Abstract
Rationale:
Obstructive sleep apnea (OSA) is a common but underdiagnosed disorder. There is a need for validated simpler modalities such as single-channel monitors to assist diagnosis of OSA.
Study Objectives:
To assess data sufficiency, agreement, and diagnostic accuracy of nasal airflow measured by a single-channel pressure transducer device (Flow Wizard, DiagnoseIT, Sydney, Australia) compared to attended full polysomnography (PSG) on the same night for OSA diagnosis.
Design:
Cross-sectional study
Setting:
Laboratory
Participants:
Subjects with possible OSA referred to the sleep laboratory for PSG were eligible.
Methods:
Nasal airflow was measured by a pressure transducer in the laboratory concurrently with PSG.
Results:
Of 226 eligible subjects who consented, 221 (97.8%; 151 males, 70 females) completed the protocol. With nasal airflow measurement, 5.3% of subjects had insufficient data, compared with 2.2% on PSG. The mean difference between PSG AHI and NF RDI was −6.2 events/h with limits of agreement (± 2 standard deviation [SD]) of 17.0 events/hr. The accuracy of the Flow Wizard for diagnosing severe OSA (PSG AHI > 30) was very good (area under the ROC curve [AUC] 0.96; 95% confidence interval [CI] 0.92 to 0.99) and for diagnosing OSA (PSG AHI > 5) was good (AUC, 0.84; 95% CI, 0.77 to 0.90). There was no difference in the rate of data insufficiency and accuracy between males and females.
Conclusion:
Nasal flow measured by a nasal pressure transducer has a low rate of data insufficiency, good agreement, and high accuracy compared to PSG for diagnosing OSA in the monitored sleep laboratory setting.
Citation:
Makarie Rofail L; Wong KKH; Unger G; Marks GB; Grunstein RR. The role of single-channel nasal airflow pressure transducer in the diagnosis of OSA in the sleep laboratory. J Clin Sleep Med 2010;6(4):349-356.
Keywords: single-channel device, pressure transducer, obstructive sleep apnea, diagnosis, women
Obstructive sleep apnea (OSA) is common but underdiagnosed disorder.1 Until recently, polysomnography has been regarded as a standard diagnostic investigation for OSA, but the combination of the high prevalence of the condition and limited health system resources has prompted the development of simpler methods of disease detection and diagnosis. However there is controversy regarding the best diagnostic approaches in different populations.
Recent professional guidelines on OSA diagnosis have classified non-EEG based diagnostic methods as either multi-channel (Type 3) or single- or dual-channel (Type 4) based recording, but neither is considered to be a definitive method of diagnosis in any population.2,3 Single-channel Type 4 devices usually measure either oxygen saturation or nasal airflow.
Nasal airflow in such devices is measured using a thermistor and/or a nasal pressure transducer. Thermistors deduce information about airflow by detecting changes in temperature. However they have low sensitivity at detecting hypopneas.4 Portable oral-nasal thermistors and thermal sensors have been developed as screening tools for OSA,5–9 but home based studies have reached diverse conclusions about the utility of these devices.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Polysomnography has been regarded as a standard diagnostic investigation for OSA, but the combination of the high prevalence of the condition and limited health system resources has prompted the development of simpler methods of disease detection and diagnosis. There is a need for validated modalities that are simple to use, cost effective and easily accessible such as single-channel nasal pressure transducers to assist diagnosis of OSA.
Study Impact: This study shows that nasal flow measured by nasal pressure transducer is accurate and has good agreement with in-laboratory PSG in both males and females, with low data loss and insufficiency rates comparable to PSG. This modality can be used to both rule in and rule out OSA in those with suspected severe disease and is thus a viable alternative to laboratory based PSG for OSA screening in carefully selected subjects.
In contrast to thermistors, nasal pressure transducers are more sensitive than thermistors in detecting hypopneas.10 There is excellent agreement between nasal pressure transducers and the reference standard pneumotachography for detection of apneas and hypopneas.11 Single-channel nasal pressure transducer devices are available, but only a few studies have examined their role in OSA diagnosis.8,12–14
Gender differences in the prevalence, pathophysiology, and clinical presentation of sleep disordered breathing have been reported in the literature.15–17 Population-based studies have shown that OSA is more common in men than in women.18 Women tend to have lower apnea hypopnea indexes than men, despite being more symptomatic, and there is a higher prevalence of REM related obstructive events in women.19 Thus gender differences in diagnostic test performance may exist. There are no data on sex differences in the use of these devices.
The Flow Wizard (DiagnoseIT, Sydney, New South Wales, Australia) is a newly developed single-channel nasal pressure transducer device that is battery operated and measures nasal airflow in patients during sleep. While preliminary results using this device to diagnose OSA have been promising,13 there is a need for an adequately powered prospective study to assess the accuracy of the device with reference to concurrently performed, in-laboratory PSG.
