Abstract
Study Objectives:
American Academy of Sleep Medicine (AASM) practice parameters indicate that split-night polysomnograms (SN-PSG) may be performed when the apnea hypopnea index (AHI) is ≥ 20 to 40, depending on clinical factors. The aim of this study was to determine the diagnostic accuracy of SN-PSG, including at the lower range of AHIs.
Methods:
We reviewed 114 consecutive full-night PSGs (FN-PSG) performed at our center between August 2006 and November 2008 on subjects enrolled in studies in which obstructive sleep apnea (OSA) was the sleep disorder of interest. We compared the AHI from the first 2 hours (2hr-AHI) and 3 hours (3hr-AHI) of sleep with the “gold standard” AHI from FN-PSG (FN-AHI), considering OSA present if FN-AHI ≥ 5.
Results:
The 2hr-AHI and 3hr-AHI correlated strongly with the FN-AHI (concordance correlation coefficient [CCC] = 0.93 and 0.97, respectively). After adjusting for percentage of sleep in stage REM sleep and in supine position, the correlation of 2 hr- and 3 hr-AHI with FN-AHI remained strong (0.92 and 0.96, respectively). The area under the receiver operating curves (AUC) for 2hr-AHI and 3hr-AHI using FN-AHI ≥ 5 were 0.93 and 0.95, respectively.
Conclusions:
The AHI derived from the first 2 or 3 hours of sleep is of sufficient diagnostic accuracy to rule-in OSA at an AHI threshold of 5 in patients suspected of having OSA. This study suggests that the current recommended threshold for split-night studies (AHI ≥ 20 to 40) may be revised to a lower number, allowing for more efficient use of resources.
Citation:
Khawaja IS; Olson EJ; van der Walt C; Bukartyk J; Somers V; Dierkhising R; Morgenthaler TI. Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med 2010;6(4):357-362.
Keywords: Obstructive sleep apnea (OSA), polysomnography (PSG), split-night polysomnography, diagnostic accuracy
Obstructive sleep apnea (OSA) is a common condition1 associated with serious health consequences.2 Increased attention to sleep disorders, particularly OSA, by health care providers and the public has stoked demand for sleep medicine evaluations. Though the number of sleep facilities has doubled per a 2002 report, referrals to sleep facilities have increased 12-fold—an imbalance that has stimulated interest in development of different evaluation strategies for OSA.3–5
Two-night attended polysomnography (PSG) for the diagnosis of OSA and titration of continuous positive airway pressure (CPAP) therapy remains the gold standard PSG practice.6 Two-night diagnostic and therapeutic PSGs theoretically maximize the opportunities to observe breathing and CPAP response across the full spectrum of sleep states and positions, yet is time-consuming for patients and resource-intensive. Combining the diagnostic and CPAP titration into a single night, often called a “split-night” PSG (SN-PSG), potentially increases convenience of patients and economy of the sleep testing process.
The original PSG indications practice parameters published in 1997 by the American Academy of Sleep Medicine (AASM) stated that SN-PSG was acceptable only when apnea hypopnea index (AHI) was ≥ 40 during a minimum of 2 h of diagnostic PSG, or when the AHI was ≥ 20 and clinical judgment suggested this more lenient threshold was appropriate.7 However, using the prevalence rates from the Wisconsin cohort study,8 approximately 60% of the population with an AHI of > 5 would not be candidates for the SN-PSG protocol using the practice parameter AHI thresholds.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Combining the diagnostic and CPAP titration polysomnography into a single night, often called a “split-night” PSG, potentially increases convenience of patients and economy of the sleep testing process. However, the current recommended threshold apnea-hypopnea index for converting to a split-night PSG (AHI ≥ 20 to 40) is high enough to exclude approximately 60% of the population with obstructive sleep apnea syndrome.
Study Impact: The AHI derived from the first 2 or 3 hours of sleep is of sufficient diagnostic accuracy (area under the receiver operating curve ≥ 0.93) to rule-in OSA at a threshold AHI of 5 in patients suspected of having OSA. These findings suggest that the current recommended threshold for split-night studies may be revised to a lower number, allowing for more efficient use of resources.
