Abstract
Study Objectives:
Insomnia is associated with poor health related quality of life (HRQOL) in depressed patients. Prior clinical trials of hypnotic treatment of insomnia in depressed patients have shown improvement in HRQOL, but in these studies HRQOL was relegated to a secondary outcome, and objective measures of sleep were not undertaken.
Design:
Double-blind, randomized, placebo-controlled clinical trial
Setting:
Outpatient clinic and sleep laboratory
Patients:
60 depressed, insomniac outpatients
Interventions:
one week of open-label fluoxetine (FLX), followed by 8 more weeks of FLX combined with either eszopiclone (ESZ) 3 mg or placebo at bedtime
Measurements:
The primary HRQOL measure was the daily living and role functioning subscale (DLRF) of the Basis-32. Other measures included the Q-LES-Q, self-reported sleep, PSG, actigraphy, depression severity (HRSD)
Results:
At the end of randomized treatment, patients receiving ESZ had lower (better) DLRF scores (0.81 ± 0.64) than those receiving placebo (1.2 ± 0.72), p = 0.01. The effect size for DLRF was 0.62, indicating a moderate effect. An advantage for ESZ was also seen in other measures of HRQOL, and most assessments of antidepressant efficacy and sleep. Women reported better end of treatment HRQOL scores than men.
Conclusions:
ESZ treatment of insomnia in depressed patients is associated with multiple favorable outcomes, including superior improvement in HRQOL, depression severity, and sleep.
ClinicalTrials.gov Identifier:
Citation:
McCall WV; Blocker JN; D'Agostino Jr R; Kimball J; Boggs N; Lasater B; Haskett R; Krystal A; McDonald WM; Rosenquist PB. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med 2010;6(4):322-329.
Keywords: Insomnia, quality of life, depression, eszopiclone, polysomnography, actigraphy
Major depressive episode (MDE) is associated with poorer health-related quality of life (HRQOL),1 and insomnia explains a portion of this deficit. Insomnia in MDE is associated with increasing problems with “daily living and role functioning” as measured by the Basis-32,2,3 compared with MDE without insomnia.4
BRIEF SUMMARY
Current Knowledge/Study Rationale: Hypnotic medications have been reported to be associated with both sleep-benefits and side effects. Measuring health-related quality of life measurements allows the simultaneous consideration of both the benefits and adverse events, and a summary assessment of the net effect on health. We contrasted the health care quality of life effects of the hypnotic eszopiclone in a sample of depressed insomniacs.
Study Impact: Despite the known association of hypnotics with a variety of adverse events, the administration of hypnotics to patients with combined insomnia and depression resulted in a net health benefit as reflected in superior health related quality of life.
Resolution of insomnia in MDE may be important for full restoration of HRQOL. Unfortunately, insomnia is the most common unresolved symptom when MDE has otherwise been successfully treated with the serotonin reuptake inhibitor (SSRI) fluoxetine (FLX).5 Investigations have examined adding hypnotic medications to SSRIs in the treatment of MDE complicated by insomnia, but none of these stipulated HRQOL as the a priori primary outcome, and none included objective measures of sleep.6–8
Targeting HRQOL for hypnotic clinical trials is especially relevant in so far as hypnotics are associated with adverse outcomes.9–12 HRQOL simultaneously weighs beneficial and adverse effects of treatment, allowing determination of net health benefit.
METHODS
Overview
Patients with depression and insomnia underwent a week of prospective baseline data collection, followed by one week of open-label FLX monotherapy, starting at 20 mg in the morning. Patients experiencing insomnia after one week of FLX were randomly assigned to either double-blind eszopiclone (ESZ) 3 mg or placebo at bedtime and continued with 8 more weeks of open label FLX. Patients with Hamilton Rating Scale for Depression13 (HRSD) scores > 15 at the end of 4 weeks of randomized treatment could double FLX to 40 mg for the next 4 weeks. At the conclusion of the randomized trial, participants were given appointments for care-as-usual.
Participants
Participants were 18-70 years old, reporting either (a) sleep latency > 30 min and sleep efficiency < 85% at least 4 nights per week, or (b) Research Diagnostic Criteria (RDC) insomnia criteria ≥ 4 nights per week.14 Phone screening confirmed a likely diagnosis of MDE with a Patient Health Questionnaire (PHQ9) score ≥ 10,15 body mass index (BMI) ≤ 35, absence of habitual snoring or daytime sleepiness, absence of significant restless leg symptoms,16 and absence of substance abuse or medical illnesses likely to interfere with sleep. The project was approved by the local institutional review board, and all participants provided written informed consent.
A DSM-IV diagnosis of unipolar MDE per Structured Clinical Interview for DSM-IV (SCID) was made at the first face-to-face visit.17 This visit also confirmed a Mini Mental State Exam (MMSE) score > 24,18 and a 24-item HRSD score ≥ 20.13
HRQOL
The primary outcome was role competence as described in the Basis-32, using the Daily Living and Role Functioning (DLRF) subscale.2,3 We also measured the degree of difficulty in managing relationships with the Relationship to Self and Others (RSO) subscale of the Basis-32. DLRF and RSO each have 7 items scaled from 0 to 4, with 0 indicating no difficulty and 4 indicating extreme difficulty. DLRF and RSO were chosen as the principal HRQOL measures based upon our prior reports that these measures separate depressed patients with insomnia from depressed patients without insomnia.4 The Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) rates 14 items with a total score between 20 and 100, with 20 indicating very poor satisfaction, and 100 indicating very high satisfaction.19,20 All HRQOL measures were self-rated and were assessed at baseline and at the end of randomized treatment.