Our aims were to assess data adequacy and agreement (bias) between nasal flow measured simultaneously by the Flow Wizard pressure transducer and full laboratory PSG with pressure transducer recording of airflow; to assess the potential role of nasal flow along with 3 questionnaire scales in prioritizing patients presenting to the sleep clinic or sleep laboratory for further assessment or treatment; to examine the effect of gender on data quality, agreement with reference standard, and accuracy of nasal flow in diagnosing OSA; and to develop a diagnostic algorithm that can aid OSA diagnosis in clinic patients.
MATERIALS AND METHODS
A cross-sectional prospective study was conducted to evaluate the test characteristics of a single-channel nasal pressure transducer for the diagnosis of OSA in the laboratory setting. All subjects were administered self-reported questionnaires. In-laboratory polysomnography, the reference standard for OSA diagnosis, was performed concurrently with single-channel nasal pressure measurements. The nasal cannulae (Pro-Flow adult nasal cannula, Pro-Tech, WA, USA) were connected to both the single-channel nasal flow monitor (Flow Wizard, DiagnoseIT, Sydney, Australia) and the PSG system pressure transducer with a T connector (Figure 1). A detailed history, examination, and demographic data were collected on all subjects at baseline. Height (to the nearest 0.5 cm) and weight (to the nearest 0.5 kg) were measured, and the body mass index (BMI) was calculated as kg/m2.
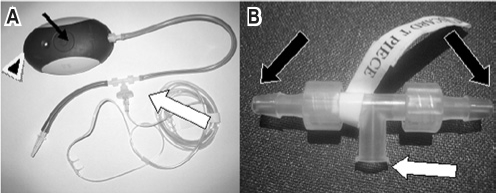
Figure 1.
The Flow Wizard Nasal Airflow Pressure Transducer in laboratory set up
The T piece is shown in detail (B), the central port is attached to nasal cannulae in (A) (large white arrow) and each arm is attached to a pressure transducer either to the Flow Wizard or to the PSG pressure transducer as shown in (B) (large black arrows). The slim black arrow in (A) shows the tactile button and the arrow head in (A) points to the USB Port.
The study was approved by the Human Research Ethics Committee of the Sydney South West Area Health Service (study number X05-0105) and was registered with the Australian Clinical Trials Registry (ACTRN012605000120673). Informed consent was obtained from all subjects.
Subjects
We recruited consecutive subjects presenting to the sleep laboratory for nocturnal in-laboratory full PSG for evaluation of possible OSA from November 2006 to May 2007. Exclusion criteria were: complex unstable medical conditions, such as severe congestive heart failure; severe chronic respiratory disease; dependence on supplemental home oxygen; neuromuscular disorder; unstable psychiatric illness and/or history of current or previous drug and alcohol dependence (including those in drug and alcohol rehabilitation); known history of other sleep disorders; inability to understand the patient information sheets; and enrollment in other clinical research studies.
Questionnaires
The Epworth Sleepiness Scale (ESS),20 the multivariate apnea index (MAP),21 and the Berlin Questionnaire22 were administered. The MAP score was calculated according to the published equation combining BMI, age, and gender, as well as the average of non missing values for the frequency of 3 self-reported symptoms (snoring, apneas, and snorting/gasping). The MAP was classified as indeterminate if all 3 symptom frequency questions were marked as “Don't Know.” The Berlin Questionnaire22 is a self-report instrument developed mainly for use in primary care and is focused on a set of known symptoms and clinical features associated with OSA. It consists of 3 main categories. Category 1 examines snoring and witnessed apneas, category 2 examines daytime sleepiness, and category 3 examines blood pressure and BMI. It classifies subjects as having high or low risk of OSA according to the score obtained for each category.
In-Laboratory PSG
Computerized attended full PSG recordings were performed (Alice 5, Respironics, Murrysville PA, USA) and included electroencephalography (EEG) (C2-A1, C3-A2, O1-A2, O2-A1); electro-oculography (EOG), and submental and tibialis anterior electromyography (EMG) for sleep staging according to Rechtschaffen and Kales criteria.23 Also, thoracic and abdominal piezoelectric respiratory movement sensors, oxygen saturation, nasal pressure via cannulae, body position, snoring, and electrocardiogram were monitored. A PSG apnea-hypopnea index (AHI) ≥ 5 classified subjects as having OSA, and PSG AHI ≥ 30 as having severe OSA according to the American Academy of Sleep Medicine Task Force diagnostic criteria.10 Apneas were defined as complete cessation of airflow, and hypopneas were defined as flow reduction > 50% associated with either a 3% desaturation or an arousal. The PSG recordings were scored independently by trained sleep technicians blinded to the portable monitor results. The technicians were instructed to perform in-laboratory PSG as usual, including applying nasal airflow cannula, calibration, checking of airflow signal on PSG, and to change the gain on the PSG flow trace to get an acceptable signal. No restriction on changing gain on the PSG was given.