The AHI threshold in the original practice PSG parameter appears to be based on 2 types of studies. The first set of studies9–11 revealed specificities of SN-PSG of 95% to 100%, suggesting that when an elevation of AHI occurs during the first part of the testing, it will remain high during the FN. These studies were performed in patients with more severe OSA. The mean AHIs were 44.2 ± 34.310 and 74.3 ± 19.69; in the study of Scharf et al., about 50% of patients had an AHI > 20, and all tested patients had an AHI > 10.11 Thus there was a paucity of data on the performance of SN-PSGs with lower diagnostic AHIs. The second set of studies suggested more reliable CPAP titration in patients with higher AHI.12,13 Iber et al.12 attempted CPAP titration in a split-night protocol applied to 412 consecutive patients with an apnea index (not AHI) ≥ 20 (mean AI: 67 ± 30). CPAP was started at 5 cm H2O and titrated in 2.5 cm H2O increments; mean titration time was 176 ± 97 min. An effective pressure was found in 320 (78%) patients per their split-night protocol. Yamashiro et al. compared a partial night CPAP titration (mean titration time 160 ± 66.0 min) with a full night of CPAP titration.13 When the AHI was ≥ 40, the final pressure was not significantly different between the 2 nights. In 69 patients with an AHI < 20, full-night titration pressures were higher by a mean of 1.5 cm H2O than split-night titration pressures. When the AHI was < 20, less than 3 h was generally available for titration, a time the authors felt was insufficient for adequate titration. When the PSG practice parameters were updated in 2005, the requirements for SN-PSG remained unchanged given the lack of significant interval data on the subject.14
At the time of the original practice parameter, SN-PSGs were a controversial clinical practice. Now it is common, and in some locations, virtually mandated by third-party payers.15 Since 1997, guidelines have been published regarding positive airway pressure (PAP) titration.6,16 There has also been standardization of the definitions and techniques for measuring sleep disordered breathing events,17 which may greatly influence the AHI.18 Given these changes, we considered it propitious to reevaluate whether the current AHI threshold of 20-40 is unnecessarily high. This study sought to determine the accuracy of split-night studies using lower AHI thresholds of 5 and 10 by comparing the AHI during selected time periods in patients who had a full night (FN) diagnostic PSG with disordered breathing events defined by more contemporary criteria.
METHODS
We retrospectively reviewed consecutive FN-PSGs performed in subjects enrolled in research studies focusing on OSA conducted at the Clinical Research Unit-Sleep Core, Mayo Clinic from August 2006 to November 2008. PSGs from normal controls, as well as those of subjects suspected of having OSA based on clinical suspicion or clinical prediction scales such as Berlin Questionnaire19 were included. PSGs from studies investigating central sleep apnea (CSA), Cheyne-stoke respiration (CSR), PAP titration studies, and unattended bedside studies were excluded. All PSGs were recorded in an attended fashion with PSG Online 2 recording software and scored using Profusion PSG 2 software (E-series, Compumedics, Abbotsford, VIC, Australia). PSGs performed on different recording systems were excluded because of limitations in transferring data on-line for the review by sleep physicians. A total of 402 PSGs on 305 subjects were performed during this time. One hundred thirty-four attended diagnostic PSGs from 114 unique subjects qualified for inclusion and were reviewed. If a subject underwent more than one study, only the first PSG was analyzed.