Measures of Sleep
Self-Reported Sleep
The first visit was followed by prospective, daily sleep diary collection of bedtime, sleep latency (SL), number of awakenings (NAW), wake after sleep onset (WASO), total sleep time (TST), and rising time for the duration of participation. Participants completed the Insomnia Severity Index (ISI) at every visit.21 The ISI has 7 items, each scored 0-4, for a maximum of 28 points. Higher scores on the ISI represent greater degrees of insomnia. We defined a decrease of 6 points as clinically meaningful.22
Objective Measures of Sleep
Participants continuously wore an actigraph (Mini Mitter Actiwatch) on their non-dominant wrist for the duration of the study. Actigraphic data were analyzed using the medium sensitivity setting at 30-sec epochs. Weekly averages of sleep variables were computed from actigraphy software algorithms.
Participants completed one night of 8-h PSG at the end of the first week of baseline data collection and at which time they had been free of psychotropic medications > 2 weeks. The PSG was started at their median bedtime as established from the prospective diary collection. The PSG montage included standard sleep staging, respiratory, and leg movement sensors, and was scored according to standard criteria,23 blind to participant identity. Latency to persistent sleep (LPS) was defined as the time from lights out to the first epoch of 10 consecutive minutes of uninterrupted sleep. REM latency was defined as the time from LPS to the first epoch of REM sleep, minus intervening wake time. Participants with clinically significant sleep apnea (apnea-hypopnea index (AHI) ≥ 15) or clinically significant periodic limb movement (PLM) disorder (PLM-arousal index (PLMAI) ≥ 15) were excluded.24
Depression, Anxiety, and Clinical Global Illness
Depression severity was tracked by the 24-item HRSD,13 blind to treatment assignment, at every visit. There are 3 sleep items in the HRSD. Therefore the HRSD was recorded as the total score (HRSD24), and as the sum of the non-sleep items (HRSD21). Response was defined as a 50% decrease in HRSD24, while remission was defined as HRSD24 ≤ 7.
The Beck Anxiety Inventory (BAI) was measured at baseline and at the end of study participation as a possible mediator of improvement in HRQOL and depressive symptoms.25
Clinical Global Impression-Severity (CGI-S) with 1 indicating normal (no illness) to 7, indicating “among the most extremely ill patients,” was assessed by a blinded research psychiatrist at each time point during FLX and randomized treatment. Similarly, the CGI-Improvement (CGI-I) was measured at the same time points, with 1 indicating very much improved, and 7 indicating very much worse.
Side Effects and Adverse Events
Adverse events (AEs) and serious adverse events (SAEs) were solicited with open-ended questions at each visit after the initiation of the open-label FLX monotherapy. The most common side effects were assessed using the UKU scale.26
Naturalistic Follow-up
Naturalistic follow up was conducted monthly by telephone for 4 months after the end of randomized treatment, and included the PHQ-9, the ISI, the RDC-Insomnia criteria, and an assessment of the adequacy of prescribed antidepressant medication.27
Data Management and Analytic Plan
HRQOL measurement formed the a priori primary endpoints. In prior work we found that the difference between insomniac and non-insomniac depressed patients in the DLRF subscale of the Basis-32 was 0.8 ± 0.8.4 Assuming that the addition of a hypnotic medication could close this gap by 60% (i.e., 0.48), the sample size required to detect group differences with α = 0.05 and 80% power would be 32 in each group, or 64 total evaluable participants. Sample size calculations were not considered for the secondary outcomes.
Descriptive and inferential statistics were performed using SAS (Version 9.2). For all analyses, a 2-sided p-value < 0.05 was considered statistically significant. Differences in baseline characteristics between groups, and simple pre-post treatment differences were examined using 2-sample t-tests for continuous measures and χ2 or Fisher exact tests for dichotomous measures. Some outcomes were measured at multiple post-randomization time points (i.e., sleep diary measures), while others were assessed at one post-randomization time point (i.e., PSG). For the outcomes with repeated measures, a mixed models approach was used to compare treatment groups; whereas for the outcomes with only pre-post assessments, a general linear models approach was used to compare treatment groups.
We fit repeated measures mixed model analysis in which participants were treated as random effects. Fixed effects in the model included time, age, gender, baseline (pre-treatment) value of the outcome, the treatment indicator, and treatment by time interactions. Nonsignificant interactions were removed from the model and the model was re-fit. We also compared effect sizes for the primary HRQOL measures using a growth model approach.28
For certain outcomes measured at several post-randomization time points, we fit Cox proportional hazards regression models to compare time until a particular event occurred (i.e., time to a 6-point drop on ISI score), with age and gender as covariates.