Nasal Flow Monitor
The Flow Wizard (FW) records nasal pressure via a set of nasal prongs (Figure 1). It is designed to sit on a bedside table or on the floor beside the bed while a patient is sleeping. The recorder has a Luer lock at one end where a standard nasal oxygen cannula is attached, and a USB port at the other end for device setup and data transfer procedures. A single tactile button is located on the center top of the device to enable users to initiate recordings with depression of the button for a 5-sec period. Once a recording is initiated (and the LED is constantly illuminated), FW recorder will acquire the signal (at 25 Hz) for a period of 9 h, then cease recording. Patients are not required to terminate the recording but can do so if this occurs within the 9-h period. Up to three 9-h recordings can be stored in the recorder before data transfer procedures are required.
The processed signal is stored in the flash memory unit of the device and loaded on to the nominated computer for analysis and interpretation via device-specific proprietary software. The nasal airflow signal can be visualized using the software. Nasal flow respiratory disturbance index (NF RDI) calculations were based on artifact-free flow recording in bed between lights-off and lights-on. Respiratory disturbances included apneas, defined as a decrease in the amplitude of the airflow signal ≥ 90% for ≥ 10 sec; and hypopneas, defined as reduction in the amplitude of the respiratory signal ≥ 50% for ≥ 10 sec. The recordings were automatically scored using the device software without manual editing. Although laboratory technicians applied the nasal airflow cannulae, they were not able to visualize if the amplitude of the nasal airflow trace on the Flow wizard (which has fixed signal gain) was sufficient. Further, the technicians did not have access to the analysis software.
Data Quality
The data from the reference standard (in-lab PSG) were regarded as sufficient if ≥ 3 h of total sleep time was obtained. Nasal airflow data was regarded as sufficient and included in the analysis if ≥ 3 h of good quality recording was obtained.
Statistical Methods
All analyses were carried out using SPSS, Version 14 software (Chicago, IL, USA). Values are expressed as mean (standard deviation, SD) and median (interquartile range, IQ) according to their distribution. The statistical level of significance was set as p < 0.05. The mean bias and limits of agreement between the PSG and nasal flow were calculated.24 Areas under receiver operator characteristics (ROC) curves (AUC) were constructed for nasal flow and MAP with respect to PSG AHI ≥ 5 and ≥ 30 events per h.25 Sensitivity (Sn), specificity (Sp), positive (LR+), and negative (LR−) likelihood ratios, as well as the post-test probability of having OSA were calculated. Stepwise logistic regression analysis was employed to derive a predictive algorithm for PSG-confirmed OSA using PSG AHI ≥ 5 as a binary variable, and with anthropometric data, questionnaire data, and nasal flow as predictive continuous variables.
RESULTS
A total of 431 subjects were referred to the sleep unit for in-laboratory PSG, of whom 227 were eligible, 226 consented, 221 (97.8%) completed the protocol, and 200 (88.5%) had evaluable data for both modalities (PSG and nasal flow). There were 190 (84.1%) with evaluable PSG and nasal flow who also had evaluable data on the MAP score. Most of the subjects were Caucasian (80%) with the following symptoms: snoring (91.6%), witnessed apneas (54.9%), excessive sleepiness (49%), and a history of hypertension (42%). For the subjects who did not complete the protocol, the mean (SD) age was 49.9 (14.8) years, BMI was 30.0 (5.8) kg/m2, ESS was 9.4 (4.9), and 66% were male. There were no differences in demographic data between the subjects who completed the protocol and those who did not. Table 1 shows the subject characteristics of those that completed the protocol.
Table 1.
Subject characteristics
| All (n = 200) Mean (SD) | Males (n = 136) Mean (SD) | Females (n = 64) Mean (SD) | Mean Difference (95% CI) | |
|---|---|---|---|---|
| Age (y) | 49.4 (14.5) | 49.3 (14.5) | 49.8 (14.7) | −0.5 (−4.9 to 3.8) |
| BMI (kg/m2) | 30.5 (6.7) | 30.5 (6.2) | 30.4 (7.8) | 0.08 (−1.9 to 2.1) |
| TST (h) | 5.8 (1.1) | 5.8 (1.0) | 5.8 (1.2) | 0.07 (−0.3 to 0.4) |
| PSG AHI (events/h) | 21.4 (25.0) | 24.5 (27.2) | 14.7 (19.0) | 9.8 (3.3 to 16.3) |
| ESS | 9.9 (5) | 10.2 (4.7) | 9.2 (5.7) | 1 (−0.4 to 2.6) |
| NF analysis duration (h) | 7.6 (0.9) | 7.6 (0.9) | 7.6(0.8) | −0.03 (−0.3 to 0.2) |
| NF quality duration (h) | 6.3 (1.5) | 6.4 (1.4) | 6.2 (1.6) | 0.2 (−0.3 to 0.6) |
| PSG AHI minus NF RDI (events/h) | −6.2 (8.5) | −6.0 (8.8) | −6.8 (7.7) | 0.8 (−1.9 to 2.8) |
BMI refers to body mass index; TST, total sleep time; PSG AHI, polysomnography derived apneas hypopnea index; ESS, Epworth Sleepiness Scale; NF, nasal flow; RDI, respiratory disturbance index.