Sleep staging and arousals were scored according to the AASM manual using the alternative montages for electroencephalography (Fz-Cz, Cz-Oz, C4-M1, C3-M2), and electrooculography (E1-Fpz and E2-Fpz).17 Breathing was assessed by nasal pressure transducer (Compumedics integrated nasal pressure transducer, Compumedics Inc, Melbourne, Australia, with Pro-tech Pro-Flow nasal cannulas, Pro-Tech Services, Woodinville, WA, USA) and/or oronasal thermocouple (Pro-Tech one-channel oro-nasal Thermal airflow sensor, Pro-Tech Services, Woodinville, WA, USA). In 88 subjects, both methods were used to assess airflow. In 26 patients, only a thermal sensor was used. Respiratory effort was monitored by calibrated respiratory impedance plethysmography (Compumedics Summit IP Inductive Respiratory Effort system, Compumedics Inc, Melbourne, Australia). Oxyhemoglobin saturation was recorded by finger pulse oximetry (Compumedics E-Series integrated pulse oximetry, Compumedics Inc, Melbourne, Australia). No specific instructions were given to the patients about sleeping either on their backs or side.
Apneas were defined as a cessation of airflow ≥ 10 sec, and hypopneas defined by a 30% decline in airflow ≥ 10 sec accompanied by an oxyhemoglobin desaturation ≥ 4%.17 Apneas unaccompanied by evidence of respiratory effort were scored as central, while those accompanied by respiratory effort were labeled obstructive or mixed. For the purposes of tabulation, mixed apneas were counted as obstructive. The AHI was calculated as the number of apneas (central, mixed, or obstructive) plus hypopneas per hour of sleep. Respiratory effort related arousals were scored episodes ≥ 10 sec of reduced airflow not meeting criteria for apneas or hypopneas and terminating with an arousal.17 The respiratory disturbance index (RDI) was calculated as the number of apneas plus hypopneas plus respiratory arousals per hour of sleep. All PSGs were conducted and scored by a registered polysomnographic technologist, and subsequently reviewed by Sleep Medicine specialists.
Portions of each PSG file were extracted from the full night PSG (FN-PSG) for comparison as follows: first 2 hours of sleep (2-hr PSG), first 3 hours of sleep (3-hr PSG), first 10 apneas plus hypopneas (10-event PSG), and first 30 apneas plus hypopneas (30-event PSG). The Mayo Clinic Institutional Review Board (IRB) approved the study.
STATISTICAL METHODS
Continuous and categorical variables are reported as mean ± standard deviation (SD) and frequency (%), respectively. Comparisons between partial-night and FN for proportions of supine and REM sleep time were computed using signed-rank test. The agreement between split-night and full-night continuous measurements (AHI and RDI) was assessed using the concordance correlation coefficient (CCC).20,21 The CCC measures variation from the y = x line, and ranges from −1 to 1, where 1 means perfect positive agreement (all points lie on the y = x line) and 0 means no agreement. This approach also allows adjustments for subject-specific covariates. Thus, we also computed CCCs adjusted for the percent of time in REM sleep and the percent of time in supine, both for the split- and full-night periods, since the agreement could be affected by variations in these measurements.
The next level of assessment involved dichotomizing patients at AHI and RDI cutoffs of 5, 10, and 15 for the full night measurements. Univariate logistic regression models were estimated using a full-night binary variable (yes vs. no for being at least as large as the cutoff value, which could indicate sleep apnea) as the response and the corresponding continuous split-night variable as the predictor. Subsequent receiver operating characteristic (ROC) curves and the area under the curves (AUC) were estimated. Sensitivities and specificities with 95% exact binomial confidence intervals are reported for the same cutoff as the full-night measurement in each particular model. Likelihood ratios with 95% confidence intervals of 2 hr and 3 hr AHI at cutoffs of 5, 10, and 15 are also reported.22
RESULTS
PSGs from 114 subjects were included in the review. Table 1 shows the subject demographics and FN-PSG data. Sixty-nine subjects (61%) were men. Two patients (2%) had chronic obstructive pulmonary disease, 10 (9%) had histories of congestive heart failure, and 4 (4%) were on chronic opiate therapy.
Table 1.