For measures that only had one post-randomization assessment we used analysis of covariance (ANCOVA) techniques to compare groups where the terms in these models included age, gender, baseline (pre-treatment) outcome, and the treatment indicator. If the outcome variable was non-normal and an appropriate transformation was not identified, the ANCOVA model was re-fit using a nonparametric approach that included the ranks of the outcome rather than the actual outcome values in the model.
The naturalistic follow-up data included missing data that appeared to be missing at random, so a mixed linear models approach was used to compare groups. We used SAS Proc Mixed for continuous outcomes and SAS Proc GLIMMIX for categorical outcomes, with fixed effects of time, age, gender, and PHQ-9, treatment group, and a time-by-treatment interaction.
RESULTS
Participant Disposition, Retention, and Treatment Adherence
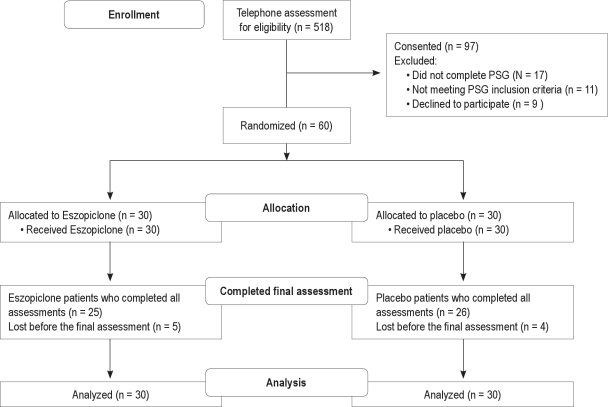
Sixty participants were randomized after successful completion of clinical and PSG screening, and 51 of these completed the study (Figure 1) No participants had resolution of their insomnia after one week of open label FLX; therefore all completing the FLX run-in week were eligible for randomization.
Figure 1.
CONSORT flow diagram
Randomized patients remained in the protocol an average of 62 days out of an expected 70 days; those who completed the protocol remained in protocol an average of 97% of the scheduled days of participation, with no differences between groups in the number of days in the protocol, or the number of research pills taken. FLX doses were doubled for 58% of participants after 4 weeks of randomized treatment, with no difference in the rate of doubling between the ESZ and placebo groups.
Participant Demographics, Baseline Symptoms and Baseline HRQOL
The overall randomized sample was middle aged, but with an unexpected imbalance in the age of the treatment groups, with ESZ patients older than placebo patients (Table 1). Women constituted two-thirds of each group, with 23% minorities and no differences between groups. The baseline severity of insomnia, as reflected in the ISI, was in the moderate range, and depression severity was in the moderate to severe range, as reflected both by the PHQ-9 and HRSD (Table 1).
Table 1.
Baseline demographics and clinical characteristics by treatment group (N = 60)
| N (col %) or Mean [SD] |
||||
|---|---|---|---|---|
| Characteristic | Total sample | Drug | Placebo | p-value* |
| Gender | 1.000 | |||
| Male | 20 (33.3) | 10 (33.3) | 10 (33.3) | |
| Female | 40 (66.7) | 20 (66.7) | 20 (66.7) | |
| Age | 41.5 [12.5] | 44.9 [11.7] | 38.0 [12.5] | 0.031 |
| Race | 0.660 | |||
| Caucasian | 46 (76.7) | 22 (73.3) | 24 (80) | |
| African American | 12 (20) | 6 (20) | 6 (20) | |
| Other | 2 (3.3) | 2 (6.7) | 0 (0) | |
| Marital Status | 0.609 | |||
| Married/ Living with Someone | 23 (38.3) | 13 (43.3) | 10 (33.3) | |
| Separated/Divorced/Widowed | 22 (36.7) | 11 (36.7) | 11 (36.7) | |
| Never Married | 15 (25) | 6 (20) | 9 (30) | |
| Years of Education | 0.104 | |||
| < High School | 5 (8.3) | 1 (3.3) | 4 (13.3) | |
| High School Diploma/GED | 22 (36.7) | 14 (46.7) | 8 (26.7) | |
| Associate Degree | 9 (15) | 4 (13.3) | 5 (16.7) | |
| Bachelor’s Degree | 16 (26.7) | 5 (16.7) | 11 (36.7) | |
| Master’s/ Doctorate Degree | 8 (13.3) | 6 (20) | 2 (6.7) | |
| Body mass index (BMI) | 27.8 [4.9] | 27.0 [4.8] | 28.6 [5.2] | 0.237 |
| Mini Mental State Exam (MMSE) | 29.4 [0.9] | 29.4 [0.8] | 29.5 [1.0] | 0.779 |
| Age at first lifetime major depressive episode | 29.9 [13.1] | 32.3 [12.8] | 27.6 [13.2] | 0.167 |
| Number of prior lifetime major depressive episodes | 0.673 | |||
| 0 Episodes | 2 (3.3) | 0 (0) | 2 (6.7) | |
| 1 Episode | 15 (25) | 7 (23.3) | 8 (26.7) | |
| 2 Episodes | 18 (30) | 8 (26.7) | 10 (33.3) | |