Data Sufficiency
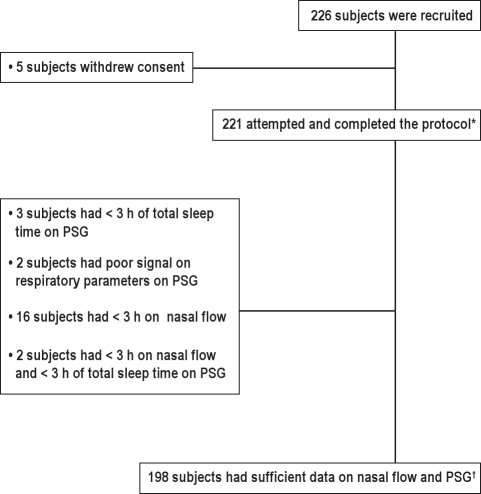
Of 221 subjects, 18 (8.1%) had < 3 h of good quality recording for nasal flow, while 5 (2.2%) subjects had insufficient data on PSG (slept < 3 h). Figure 2 shows subject participation and data insufficiency rates for the FW. Approximately one-third of the insufficient nasal flow data was due to pressure leakage in the connection between the Flow Wizard device and the PSG system. This resulted in a poor trace on the Flow wizard device, as the device has signal amplification to a fixed gain to enable use in the unattended setting (in contrast to the PSG nasal airflow signal with variable gain, which the technicians can adjust as necessary). The pressure leakage was rectified during the course of the study. The rate of insufficient data was lower after rectifying the leakage (5.3% vs. 8.1%, p < 0.0001). Mean duration of analyzable data was 7.6 h, and total good quality recording duration was 5.8 h on nasal flow.
Figure 2.
Subject participation and data sufficiency at various stages in the laboratory
PSG, polysomnography. *Data included in the data quality analysis. †Data included in the in laboratory nasal flow and oximetry accuracy analysis.
Agreement of Nasal Flow and Oximetry with PSG
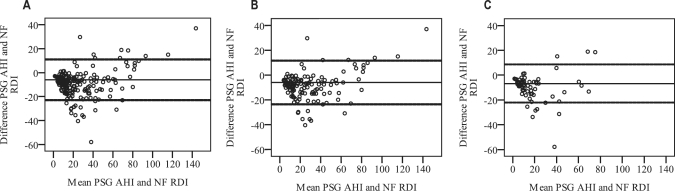
Figure 3 shows that the difference between PSG AHI and NF RDI increased with OSA severity. The mean difference between PSG AHI and NF RDI was –6.2 (8.5) events/h with limits of agreement (2 SD) −23.2 to 11.0 events/h.
Figure 3.
Bland Altman plots showing the mean difference (thin lines) and the limits of agreement ([2SD] thick lines) for polysomnography apnea-hypopnea index (PSG AHI) and nasal flow respiratory disturbance index (NF RDI) for all subjects (A), for males (B) and for females (C)
Overall NF RDI slightly overestimated AHI severity. The limits of agreement were large but were generally lower at low AHI. There was no difference between genders in the degree of AHI overestimation. The limits of agreement were also comparable for both genders.
Diagnostic Accuracy of Nasal Flow for Diagnosing OSA
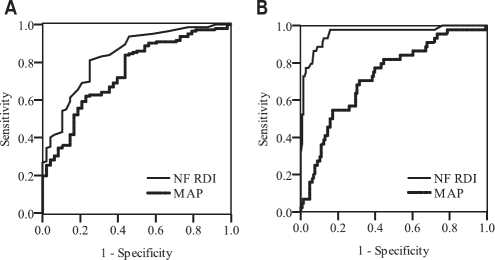
The device was more accurate for diagnosing severe OSA (defined as AHI > 30) than for diagnosing OSA (defined as PSG AHI ≥ 5), with the AUC for in-laboratory NF RDI being 0.96 (95% confidence interval (CI) 0.92-0.99) and 0.84 (95% CI 0.77-0.90), respectively. Table 2 shows the operating characteristics of nasal flow for diagnosing OSA and severe OSA at different thresholds. Overall nasal flow had high sensitivity and specificity for diagnosing severe OSA. If NF RDI ≥ 30 events, PSG-confirmed diagnosis of OSA is highly likely; and if NF RDI < 30 events/h, PSG-confirmed severe OSA is very unlikely. Furthermore if NF RDI < 10 events/h, PSG-confirmed diagnosis of any OSA is unlikely. Figure 4 shows the AUC curve for Nasal Flow compared to PSG for diagnosing OSA and severe OSA.
Table 2.