Demographic and full night polysomnogram data (n = 114 subjects)
| Variable | Mean ± SD | Range |
|---|---|---|
| Age | 50.9 ± 17.7 | 18-81 |
| Male gender N, (% of total) | 69 (61%) | |
| BMI | 29 ± 6 | 18.8–52.9 |
| AHI | 11.3 ± 17.3 | 0–90 |
| RDI | 23.2 ± 20.5 | 0.3–91 |
| RERA index | 11.9 ± 9.5 | 0–48.1 |
SD refers to standard deviation; BMI, body mass index (kg/m2); AHI, apnea hypopnea index (events/hour); RDI, respiratory disturbance index (events/hour); RERA index, respiratory effort related arousal index (events/hour)
Polysomnographic parameters from partial-night PSG and FN-PSGs are compared in Table 2. The percentage of supine sleep was higher for the FN than 2 hr PSG, but not 3-hr PSG conditions (p = 0.037 and 0.201, respectively). The percentage of REM sleep was higher for the FN than 2 hr and 3-hr PSG (p < 0.001 for both).
Table 2.
Polysomnogram data from full-night and partial-night conditions (n = 114 subjects)*
| FN-PSG | 2hr-PSG | 3hr-PSG | 10 event PSG | 30 event PSG | |
|---|---|---|---|---|---|
| AHI | 11.3 ± 17.3 | 9.7 ± 17.4 | 10.6 ± 17.7 | 16.1 ± 24.8 | 20.5 ± 22.1 |
| RDI | 23.2 ± 20.6 | 22.6 ± 22.9 | 23.1 ± 22.0 | 34.2 ± 31.2 | 39.3 ± 27.5 |
| TST supine | 128.0 ± 101.6 | 39.7 ± 41.2 | 66.3 ± 55.1 | 33.4 ± 46.2 | 60.3 ± 67.8 |
| TST non-supine | 223. ± 99.3 | 80.3 ± 41.2 | 113.3 ± 55.0 | 77.3 ± 69.4 | 111.7 ± 90.7 |
| % REM | 17.9 ± 6.5 | 9.0 ± 8.2 | 11.0 ± 7.3 | 6.4 ± 7.3 | 11.3 ± 10.2 |
| % Supine | 35.7 ± 26.5 | 33.1 ± 34.3 | 36.9 ± 30.6 | 36.2 ± 37.6 | 38.8 ± 33.6 |
Data presented as mean ± standard deviation.
FN, full night; PSG, polysomnogram; AHI, apnea hypopnea index (events/hour); RDI, respiratory disturbance index (events/hour); TST, total sleep time (minutes); % REM, % of total sleep scored as rapid eye movement; % Supine, % of total sleep time spent in supine position.
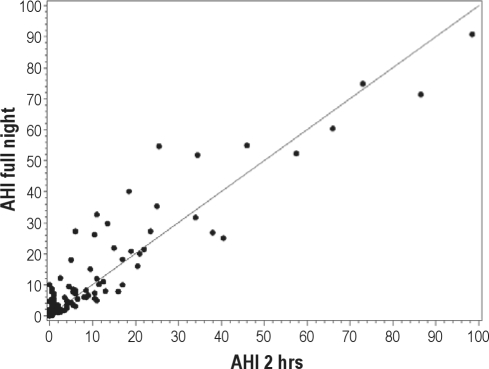
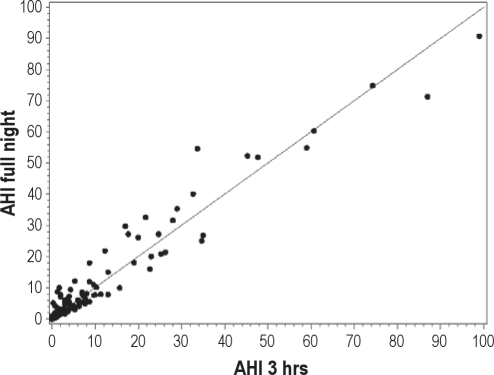
The relationships between the 2hr-AHI, 3hr-AHI, and FN AHI are explored in Figures 1A and 1B, and in Table 3. The adjusted concordance correlation coefficient (CCC) for the 2-hr data (adjusted for time spent supine and in stage REM) was 0.93 and for the 3-hour data was 0.96 indicating a high degree of both precision and accuracy (see below). Very few of our patients who had an AHI ≥ 5 on the 2-hr PSG had an AHI < 5 on the FN-PSG (Specificity = 93.1 (95% CI = 83.3, 98.1), and results were similar for the 3-hr data (Specificity = 91.2 (95% CI = 80.7, 97.1). The scatterplots for RDI showed similar patterns and are not shown. Judging from the graphs (Figures 1A and 1B), measures from the 3hr-PSG are slightly more precise and accurate than those from the 2hr-PSG. This is reflected numerically in the higher CCC for 3hr-PSG data (Table 3). The other visually notable finding is the greater scatter