA. Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand 1. Infect Immun. 2009;77:4018–27. doi: 10.1128/IAI.00527-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlyon JA, Chan WT, Galan J, Roos D, Fikrig E. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J Immunol. 2002;169:7009–18. doi: 10.4049/jimmunol.169.12.7009. [DOI] [PubMed] [Google Scholar]
- 24.Scorpio DG, Caspersen K, Ogata H, Park J, Dumler JS. Restricted changes in major surface protein-2 (msp2) transcription after prolonged in vitro passage of Anaplasma phagocytophilum. BMC Microbiol. 2004;4:1. doi: 10.1186/1471-2180-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Choi KS, Dumler JS. Major surface protein 2 of Anaplasma phagocytophilum facilitates adherence to granulocytes. Infect Immun. 2003;71:4018–25. doi: 10.1128/IAI.71.7.4018-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borjesson DL, Simon SI, Hodzic E, DeCock HE, Ballantyne CM, Barthold SW. Roles of neutrophil beta 2 integrins in kinetics of bacteremia, extravasation, and tick acquisition of Anaplasma phagocytophila in mice. Blood. 2003;101:3257–64. doi: 10.1182/blood-2002-04-1019. [DOI] [PubMed] [Google Scholar]
- 27.Carlyon JA, Ryan D, Archer K, Fikrig E. Effects of Anaplasma phagocytophilum on host cell ferritin mRNA and protein levels. Infect Immun. 2005;73:7629–36. doi: 10.1128/IAI.73.11.7629-7636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 29.Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, et al. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol. 2009;191:4232–42. doi: 10.1128/JB.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voth DE, Howe D, Heinzen RA. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun. 2007;75:4263–71. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–82. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- 32.Bannantine JP, Griffiths RS, Viratyosin W, Brown WJ, Rockey DD. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell Microbiol. 2000;2:35–47. doi: 10.1046/j.1462-5822.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 33.Rockey DD, Alzhanov D. Proteins in the Chlamydial Inclusion Membrane. In: Bavoil PM, Wyrick PB, editors. Chlamydia Genomics and Pathogenesis. Norfolk, UK: Horizon Bioscience; 2006. pp. 235–54. [Google Scholar]
- 34.de la Fuente J, Garcia-Garcia JC, Barbet AF, Blouin EF, Kocan KM. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet Microbiol. 2004;98:313–22. doi: 10.1016/j.vetmic.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Goodman JL. Human Granulocytic Anaplasmosis. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Washington, D. C.: ASM Press; 2005. pp. 218–38. [Google Scholar]
- 36.Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174:6364–72. doi: 10.4049/jimmunol.174.10.6364. [DOI] [PubMed] [Google Scholar]
- 37.Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol. 2010 doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- 38.Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–57. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 39.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10:1209–20. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 40.de Jong MF, Sun YH, den Hartigh AB, van Dijl JM, Tsolis RM. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol Microbiol. 2008;70:1378–96. doi: 10.1111/j.1365-2958.2008.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson CM, Herron MJ, Felsheim RF, Schloeder BR, Grindle SM, Chavez AO, et al. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics. 2008;9:364. doi: 10.1186/1471-2164-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petsko GA, Ringe D. Protein Structure and Function. 1. London, UK: New Science Press; 2004. From sequence to structure. [Google Scholar]
- 43.Luo T, Zhang X, Wakeel A, Popov VL, McBride JW. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun. 2008;76:1572–80. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu XJ, Crocquet-Valdes P, Cullman LC, Walker DH. The recombinant 120-kilodalton protein of Ehrlichia chaffeensis, a potential diagnostic tool. J Clin Microbiol. 1996;34:2853–5. doi: 10.1128/jcm.34.11.2853-2855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu XJ, Crocquet-Valdes P, Walker DH. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene. 1997;184:149–54. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 46.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77:1734–45. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, et al. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A. 2004;101:10166–71. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Futse JE, Brayton KA, Nydam SD, Palmer GH. Generation of antigenic variants via gene conversion: Evidence for recombination fitness selection at the locus level in Anaplasma marginale. Infect Immun. 2009;77:3181–7. doi: 10.1128/IAI.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussain M, Haggar A, Peters G, Chhatwal GS, Herrmann M, Flock JI, et al. More than one tandem repeat domain of the extracellular adherence protein of Staphylococcus aureus is required for aggregation, adherence, and host cell invasion but not for leukocyte activation. Infect Immun. 2008;76:5615–23. doi: 10.1128/IAI.00480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ijdo J, Carlson AC, Kennedy EL. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol. 2007;9:1284–96. doi: 10.1111/j.1462-5822.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 51.Wistedt AC, Ringdahl U, Muller-Esterl W, Sjobring U. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol Microbiol. 1995;18:569–78. doi: 10.1111/j.1365-2958.1995.mmi_18030569.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhu B, Nethery KA, Kuriakose JA, Wakeel A, Zhang X, McBride JW. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect Immun. 2009;77:4243–55. doi: 10.1128/IAI.00376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakeel A, Zhang X, McBride JW. Mass spectrometric analysis of Ehrlichia chaffeensis tandem repeat proteins reveals evidence of phosphorylation and absence of glycosylation. PLoS One. 2010;5:e9552. doi: 10.1371/journal.pone.0009552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vergunst AC, van Lier MC, den Dulk-Ras A, Stuve TA, Ouwehand A, Hooykaas PJ. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci U S A. 2005;102:832–7. doi: 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delepelaire P. Type I secretion in gram-negative bacteria. Biochim Biophys Acta. 2004;1694:149–61. doi: 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]