Operating characteristics of NF RDI for diagnosing OSA and severe OSA
| Cut Off | Sensitivity (95% confidence interval) | Specificity (95% confidence interval) | LR+ | LR− | Post-test probability |
|
|---|---|---|---|---|---|---|
| Of OSA if test positive | Of no OSA if test negative | |||||
| For diagnosing OSA (PSG AHI ≥ 5)i | ||||||
| 10 | 0.94 (0.92-0.98) | 0.62 (0.47-0.77) | 2.5 | 0.10 | 0.90 | 0.74 |
| 20 | 0.79 (0.72-0.85) | 0.79 (0.66-0.91) | 3.8 | 0.27 | 0.93 | 0.51 |
| 30 | 0.47 (0.41-0.53) | 0.94 (0.88-1.00) | 7.8 | 0.56 | 0.97 | 0.33 |
| For diagnosing OSA (PSG AHI ≥ 30)ii | ||||||
| 20 | 0.98 (0.91-1.00) | 0.60 (0.49-0.74) | 2.5 | 0.03 | 0.46 | 0.99 |
| 30 | 0.98 (0.92-1.00) | 0.81 (0.76-0.88) | 5.2 | 0.02 | 0.64 | 0.99 |
PSG AHI, polysomnography derived apnea-hypopnea index; LR+, positive likelihood ratio; LR−, negative likelihood ratio; NF RDI, nasal flow respiratory disturbance index;
Prevalence (Pre test probability) = 0.78;
Prevalence (Pre test probability) = 0.26.
Figure 4.
Receiver operator characteristics (ROC) curves for in-laboratory nasal flow respiratory disturbance index (NF RDI) and multivariate apnea index (MAP) for diagnosing OSA defined by polysomnography apnea-hypopnea index (PSG AHI) ≥ 5 (A) and for diagnosing severe OSA, defined as PSG AHI ≥ 30 in (B)
Role of Gender
Table 1 shows that the mean PSG AHI was lower for females than males in this study. There was no difference in the duration of recording or good quality data on nasal flow between males and females. There were equal numbers of male (n = 9) and female (n = 9) subjects with insufficient data. There was also no difference in total sleep duration between males and females on the PSG night.
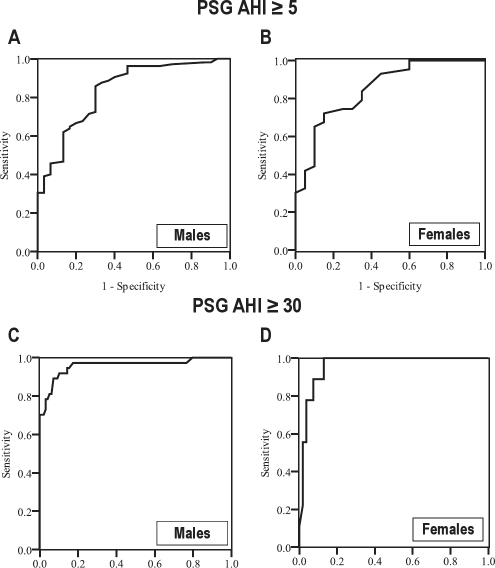
Table 1 and Figure 5 show the mean bias between PSG AHI and NF RDI is similar in males and females. Also, Figure 4 shows there is no difference between males and females in the AUC for diagnosing OSA (AUC = 0.84 [0.75-0.92] and 0.85 [0.75-0.95], respectively; AUC difference −0.01; 95% CI [−0.15 to 0.12]; p value = 0.83) and severe OSA (AUC = 0.96 [0.92-1.0] and 0.96 [0.92-1.0], respectively; AUC difference < 0.01; 95% CI [−0.07 to 0.06]; p value = 0.93).
Figure 5.
Receiver operator characteristics (ROC) curves for in-laboratory nasal flow respiratory disturbance index (NF RDI) in males and females for diagnosing OSA defined by polysomnography apnea-hypopnea index (PSG AHI) ≥ 5, panels (A) and (B), and for diagnosing severe OSA, defined as PSG AHI ≥ 30, panels (C) and (D), respectively
The Value of Questionnaires for Diagnosing OSA
Figure 4 shows that the MAP had a lower AUC than NF RDI for diagnosing OSA (AUC difference −0.09; 95% CI of difference −0.18 to 0.01, p = 0.045) and for diagnosing severe OSA (AUC difference −0.23; 95% CI of difference −0.32 to −0.15, p < 0.001). At the optimal threshold (MAP = 0.4) for diagnosing OSA (PSG AHI ≥ 5), the sensitivity was 84% and specificity was 56%, with positive and negative likelihood ratios of 1.9 and 0.28, respectively. The Berlin questionnaire had a sensitivity of 82% and specificity of 50%, with positive and negative likelihood ratios of 1.6 and 0.37, respectively.
Development of a Predictive Algorithm
We used stepwise logistic regression to develop an algorithm to estimate the probability of OSA based on anthropometrics, questionnaire responses, and in-laboratory NF RDI. Gender, BMI, ESS, Berlin questionnaire, MAP, and nasal flow were entered into the model, as they all showed significant univariate associations with PSG AHI (Table 3).
Table 3.
Correlation of variables with PSG AHI
| Variable | Pearson correlation | p value |
|---|---|---|
| Age | 0.07 | 0.31 |
| Gender | 0.18 | 0.01 |
| BMI | 0.48 | < 0.01 |
| ESS | 0.18 | 0.01 |
| Berlin | 0.27 | < 0.01 |
| MAP | 0.41 | < 0.01 |
| NF RDI | 0.89 | < 0.01 |
PSG AHI, polysomnography derived apnea-hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; NF, nasal flow; RDI, respiratory disturbance index; MAP, multivariate apnea index.