from the line of unity at the lower AHI than at the higher AHI values. Using a Bland-Altman analysis of those AHI pairs (2-hr vs. FN PSG data) when the AHI on the 2-hour study was < 20, the mean difference between the pairs was very small (mean difference −1.7, 95% confidence intervals of the difference were −2.6 and −0.8) and there is a tendency for the 2-hr AHI to underestimate the actual value. In addition, there is somewhat less agreement between test results when the 2-hr AHI is < 20 (CCC = 0.65, 95% CI 0.52-0.74). Closely examining the portion of the graph with AHI or RDI < 10, one may see that most of the FN-PSG results are nearly identical or exceed the SN-PSG results. Table 3 lists the CCC for 2hr-PSG and 3hr-PSG compared to FN-PSG results for both AHI and RDI. The uncorrected CCC is listed first, and realizing that there was a statistically different duration of time spent supine and in REM sleep between some SN-PSG and FN-PSG, subsequently the CCC adjusted for time supine and in REM is listed. The unadjusted and adjusted CCC values are very similar (Table 3). The data show that 2hr-AHI and 3hr-AHI correlated very well with the FN AHI after adjustment for percent REM sleep and percent supine position sleep (adjusted CCC = 0.92 and 0.96, respectively). There was also a strong correlation between 2hr RDI and 3hr RDI with FN RDI (adjusted CCC = 0.92 and 0.97, respectively).
Figure 1A.
Scatter plot of the apnea hypopnea index (AHI) derived from the 2-hr PSG versus the FN-PSG along with the line of unity
The adjusted concordance correlation coefficient (CCC) for these data (adjusted for time spent supine and in stage REM) was 0.93, indicating a high degree of both precision and accuracy. Very few of our patients with an AHI ≥ 5 on the 2-hr PSG had an AHI < 5 on the FN-PSG (specificity = 93.1 (95% CI = 83.3, 98.1).
Figure 1B.
Scatter plot of the AHI derived from the 3-hr PSG versus the FN-PSG along with the unity line
The adjusted CCC for the 3-hour data was 0.96, again indicating a high degree of both precision and accuracy. Very few patients with an AHI ≥ 5 on the 3-hr PSG had an AHI < 5 on the FN-PSG (specificity = 91.2 (95% CI = 80.7, 97.1).
Table 3.
Concordance correlation coefficients of the respiratory indexes between 2-hour, 3-hour and full-night polysomnography
| Comparison | Unadjusted CCC | Adjusted* |
|
|---|---|---|---|
| (CCC) | 95% CI | ||
| AHI: full vs. 2 hr | 0.93 | 0.92 | 0.88–0.94 |
| AHI: full vs. 3 hr | 0.97 | 0.96 | 0.94–0.98 |
| RDI: full vs. 2 hr | 0.92 | 0.92 | 0.88–0.94 |
| RDI: full vs. 3 hr | 0.97 | 0.96 | 0.95–0.98 |
Adjusted for the percentage of REM sleep and percentage of sleep in the supine position (see text).
CCC, concordance correlation coefficient; AHI, apnea hypopnea index; RDI, respiratory disturbance index; CI, confidence interval.