| 3 Episodes | 11 (18.3) | 6 (20) | 5 (16.7) | |
| 4 Episodes | 7 (11.7) | 4 (13.3) | 3 (10) | |
| 5 Episodes | 7 (11.7) | 5 (16.7) | 2 (6.7) | |
| Duration of present episode of major depression (in weeks) | 169.6 [310.1] | 185.5 [349.4] | 153.8 [270.3] | 0.695 |
| Duration of insomnia complaint (in weeks) | 165.5 [322.7] | 207.2 [400.2] | 123.8 [219.2] | 0.322 |
| SCID: MDE Specifier | 0.707 | |||
| None | 17 (28.3) | 8 (26.7) | 9 (30) | |
| Melancholic | 39 (65) | 19 (63.3) | 20 (66.7) | |
| Atypical | 4 (6.7) | 3 (10) | 1 (3.3) | |
| Psychiatric DOS | ||||
| Dysthymic Disorder (No) | 60 (100) | 30 (100) | 30 (100) | --- |
| Presence of any lifetime dependence or abuse (yes) | 22 (36.7) | 11 (36.7) | 11 (36.7) | 1.000 |
| Presence of any anxiety disorder (yes) | 32 (53.3) | 13 (43.3) | 19 (63.3) | 0.120 |
| Index | Total sample mean [SD] | Drug (N = 30) mean [SD] | Placebo (N = 30) mean [SD] | p-value* |
| 24-item Hamilton Rating Scale for Depression | 27.1 [3.9] | 27.3 [3.3] | 26.9 [4.5] | 0.744 |
| 21-item HRSD (no insomnia items) | 22.4 [3.8] | 22.6 [3.3] | 22.3 [4.3] | 0.762 |
| Sum of HRSD insomnia items | 4.7 [1.2] | 4.7 [1.1] | 4.7 [1.2] | 0.912 |
| PHQ-9 Total Score | 16.6 [4.3] | 16.7 [4.3] | 16.5 [4.4] | 0.860 |
| Beck Anxiety Index | 16.0 [8.5] | 15.1 [7.5] | 6.8 [9.4] | 0.442 |
| ISI Total Score | 20.7 [4.0] | 21.1 [4.0] | 20.2 [4.1] | 0.429 |
| Basis 32 Daily Living and Role Function (DLRF) Visit 2 | 2.0 [0.7] | 1.9 [0.7] | 2.0 [0.7] | 0.628 |
| Basis 32 Relationship to Self and Others (RSO) Visit 2 | 1.9 [0.8] | 1.8 [0.7] | 2.0 [0.8] | 0.582 |
| Q-LES-Q Total Score | 38.7 [6.9] | 38.8 [7.2] | 38.6 [6.7] | 0.897 |
p-value from Fisher exact test for categorical variables and t-test for continuous variables. Tests compared drug and placebo participant groups.
The average impairments at baseline in the domains of DLRF and RSO were in the “moderate difficulty” range, with no significant group differences (Table 1). Similarly, baseline Q-LES-Q scores were in the “poor” range, with no significant group differences.
Primary Hypothesis: End-of-treatment HRQOL
There were no significant interaction terms in any of the HRQOL models. Final DLRF scores were lower (better) in the ESZ group (0.81 ± 0.64) than the placebo group (1.2 ± 0.72), and were lower in women (0.85 ± 0.72) than in men (1.33 ± 0.57). A model for end-of-treatment DLRF, controlling for age, gender and baseline DLRF, showed that treatment assignment (p = 0.01) and gender (p < 0.01) made significant contributions. The effect size for DLRF was 0.62, indicating a moderate effect. The presence or absence of a prior history of substance abuse was not related to DLRF.
Final RSO scores were lower (better) in the ESZ group (0.74 ± 0.71) than in the placebo group (1.04 ± 0.77), and were lower in women (0.73 ± 0.69) than in men (1.2 ± 0.78). A model for end-of-treatment RSO, controlling for age, gender and baseline RSO, showed that treatment assignment (p < 0.05) and gender (p < 0.05) made significant contributions. The effect size for RSO was 0.44, indicating a moderate effect.
Q-LES-Q scores were higher (better) in the ESZ group (50.2 ± 8.11) than in the placebo group (46.9 ± 9.0), and were also higher in women (50.7 ± 8.5) than in men (44.6 ± 7.5). A model for end-of-treatment Q-LES-Q, controlling for age, gender, and baseline Q-LES-Q, showed that treatment assignment made a near significant contribution (p = 0.08), and gender (p < 0.01) made a significant contribution. The effect size for Q-LES-Q was 0.38, indicating a small effect.
Secondary Endpoint: Measures of Sleep
Self-Reported Sleep
There was no time-by-group interaction for any of the sleep diary variables. There were significant main effects across the randomized period for sleep latency, number of awakenings, and total sleep time, all favoring the ESZ group (Table 2).
Table 2.