The derived final equation for the probability of OSA (P (OSA)) was:
P(OSA) = ex/(1 + x), where
x = −30 + (0.15 × NFRDI) + (3.0 × MAP)
Table 4 shows the logistic regression model for predicting OSA. For severe OSA, neither anthropometric measures nor questionnaire data were significant predictors of OSA, when added to NF RDI.
Table 4.
Logistic regression model combining NF RDI and MAP for predicting OSA (PSG AHI ≥ 5)
| Units | Odds ratio (95% CI) | p value | |
|---|---|---|---|
| NF RDI | 1 event per h increase | 1.15 (1.05 to 1.25) | < 0.01 |
| MAP | 1 percent increase | 1.03 (1.01 to 1.05) | < 0.01 |
OSA, obstructive sleep apnea; PSG AHI, polysomnography derived apnea-hypopnea index; NF, nasal flow; RDI, respiratory disturbance index; MAP, multivariate apnea index.
DISCUSSION
Our data demonstrate that single-channel nasal flow measured with a pressure transducer (Flow Wizard) has a low data insufficiency rate and high accuracy for diagnosing severe OSA in patients referred to a sleep clinic. Therefore, such a device has a potential role in triage of subjects presenting to the clinic with possible OSA. We found no differences between males and females in data sufficiency, agreement with the reference standard in-laboratory PSG, and accuracy for diagnosing OSA and severe OSA. A prediction algorithm using the MAP questionnaire in combination with NF RDI may increase the accuracy of OSA diagnosis in clinic patients.
Current recommendations3 do not endorse the use of single-channel monitoring for screening for OSA because of lack of sufficient evidence regarding their utility. Hence, the aim of our study was to provide evidence in relation to the role of single-channel nasal pressure transducer device initially as a triaging tool in those who present to the sleep disorders clinic, and also to derive an evidence-based algorithm involving single-channel monitoring to simplify the diagnostic process.
Single-channel self-applied diagnostic devices are a viable alternative to multichannel devices because of their portability, low cost, and low demand on patient and technician time, as well as ease of accessibility to those patients in remote and regional areas. Only a few studies have examined single-channel nasal flow measured by a pressure transducer; these are compared to our current study in Table 5. A previous study13 using the same device (FW) obtained similar results to the present study.
Table 5.
Comparison of the present and previously published studies on single-channel nasal pressure transducers
| Subjects, study location, gender | Device | Mean bias PSG AHI and NF RDI (SD) | PSG AHI (Cut off) | AUC | Sn (%) | Sp (%) | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|---|
| DeAlmeida et al.,14 2006 | 30 sleep clinic subjects, in-lab study, 77% male | Sleep-Checki | −27.4 (13.3) | 5 | 0.89 | 86 | 75 | 3.4 | 0.19 |
| Nakano et al.,8 2007 | 117 sleep lab subjects, in-lab study, retrospective | PTAFii | −2.5 (6.5) | 5 | 0.95 | 97 | 77 | 4.2 | 0.04 |
| Erman et al.,12 2007 | 63 diabetes clinic, in-lab and at home studies, 52% male | Apnea-Linkiii | Approx −4 (10) | 5 | 0.86 | 85 | 50 | 1.7 | 0.30 |
| Wong et al.,13 2007 | 34 sleep lab subjects, in-lab and at home studies, 97% male | Flow Wizardiv | −7.1 (11.1) | 10 | 0.95 | 96 | 71 | 3.3 | 0.05 |
| 30 | 0.89 | 91 | 75 | 3.64 | 0.12 | ||||
| Grover et al.,27 2008 | 25 sleep lab subjects, in-lab | RUSleepingv | 2.6 (19.9) | 5 | 0.94 | 89 | 86 | 6.2 | 0.13 |
| Present Study | 200 sleep lab subjects, in lab study, 68% male | Flow Wizardvi | −6.2 (8.5) | 5 | 0.84 | 94 | 62 | 2.5 | 0.10 |
| 30 | 0.96 | 90 | 89 | 8.5 | 0.11 |
OSA, obstructive sleep apnea; PSG, polysomnography; AHI, apnea-hypopnea index; SD, standard deviation; Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AUC, area under receiver operator characteristics; RDI, respiratory disturbance index; PSG, polysomnography.
IM systems Inc, Baltimore, USA;
Pro-Tec Services, Washington, USA;
ResMed Corporation, California, USA;
DiagnoseIT, Sydney, Australia;
Respironics, Murrysville, PA;
DiagnoseIT, Sydney, Australia
Data Insufficiency
After rectifying the nasal airflow leakage which occurred in the study initially, the rate of data insufficiency was low and was comparable to similar studies in the home setting utilizing a different single-channel nasal pressure transducer (6%)12 and other portable PSG monitoring devices (5%).26 It is possible that data sufficiency could be lower in the unattended setting due to cannulae displacement during sleep.