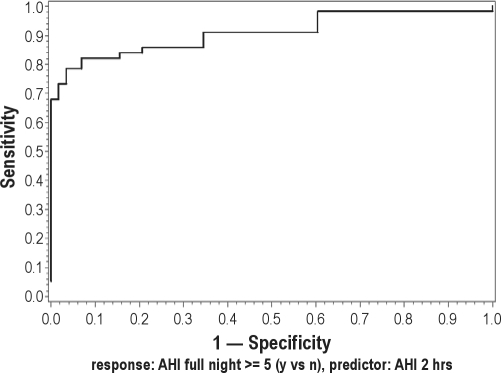
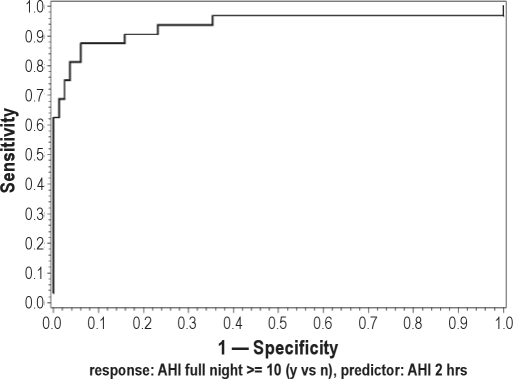
Following dichotomization of the subjects based upon threshold AHI values ≥ 5, 10, or 15, likelihood ratios (LR), sensitivities, specificities, ROCs, and AUC of 2hr- and 3hr-PSG at various AHI cutoff points were calculated and are shown in Table 4. The results for the RDI models were similar and are not shown. Specificities, the measure of interest for “ruling in” disease for all SN-PSGs at all thresholds tested exceeded 90%. The ROC for the calculated from the 2hr-PSG and FN-PSG using an AHI threshold of 5 and 10 is shown in Figure 2. A perfect test has a ROC with an AUC = 1. The ROCs from the 2hr AHI logistic regression model, even at the low AHI cutoffs of 5 and 10 shown in Figure 2 have AUC values exceeding 0.92, indicating a clinically discriminative test.
Table 4.
Likelihood ratios, sensitivities, specificities, and area under the curves of apnea hypopnea indices (AHI) generated at 2 hours and 3 hours per different AHIs*
| Measurement | Cutoff | LR+ (95% CI) | LR− (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | AUC* |
|---|---|---|---|---|---|---|
| 2hr AHI | 5 | 11.65 (0.53, 22.77) | 0.21 (0.10, 0.32) | 80.4 (67.6, 89.8) | 93.1 (83.3, 98.1) | 0.925 |
| 10 | 13.84 (1.90, 25.77) | 0.17 (0.03, 0.30) | 83.4 (67.2, 94.8) | 93.9 (86.3, 98) | 0.944 | |
| 15 | 33.85 (0, 80.76) | 0.24 (0.07, 0.40) | 76.9 (56.4, 91.0) | 97.7 (92.0, 99.72 | 0.972 | |
| 3hr AHI | 5 | 9.36 (1.44, 17.29) | 0.20 (0.08, 0.31) | 82.1 (69.6, 91.1) | 91.2 (80.7, 97.1) | 0.954 |
| 10 | 22.78 (0, 48.31) | 0.16 (0.03, 0.29) | 84.4 (67.2, 94.7) | 96.3 (89.6, 99.2) | 0.972 | |
| 15 | 79.96 (0, 227.31) | 0.12 (0, 0.24) | 88.5 (69.8, 97.6) | 98.9 (93.8, 100.0) | 0.995 |
From a logistic regression model where split-night AHI is a continuous predictor.
Abbreviations: LR+, likelihood ratio for the presence of disease when the condition is met; LR−, likelihood ration for the presence of disease when the condition is not met; AUC, area under the curve; AHI, apnea hypopnea index
Figure 2A.
Receiver operating curve (ROC) for the apnea hypopnea index (AHI) calculated from the 2-hr PSG at an AHI cutoff of 5. The area under the curve (AUC) was 0.925
Figure 2B.
ROC for the 2-hr PSG using an AHI cutoff of 10 (AUC = 0.944)
DISCUSSION
The main conclusion from this study is that the AHI from the first 2 or 3 hours of sleep during polysomnography performed in patients suspected of having obstructive sleep apnea provides a very accurate estimate of the AHI from full-night polysomnography as evidenced by a number of statistical parameters, including high estimates of CCC.