Repeated measures mixed modeling comparing the eszopiclone group to placebo group—main effects
| Sleep Diary V2 - V8 | β* | SE | t | p-value |
|---|---|---|---|---|
| Latency | −19.64 | 5.71 | −3.44 | 0.0008 |
| Awakenings | −0.81 | 0.20 | −4.00 | 0.0001 |
| Total Awake Time WASO | −11.81 | 8.41 | −1.41 | 0.1623 |
| Total Sleep Time | 28.13 | 13.63 | 2.06 | 0.0411 |
| Number of Naps | −0.02 | 0.05 | −0.38 | 0.7009 |
| Nap Time | −0.64 | 4.62 | −0.14 | 0.8897 |
| Actigraphy V2 - V8 | ||||
| Number of awakenings | −0.62 | 1.56 | −0.40 | 0.6928 |
| Sleep efficiency | 2.15 | 1.44 | 1.50 | 0.1370 |
| Wake time after sleep onset | 4.00 | 3.56 | 1.12 | 0.2639 |
| Total sleep time | 8.98 | 10.45 | 0.86 | 0.3919 |
| SI V1 - V8 | ||||
| ISI Total Score | −4.41 | 1.43 | −3.09 | 0.0024 |
| ISI Improvement (6-point drop) V3-V8 (Y/N) | 7.21† | (1.51,34.4)† | 2.50 | 0.0136 |
| HRSD V1 - V8 | ||||
| 24-Item Hamilton | −3.50 | 1.46 | −2.40 | 0.0177 |
| 21-Question Hamilton | −2.20 | 1.36 | −1.62 | 0.1064 |
| CGI | ||||
| CGI Severity V1-V8 | −0.52 | 0.22 | −2.36 | 0.0194 |
| CGI Improvement V3-V8 | −0.46 | 0.20 | −2.30 | 0.0229 |
V refers to Visit;
Models adjusted for treatment, time, baseline values, age, gender;
Adjusted odds ratio and 95% confidence interval.
There was no time-by-group interaction for the ISI score. There was a main effect across the randomized period for the ISI total score, favoring the ESZ group (Table 2). The likelihood of experiencing an ISI drop ≥ 6 points across the randomized period was significantly greater in the ESZ group than the placebo group (AOR = 7.2; 95% CI: 1.51-34.4; p = 0.01) (Table 2). Cox proportional hazards regression models comparing the adjusted time to a 6-point drop between groups also showed an advantage for ESZ (hazard ratio = 2.3, χ2 = 5.8, p < 0.05).
Objective Measures of Sleep
Fifty-nine of the 60 randomized persons agreed to wear an actigraph, for a grand total of 3631 nights, and usable actigraphic data was obtained for 82% of nights. There was a significant time-by-group interaction (p < 0.05) for actigraphic sleep latency and for treatment groups across the randomized period (p < 0.01), showing that while sleep latency initially showed superior improvement during the first week of ESZ, this advantage regressed back to the placebo group thereafter. There were no other time-by-group interactions for any other actigraphy variables, and no significant main effects for treatment group (Table 2).
All study completers had a PSG at baseline and at the end of randomized treatment (Table 3). Pre-post PSG differences failed to reveal an advantage for ESZ for either latency to the first epoch of sleep or LPS. However, WASO was lower in the ESZ group at the end of treatment, as compared with placebo. Sleep efficiency and TST were higher at the end of randomized treatment in the ESZ group as compared with the placebo group. REM latency approximately doubled in both groups between baseline and the end of treatment, consistent with the known effects of FLX on REM sleep,29 but there were no differences in REM latency between groups.
Table 3.
Descriptive statistics of polysomnography at baseline and end of randomized treatment and regression analysis of polysomnography comparing treatment groups at end of randomized treatment
| PSG Continuous Measures | Time | Eszopiclone N=30 | Placebo N=30 | p value1,2 |
|---|---|---|---|---|
| Latency to 1st Epoch of Sleep (min) | Pre | 10.8 [24.5] | 17.3 [24.0] | |
| Post1 | 17.0 [14.5] | 17.0 [22.0] | 0.535 | |
| Latency to Persistent Sleep (min) | Pre | 20.5 [35.5] | 31.5 [27.0] | |
| Post1 | 20.0 [21.5] | 22.0 [23.5] | 0.471 | |
| Wakefulness after Sleep Onset (min) | Pre | 73.09 (8.8) | 44.47 (8.8) | |
| Post2 | 30.56 (5.5) | 59.18 (5.3) | 0.001 | |
| Total Sleep Time (min) | Pre | 388.5 [108.0] | 411.5 [61.0] | |
| Post1 | 426.5 [52.5] | 396.5 [65.5] | 0.007 | |
| Sleep Efficiency (%) | Pre | 80.8 [22.5] | 85.7 [12.8] | |
| Post1 | 88.9 [11.0] | 82.6 [13.6] | 0.006 | |
| REM Latency (min) | Pre | 83.7 (9.0) | 80.1 (8.9) | |
| Post2 | 154.4 (18.2) | 187.6 (17.6) | 0.186 | |
| Stage 1 Minutes | Pre | 32.4 (6.5) | 40.0 (6.4) | |
| Post2 | 50.1 (7.1) | 42.9 (7.0) | 0.464 | |
| Stage 1 Percent of Total Sleep Time | Pre | 6.6 [7.0] | 7.3 [10.2] | |