Agreement with PSG AHI
Nasal pressure transducers vary in the degree of overestimation of OSA. The bias of the Flow Wizard estimated RDI with reference to the PSG AHI was similar to that observed using some nasal flow devices and smaller than others (Table 5). For example, the SleepCheck (IM Systems Inc., Baltimore, USA) device14 has been reported to overestimate apneas and hypopneas compared with in-laboratory PSG by 27.4 events/h.
The hypothesized factors that could have contributed to the bias between the Flow Wizard RDI and the reference in laboratory PSG are overscoring of respiratory variation during REM, scoring of events occurring after an arousal or periodic limb movement, and scoring of respiratory effort related arousals and periods of reduced airflow in the absence of arousals or desaturation not meeting standard criteria for hypopnea. Other factors include nasal obstruction and full or partial mouth breathing.
The degree of variability around the mean (2 SD) reported in our study was similar to some previous studies and lower than others12–14 examining PSG AHI and NF RDI agreement in the laboratory.
Accuracy of OSA Diagnosis
Nasal flow had a high accuracy for diagnosing severe OSA in this study. The post-test probability of no severe OSA after a negative test is very low (i.e., high negative predictive value). Therefore nasal flow can be used to rule out severe OSA in subjects awaiting PSG. Conversely, the post-test probability of having OSA after a positive test (defined as NF RDI ≥ 30 events per h) was 3-fold that of pre-test probability. Thus nasal flow can also be used to rule in severe OSA facilitating better resource allocation to the subjects who have severe disease in need of urgent treatment. However, these results can be affected by the high prevalence of disease in the sleep clinic population. Likelihood ratios are useful as they are not dependent on the prevalence of disease. Our results again reflect a high positive likelihood ratio (8.5) and a low negative likelihood ratio (0.1) for diagnosing severe OSA.
On the other hand, if NF RDI is used for screening purposes, the threshold for NF RDI can be set at a level to optimize the sensitivity to help rule out any degree of OSA (PSG AHI ≥ 5). A threshold of 10 events/h has a sensitivity of 94% and negative likelihood ratio of 0.1. Similar to our findings, published studies conducted in the laboratory comparing single-channel nasal pressure transducers to PSG8,12–14 showed the overall sensitivity (85% to 97%) was higher than specificity (50% to 77%) for OSA diagnosis; the overall negative likelihood ratios was also low (0.04-0.3), indicating that nasal flow measured by a pressure transducer can be used to rule out OSA (Table 4).
Value of NF RDI in Women
Our study shows that despite the lower OSA severity found in women there was no gender difference in agreement between PSG AHI and NF RDI. Also, there was no difference in accuracy of OSA diagnosis. This is an important finding, because despite the lack of previous evidence, these devices are often used to diagnose OSA in women. This is encouraging, as it increases the likelihood of the device having a high accuracy in similar groups with lower OSA severity (such as primary care).
Role of Questionnaires in OSA Diagnosis
The questionnaires had poorer operating characteristics for diagnosing OSA than NF RDI. The positive likelihood ratios were lower and the negative likelihood ratios were higher compared to nasal flow. Interestingly the Berlin questionnaire had a lower specificity and positive likelihood ratio in our study than that previously reported for diagnosing OSA. However, it has been validated for use in primary care22 rather than in sleep laboratory subjects. In contrast, the MAP was validated on sleep laboratory subjects. Not surprisingly the operating characteristics were similar to that reported in the original study, albeit with a different threshold, as our cut-off is based on OSA defined as PSG AHI ≥ 5 (rather than PSG AHI ≥ 10 reported in the original study).
Role of Prediction Models
The role of prediction models combining anthropometrics, questionnaires, and NF RDI has not been fully explored. Only one study13 examined the role of single-channel nasal flow and the MAP questionnaire. They reported that multivariate logistic regression did not show a significant improvement in OSA diagnosis with the addition of the MAP to NF RDI. However the study had a much small number of subjects with severe OSA than the present study (and only one female subject). Our study indicates that there may be an additional role for the MAP in diagnosing any OSA, but there is no additional role in diagnosing severe disease. However our algorithm needs to be validated on a separate group of laboratory subjects, perhaps separated by time, to evaluate its true utility.
Limitations
The main limitation of this study is that the in-laboratory design may not reflect the true clinical utility of a device that is designed for use in the home setting. However, simultaneous laboratory recording enables assessment of agreement under ideal conditions without the inclusion of extraneous sources of variation, such a night-to-night variability in AHI and differences due to sleep position and sleep stages between nights. Our aim was to validate a new device against the reference standard in-laboratory PSG without the addition of other possible sources of variation. A consequence of this study design is that it is only directly applicable to the laboratory setting. There is still a need to perform studies to assess data sufficiency and accuracy of nasal flow at home. The study was performed on highly selected subjects presenting to the sleep laboratory for possible OSA, with no possible other sleep disorder diagnosis, and we have excluded subjects with severe cardiac respiratory and neurological disorders. Thus the results cannot be generalized to all subjects presenting to the sleep laboratory, especially those with a high likelihood of Cheyne Stokes respiration, central sleep apnea, and periodic leg movements or to primary care populations.