The CCC incorporates both a measure of precision and accuracy and has therefore been regarded as one of the best measures of agreement between two measures of continuous data.20 It has been suggested that in general, for various correlation tests assessing agreement between measures or observations, correlation coefficients > 0.9 connote excellent agreement for clinical purposes.23,24 Using this as a general guide, SN-PSG had excellent agreement with FN-PSG AHI and RDI measurements. The unadjusted CCC of 2h-AHI and 3hr-AHI with FN-AHI were also excellent, at 0.93 and 0.97, respectively.
The findings suggest that SN-PSG may be extended to a broader population of patients with suspected sleep disordered breathing, not restricted to those with AHIs greater than 20-40, as previously recommended. This should have important economic, convenience, and resource utilization implications for patients and providers.
This study has several strengths. As compared to other studies on SN-PSG accuracy, we included a significant number of subjects with milder OSA (AHI = 11.3 ± 17.3) and showed the 2hr- and 3hr-AHIs are accurate across a wider spectrum of disease than previous studies.5,13,25–28 The impact of sleep stage and position were accounted for in the analysis by adjusting for percentage of REM and supine position sleep. In contrast to prior studies, contemporary definitions of disordered breathing events were employed in the analysis.17 Original scoring of the PSGs were performed by technologists unaware of the purpose of this study, so although we hope great care would have been used to ensure accuracy as a matter of good practice, no systematic bias toward better scoring of the first portion of testing would have been likely.
This study has limitations as well. Studies from subjects with congestive heart failure or with suspected central sleep apnea were excluded from the study, limiting the applicability of the results to less complicated patients with suspected obstructive sleep apnea. In 26/114 (23%) subjects, only thermocouple or nasal pressure data were available. To quantify the impact of sensor method on diagnostic discrimination, we developed ROCs for thermocouple only and for thermocouple plus nasal pressure transducer studies. The AUC for ROCs pertaining to the 2-hr test, using cutoffs of AHI = 5, 10, or 15, and only a thermocouple were 0.869, 0.920, and 0.920, respectively; compared with the results of combined patients (both thermocouple only and those with thermocouple + nasal pressure transducer) of 0.940, 0.950, and 0.977, respectively. Thus, there is a small but clinically inconsequential reduction in the discriminative power of the test. Finally, in contrast to the usual clinical circumstance with split-night studies, our studies were scored off-line. Conceivably, scoring on-line could be less precise due to time constraints, leading to worse correlation between split-night scores and full-night scores. A different study design would be required to test this. This study was also not designed to assess the impact of the adequacy of CPAP titration during SN-PSG using lower AHI thresholds. Yet, if a 2-hr AHI is accurate in patients with milder OSA, this would imply that balance of the night could be confidently devoted to a CPAP titration attempt within recommended guidelines6 rather than needlessly waiting for more diagnostic sleep time or uniformly requiring that all milder OSA patients must return for a full-night CPAP titration attempt.
CONCLUSION
The AHIs derived from the first 2 or 3 hours of diagnostic polysomnography appear to provide an accurate estimate of the full-night AHI across the OSA disease spectrum. This study suggests that the current AHI threshold of 40, or greater than 20 under certain circumstances, may be too stringent; consideration should be given to revising the threshold to a lower level, which should have a positive impact on the economy and convenience of laboratory-based PSG.