| Post1 | 8.6 [14.9] | 10.5 [9.0] | 0.807 | |
| Stage 2 Minutes | Pre | 32.4 (6.5) | 40.0 (6.4) | |
| Post2 | 50.1 (7.1) | 42.9 (7.0) | 0.464 | |
| Stage 2 Percent of Total Sleep Time | Pre | 52.1 (2.6) | 50.6 (2.5) | |
| Post2 | 55.1 (3.4) | 54.3 (3.3) | 0.866 | |
| Slow Wave Sleep Minutes | Pre | 69.1 (5.9) | 62.7 (5.9) | |
| Post2 | 61.2 (6.8) | 63.3 (6.6) | 0.818 | |
| Slow Wave Sleep Percent of Total Sleep Time | Pre | 18.0 (1.5) | 15.6 (1.5) | |
| Post2 | 14.4 (1.6) | 16.6 (1.6) | 0.327 | |
| REM Minutes | Pre | 71.2 (6.5) | 87.9 (6.4) | |
| Post2 | 69.0 (7.2) | 59.8 (7.1) | 0.358 | |
| REM Percent of Total Sleep Time | Pre | 18.7 (1.5) | 21.7 (1.5) | |
| Post2 | 16.3 (1.7) | 15.0 (1.6) | 0.561 | |
| PSG Dichotomized Measures | Time & Cut Point | Eszopiclone N (%) | Placebo N (%) | p value3 |
| PLM Index (rate #/hr) | Pre 0–5 | 23 (76.7) | 23 (76.7) | |
| Pre > 5 | 7 (23.3) | 7 (23.3) | ||
| Post 0–5 | 11 (47.8) | 11 (47.8) | 0.663 | |
| Post > 5 | 12 (52.2) | 12 (52.2) | ||
| PLM Arousal Index (rate #/hr) | Pre 0–5 | 26 (86.7) | 27 (90.0) | |
| Pre > 5 | 4 (13.3) | 3 (10.0) | ||
| Post 0–5 | 18 (78.3) | 17 (73.9) | 0.807 | |
| Post > 5 | 5 (21.7) | 6 (26.1) | ||
| Apnea/hypopnea index (rate #/hr) | Pre 0–5 | 26 (86.7) | 27 (90.0) | |
| Pre > 5 | 4 (13.3) | 3 (10.0) | ||
| Post 0–5 | 19 (82.6) | 18 (78.3) | 0.790 | |
| Post > 5 | 4 (17.4) | 5 (21.7) | ||
| Number of desaturations (count) | Pre 0–5 | 26 (86.7) | 30 (100) | |
| Pre > 5 | 4 (13.3) | 0 (0) | ||
| Post 0–5 | 20 (87.0) | 18 (78.3) | 0.171 | |
| Post > 5 | 3 (13.0) | 5 (21.7) | ||
| Lowest saturation (%) | Pre 0–90 | 27 (90.0) | 26 (86.7) | |
| Pre > 90 | 3 (10.0) | 4 (13.3) | ||
| Post 0–90 | 21 (91.3) | 22 (95.7) | 0.267 | |
| Post > 90 | 2 (8.7) | 1 (4.3) | ||
| Number of Spontaneous Arousals (count) | Pre 0–50 | 18 (60.0) | 15 (50.0) | |
| Pre > 50 | 12 (40.0) | 15 (50.0) | ||
| Post 0–50 | 6 (26.1) | 10 (43.5) | 0.384 | |
| Post > 50 | 17 (72.9) | 13 (56.5) | ||
| Spontaneous Arousal Index (rate #/hr) | Pre 0–10 | 20 (66.7) | 19 (63.3) | |
| Pre > 10 | 10 (33.3) | 11 (36.7) | ||
| Post 0–10 | 8 (34.8) | 11 (47.8) | 0.713 | |
| Post > 10 | 15 (65.2) | 12 (52.2) |
Pre and post medians and Interquartile ranges are presented. p-values from Generalized Linear Models on the rank of the post value compare treatment groups and adjust for baseline, age and gender.
Pre and post least square means and standard errors are presented. p-values from Generalized Linear Models of the post value compare treatment groups and adjust for baseline, age and gender.
Categorized pre and post values are presented. p-values from Logistic Regression Models of the binary post data compare treatment groups and adjust for baseline, age and gender.
Numbers in ( ) = percent; numbers in [ ] = SD.
Secondary Endpoint Measure: HRSD
There were no time-by-group interactions for the HRSD scores, but there was an effect of group over the randomized period for the 24-item HRSD, favoring ESZ (Table 2). The 21-item HRSD (without the 3 insomnia items) was numerically lower in the ESZ group than the placebo group, but this was not statistically significant. The final response rate in the ESZ group (80%) was greater than that of the placebo group (38%) (χ2 = 8.4, df = 1, p < 0.01). The presence or absence of a prior history of substance abuse was unrelated to final HRSD score.
The final remission rate for the entire sample was low (25%); while it was numerically greater in the ESZ group (32%) than the placebo group (19%), this difference was not statistically significant. Adjusted Cox proportional hazards regression models comparing the time of initial response between groups also indicated an advantage for ESZ (hazard ratio = 2.6, χ2 = 6.7, p < 0.01).
Secondary Endpoint Measure: CGI
There were no time-by-group interactions for either CGI-severity or CGI-improvement. There were significant group effects for CGI-severity and CGI-improvement, favoring ESZ (Table 2). Adjusted Cox proportional hazards regression survival curves also reflected a significant advantage for ESZ for CGI severity (hazard ratio = 3.0, χ2 = 6.9, p < 0.01) and a trend favoring ESZ for CGI-improvement for time to initial response (hazard ratio = 1.7, χ2 = 2.3, p < 0.2).