The lack of EEG evidence of sleep may have some implications. For example, if the subject reports that they did not sleep, the study could be repeated or a PSG could be organized. Other limitations include lack of sleep position data; however, the Flow Wizard has the capability of recording multiple nights therefore it is unlikely that SDB in a particular position will be missed.
CONCLUSION
Nasal flow measured by nasal pressure transducer is accurate and has good agreement with in-laboratory PSG in both males and females, with low data loss and insufficiency rates comparable to PSG. These transducers have a high sensitivity and thus can be used to rule out OSA in sleep laboratory subjects and can also be used to both rule in and rule out OSA in those with suspected severe disease. Thus they are a viable alternative to laboratory based PSG for OSA screening. The addition of the MAP added predictive value to nasal airflow for diagnosing OSA.
DISCLOSURE STATEMENT
This was not an industry support study. Mr. Unger and Dr. Grunstein are consultants to and stockholders of Diagnose-It, the manufacturer of the Flow Wizard devices. Dr. Grunstein's department has received grant-in-aid and /or equipment use from Respironics, Resmed, Fisher-Paykel, Sanofi-Aventis, Actelion, Impax, Diagnose-It, and Arena. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to all the patients and the technical and nursing staff for participating and assisting in this study.
Institution at which the work was performed: NHMRC Centre for Respiratory and Sleep Medicine Research Excellence (CCRE), Woolcock Institute of Medical Research, Royal Prince Alfred Hospital and The University of Sydney, Australia, 2050
Support for this study was provided by the following: NHMRC Centre for Clinical Research Excellence (CCRE) Scholarship to L Makarie Rofail, NHMRC Practitioner Fellow awards to G Marks and R Grunstein, NHMRC CCRE Fellowship to K Wong, University of Sydney Bridging Support Grant 2007, NHMRC Project Grant 2008.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea - A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Ferber R, Millman R, Coppola M, et al. Portable recording in the assessment of obstructive sleep-apnea. Sleep. 1994;17:378–92. doi: 10.1093/sleep/17.4.378. [DOI] [PubMed] [Google Scholar]
- 3.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 4.Farre R, Montserrat JM, Rotger M, et al. Accuracy of thermistors and thermocouples as flow-measuring devices for detecting hypopnoeas. Eur Respir J. 1998;11:179–82. doi: 10.1183/09031936.98.11010179. [DOI] [PubMed] [Google Scholar]
- 5.Shochat T, Hadas N, Kerkhofs M, et al. The SleepStrip (TM): an apnoea screener for the early detection of sleep apnoea syndrome. Eur Respir J. 2002;19:121–6. doi: 10.1183/09031936.02.00227302. [DOI] [PubMed] [Google Scholar]
- 6.Hollingworth L, Tooby M, Roberts D, et al. Practicality of the Sleepstrip (TM) in postal screening for obstructive sleep apnoea. J Sleep Res. 2003;12:157–9. doi: 10.1046/j.1365-2869.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 7.Pang KP, Gourin CG, Podolsky R, et al. A comparison of polysomnography and the SleepStrip in the diagnosis of OSA. Otolaryngol Head Neck Surg. 2006;135:265–8. doi: 10.1016/j.otohns.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Nakano H, Tanigawao T, Furukawa T, et al. Automatic detection of sleep-disordered breathing from a single-channel airflow record. Eur Respir J. 2007;29:728–36. doi: 10.1183/09031936.00091206. [DOI] [PubMed] [Google Scholar]
- 9.Nakano H, Tanigawa T, Ohnishi Y, et al. Validation of a single-channel airflow monitor for screening of sleep-disordered breathing. Eur Respir J. 2008;32:1060–7. doi: 10.1183/09031936.00130907. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 11.Heitman SJ, Atkar RS, Hajduk EA, et al. Validation of nasal pressure for the identification of apneas/hypopneas during sleep. Am J Respir Crit Care Med. 2002;166:386–91. doi: 10.1164/rccm.2105085. [DOI] [PubMed] [Google Scholar]
- 12.Erman MK, Stewart D, Einhorn D, et al. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Wong KKH, Jankelson D, Reid A, et al. Diagnostic test evaluation of a nasal flow monitor for obstructive sleep apnea detection in sleep apnea research. Behav Res Methods. 2008;40:360–6. doi: 10.3758/brm.40.1.360. [DOI] [PubMed] [Google Scholar]
- 14.De Almeida F, Ayas N, Otsuka R, et al. Nasal pressure recordings to detect obstructive sleep apnea. Sleep Breath. 2006:1–8. doi: 10.1007/s11325-005-0042-x. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 16.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–96. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med. 2004;25:257–68. doi: 10.1016/j.ccm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness - the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service; 1977. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 26.Portier F, Portmann A, Czernichow P, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162:814–8. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 27.Grover SS, Pittman SD. Automated detection of sleep disordered breathing using a nasal pressure monitoring device. Sleep Breath. 2008;12:339–45. doi: 10.1007/s11325-008-0181-y. [DOI] [PubMed] [Google Scholar]