DISCLOSURE STATEMENT
This was not an industry support study. Dr. Somers has consulted for Resmed, Boston Scientific, and Cardiac Concepts. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to acknowledge Nancy Slocomb and Diane D. Davison for their assistance as study coordinators for the patients and data involved in this study.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.White D. Sleep apnea. Proc Am Thorac Soc. 2006;3:124–8. doi: 10.1513/pats.200510-116JH. [DOI] [PubMed] [Google Scholar]
- 3.Namen AM, Dunagan DP, Fleischer A, et al. Increased physician-reported sleep apnea. Chest. 2002;121:1741–7. doi: 10.1378/chest.121.6.1741. [DOI] [PubMed] [Google Scholar]
- 4.Patel N, Ahmed M, Rosen I. Split-night polysomnography. Chest. 2007;132:1664–71. doi: 10.1378/chest.06-1801. [DOI] [PubMed] [Google Scholar]
- 5.Deutsch PA, Simmons MS, Wallace JM. Cost-effectiveness of split-night polysomnography and home studies in the evaluation of obstructive sleep apnea syndrome. J Clin Sleep Med. 2006;2:145–53. [PubMed] [Google Scholar]
- 6.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22. [PubMed] [Google Scholar]
- 8.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 9.Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994;106:1695–701. doi: 10.1378/chest.106.6.1695. [DOI] [PubMed] [Google Scholar]
- 10.Sanders MH, Black J, Costantino JP, Kern N, Studnicki K, Coates J. Diagnosis of sleep-disordered breathing by half-night polysomnography. Am Rev Respir Dis. 1991;144:1256–61. doi: 10.1164/ajrccm/144.6.1256. [DOI] [PubMed] [Google Scholar]
- 11.Scharf SM, Garshick E, Brown R, Tishler PV, Tosteson T, McCarley R. Screening for subclinical sleep-disordered breathing. Sleep. 1990;13:344–53. [PubMed] [Google Scholar]
- 12.Iber C, O'Brien C, Schluter J, Davies S, Leatherman J, Mahowald M. Single night studies in obstructive sleep apnea. Sleep. 1991;14:383–5. [PubMed] [Google Scholar]
- 13.Yamashiro Y, Kryger MH. CPAP titration for sleep apnea using a split-night protocol. Chest. 1995;107:62–6. doi: 10.1378/chest.107.1.62. [DOI] [PubMed] [Google Scholar]
- 14.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Moyer CA, Palmisano J, et al. Sleep-disordered breathing in Michigan: a practice pattern survey. Sleep Breath. 2003;7:95–104. doi: 10.1007/s11325-003-0095-7. [DOI] [PubMed] [Google Scholar]
- 16.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157:858–65. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Torbeck LD. Coefficient of accuracy and concordance correlation coefficient: new statistics for methods comparison. PDA J Pharm Sci Technol. 1998;52:55–9. [PubMed] [Google Scholar]
- 21.Carrasco J, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics. 2003;59:849–58. doi: 10.1111/j.0006-341x.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 22.Noether G. Two confidence intervals for the ratio of two probabilities and some measures of effectiveness. J Am Stat Assoc. 1957;52:35–45. [Google Scholar]
- 23.Bowling A, Ebrahim S. Handbook of health research methods: investigation, measurement and analysis. Maidenhead, England, New York, NY: Open University Press; 2005. [Google Scholar]
- 24.Reiman MP, Manske RC. Functional testing in human performance. Champaign, IL: Human Kinetics; 2009. [Google Scholar]
- 25.Elshaug AG, Moss JR, Southcott AM. Implementation of a split-night protocol to improve efficiency in assessment and treatment of obstructive sleep apnoea. Intern Med J. 2005;35:251–4. doi: 10.1111/j.1445-5994.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 26.Minai OA, Paracha MI, Golish JA, et al. Adequacy of CPAP titration during split-night polysomnogram (PSG) Chest. 1998;114:379S. [Google Scholar]
- 27.Sanders MH, Costantino JP, Strollo PJ, Jr, Studnicki K, Atwood CW., Jr The impact of split-night polysomnography for diagnosis and positive pressure therapy titration on treatment acceptance and adherence in sleep apnea/hypopnea. Sleep. 2000;23:17–24. doi: 10.1093/sleep/23.1.17. [DOI] [PubMed] [Google Scholar]
- 28.Yamashiro Y, Suganuma Y, Miyasaka T, Hosaka K, Uchida K. Nasal continuous positive airway pressure (nCPAP) treatment using a split-night protocol for sleep breathing disorder. Kokyu To Junkan. 1997;45:1011–4. [Google Scholar]