Examination of Mediators: BAI and ISI
Given that ESZ acts at the benzodiazepine receptor, it seemed possible that the advantages of ESZ for HRQOL and mood might be mediated through anxiolytic effects, rather than by sleep effects. We tested this by examining a series of linear regression models testing whether (a) treatment group was a predictor of change in BAI, and (b) whether BAI was a predictor of final DLRF or HRSD score while controlling for treatment. The critical ratio of the Sobel Test was calculated as a test of whether the mediated effect of the independent variable on the dependent variable is statistically different from 0. In this case, it was not (p > 0.7). After excluding anxiolysis as a mediating variable for HRQOL or depression severity, we next examined insomnia severity as a mediating variable, using the ISI. Following a similar analytic strategy, we found near-significant evidence for ISI as a mediator of DLRF (p = 0.09) and HRSD (p = 0.09).
Exploratory Endpoints: Naturalistic Follow-Up
The decision to continue or stop hypnotic therapy at the end of randomized treatment was a clinical decision that took into account the preferences of the participant.
Forty-three (72%) of the 60 randomized patients were reached by phone for a naturalistic follow-up assessment at 1 month post-randomization, 35 (58%) at 2 months, 32 (53%) at 3 months, and 28 (47%) at 4 months. There were no significant differences in age, gender, or original treatment assignment between those patients who could and could not be reached for naturalistic phone follow-up. The reported use of hypnotics fell to a rate of 28% to 43% over follow-up. Women were more likely to still be taking a hypnotic than men (p < 0.05). For example, at the first month of naturalistic follow up, 57% of the women we reached reported using a hypnotic, while only 23% of the men we reached reported using a hypnotic. Correspondingly, over the period of naturalistic follow up, compared with men, the women we reached had lower PHQ-9 scores (p < 0.05), lower ISI scores (p < 0.05), and were less likely to meet RDC criteria for insomnia (p < 0.05). Fully two-thirds of the patients we reached met RDC criteria for insomnia by the first month of follow-up, and average ISI scores during follow-up were about 10. Original randomized treatment assignment was not related to meeting RDC criteria for insomnia, or PHQ-9 or ISI scores during the naturalistic follow-up.
Side Effects and Adverse Events
Except for 46% of the ESZ group which reported an unpleasant taste, there were no meaningful side effects or adverse events.
DISCUSSION
This is the first clinical trial of hypnotic medication to stipulate HRQOL as the a priori primary endpoint, and the first study to evaluate the impact of hypnotic treatment on objective measures of sleep in patients with MDE and the symptom of insomnia. We found a significant advantage for ESZ treatment for the primary endpoint of DLRF. Others have examined HRQOL as a secondary endpoint in hypnotic treatment of insomnia during MDE, finding an advantage for hypnotic treatment.7,8 Our finding of a better HRQOL at the end of antidepressant treatment in women was not anticipated, and to our knowledge has not been previously described in depression clinical trials,30,31 and will require replication.
There were multiple significant sleep effects favoring ESZ, but not every difference was statistically significant; this is not surprising given that (1) this investigation was not powered to detect differences for the sleep outcomes, and (2) we did not have any PSG or actigraphy severity criteria for inclusion into the study; our study was not enriched for long PSG sleep latency, WASO, etc. Our finding of PSG effects favoring ESZ for WASO, TST, and sleep efficiency are important as depressed patients have been previously shown to report improvement in sleep as depression improved, even when such reported improvement in sleep did not correspond to PSG improvement.29 Thus it is relevant that we found that patient-reported improvements in sleep were accompanied by PSG improvements.
Regarding actigraphy, the most remarkable finding was the adherence of our participants to a lengthy period of continuous actigraphic measurement—10 weeks, perhaps the longest continuous period ever employed in a clinical trial. Still, actigraphy failed to reveal any significant treatment effects.
We also found superior outcomes for ESZ treatment for overall depression symptoms as reflected in HRSD total score and percent responders. This is consistent with our earlier report of ESZ-treatment of MDE patients on open-label FLX.6
In general, the participants' elective use of hypnotic during the naturalistic follow-up period was relatively low. This may have been related to participants assuming the costs of medication during naturalistic follow-up, or encouragement from the investigators to try to discontinue hypnotic based upon the observation that benefit may persist after discontinuation.32 Women elected to continue with a hypnotic at a higher rate than men during naturalistic follow-up. This may be related to women's report of greater HRQOL benefit during randomized treatment, as compared with men's report.
This study has a number of limitations. The sample size was modest, and was powered to detect significant differences in only the primary endpoint of HRQOL. Therefore, results for all of the secondary endpoints, both those that were statistically significant as well as those which were not, should be viewed with some caution. Second, the duration of the randomized period was relatively short at 8 weeks, and the naturalistic follow-up was limited in its scope. While this study was focused on the impact of ESZ, we recognize that other hypnotics may produce a similar result.
Further, the finding of superior end-of-treatment HRQOL in those persons receiving ESZ was a group effect, and does not rule out the possibility of loss of HRQOL in some individuals who use ESZ or other hypnotics. For these reasons, the group effect of superior HRQOL should be tempered with the understanding of the specific risks of bad outcomes in prescribing hypnotics to some patients with depression and insomnia.
DISCLOSURE STATEMENT
This was not an industry support study. Dr. McCall has participated in speaking engagements for Sepracor and Sanofi; has received research support from Sealy, Sepracor, Mini Mitter, Corcept, and Sanofi; and is an advisor to Sealy. Dr. Krystal has received research support from Sanofi-Aventis, Cephalon, GlaxoSmithKline, Merck, Neurocrine, Pfizer, Sepracor, Somaxon, Takeda, Transcept, Respironics, Neurogen, Evotec, and Astellas and has consulted for Abbott, Actelion, Arena, Astellas, Axiom, Eli Lilly, GlaxoSmithKline, Jazz, Johnson and Johnson, Merck, Neurocrine, Neurogen, Neuronetics, Novartis, Organon, Ortho-McNeil-Janssen, Pfizer, Respironics, Roche, Sanofi-Aventis, Sepracor, Somaxon, Takeda, and Transcept. Dr. Rosenquist has received research support from Cyberonics, Aspect Medical Systems, Sanofi, and Astra-Zeneca. Dr. Kimball has received research support from Corcept and has participated in speaking engagements for Astra Zeneca and Pfizer. Dr. McDonald has received research support from Neuronetics, GlaxoSmithKline, and Wyeth. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Institution where work performed: Wake Forest University Health Sciences.
This study was funded by NIH MH70821 and M01-RR07122, as well as funding and medications from Sepracor, and funding and material support from Mini Mitter.
REFERENCES
- 1.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262:914–9. [PubMed] [Google Scholar]
- 2.Eisen SV, Dill DL, Grob MC. Reliability and validity of a brief patient-report instrument for psychiatric outcome evaluation. Hosp Community Psychiatry. 1994;45:242–7. doi: 10.1176/ps.45.3.242. [DOI] [PubMed] [Google Scholar]
- 3.Eisen SV. Assessment of subjective distress by patients' self-report versus structured interview. Psychol Rep. 1995;76:35–9. doi: 10.2466/pr0.1995.76.1.35. [DOI] [PubMed] [Google Scholar]
- 4.McCall WV, Reboussin BA, Cohen W. Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res. 2000;9:43–48. doi: 10.1046/j.1365-2869.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- 5.Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60:221–5. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 6.Fava M, McCall V, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999;60:668–76. doi: 10.4088/jcp.v60n1005. [DOI] [PubMed] [Google Scholar]
- 8.Ji J, Liu WJ, Chen ZQ, et al. Effects of paroxetine with or without zolpidem on depression with insomnia: a multi-center randomized comparative study. Zhonghua Yi Xue Za Zhi. 2007;87:1585–9. [PubMed] [Google Scholar]
- 9.Herings R, Stricker B, de Boer A, et al. Benzodiazepines and the risk of falling leading to femur fractures. Arch Intern Med. 1995;155:1801–7. [PubMed] [Google Scholar]
- 10.Dealberto MJ, Mcavay GJ, Seeman T, et al. Psychotropic drug use and cognitive decline among older men and women. Int J Geriatr Psychiatry. 1997;12:567–74. [PubMed] [Google Scholar]
- 11.Hemmelgarn B, Suissa S, Huang A, et al. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278:27–31. [PubMed] [Google Scholar]
- 12.Mallon L, Broman J, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10:279–86. doi: 10.1016/j.sleep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen R, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993;29:321–6. [PubMed] [Google Scholar]
- 20.Ritsner M, Kurs R, Kostizky H, et al. Subjective quality of life in severely mentally ill patients: a comparison of two instruments. Qual Life Res. 2002;11:553–61. doi: 10.1023/a:1016323009671. [DOI] [PubMed] [Google Scholar]
- 21.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Morin C, Schaefer K, et al. Defining the minimally important difference for the Insomnia Severity Index. Sleep. 2008;31(supplement):A342. [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson A, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.McCall WV, Kimball J, Boggs N, et al. Prevalence and prediction of primary sleep disorders in a clinical trial of depressed patients with insomnia. J Clin Sleep Med. 2009;5:454–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Roemer L. Measures for anxiety and related constructs. practitioner's guide to empirically based measures of anxiety. Dordrecht, Netherlands: Kluwer Academic Publishers; 2001. pp. 49–83. [Google Scholar]
- 26.Lingjaerde O, Ahlfors U, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 27.Prudic J, Haskett RF, Mulsant B, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153:985–92. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- 28.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as classical analysis. Psychol Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillin JC, Rapaport M, Erman MK, et al. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry. 1997;58:185–92. doi: 10.4088/jcp.v58n0502. [DOI] [PubMed] [Google Scholar]
- 30.Kornstein S, Schatzberg A, Thase ME, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 31.Watson H, Nathan P. Role of gender in depressive disorder outcome for individual and group cognitive-behavioral treatment. J Clin Psychol. 2008;64:1323–37. doi: 10.1002/jclp.20524. [DOI] [PubMed] [Google Scholar]
- 32.Krystal A, Fava M, Rubens R, et al. Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J Clin Sleep Med. 2007;3:48–55. [PubMed] [Google Scholar]