Abstract
Background:
We previously described a significant correlation between plasma aldosterone concentration (PAC) and severity of obstructive sleep apnea (OSA) in patients with resistant hypertension. This investigation examines the relationship between aldosterone status and OSA in patients with resistant hypertensive—with and without hyperaldosteronism.
Methods and Results:
One hundred and nine consecutive patients with resistant hypertension were prospectively evaluated with plasma renin activity (PRA), PAC, 24-hour urinary aldosterone excretion (UAldo), and polysomnography. Hyperaldosteronism (PRA < 1 ng·mL-1·h-1 and UAldo ≥ 12 μg/24-h) prevalence was 28% and OSA prevalence was 77%. In patients with hyperaldosteronism, OSA prevalence was 84%, compared with 74% in hypertensive patients with normal aldosterone levels. There were no significant differences in body mass index or neck circumference between aldosterone groups. PAC and UAldo were both significantly correlated with apnea-hypopnea index (AHI) in the high-aldosterone group (ρ = 0.568, p = 0.0009; ρ = 0.533, p = 0.002, respectively). UAldo correlated weakly with apnea-hypopnea index in the normal-aldosterone group, but there was no significant correlation between PAC and AHI in the normal-aldosterone group (ρ = 0.224, p = 0.049; ρ = 0.015, p = 0.898, respectively).
Conclusions:
Our analysis of patients with resistant hypertension confirms a markedly high prevalence of OSA in this group. Furthermore, severity of OSA was greater in those patients with hyperaldosteronism and related to the degree of aldosterone excess. The correlation between OSA severity and aldosterone supports the hypothesis that aldosterone excess contributes to greater severity of OSA.
Citation:
Gonzaga CC; Gaddam KK; Ahmed MI; Pimenta E; Thomas SJ; Harding SM; Oparil S; Cofield SS; Calhoun DA. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med 2010;6(4):363-368.
Keywords: Obstructive sleep apnea, aldosterone, resistant hypertension, sleep disorder, cardiovascular disease
Obstructive sleep apnea (OSA) and hypertension are both independently associated with increased cardiovascular risk.1–7 Furthermore, approximately 50% of patients with OSA have a diagnosis of hypertension, whereas 30% of hypertensive patients have OSA.8–11 Recently published evidence-based hypertension management guidelines identified OSA as an important identifiable cause of hypertension.12 The association of OSA and hypertension is particularly marked among patients with resistant hypertension, with studies reporting an OSA prevalence of 80% to 85% in these patients.13,14
BRIEF SUMMARY
Current Knowledge/Study Rationale: We previously described a significant correlation between plasma aldosterone concentration and severity of obstructive sleep apnea in patients with resistant hypertension. This investigation examines the relationship between aldosterone status and obstructive sleep apnea in patients with resistant hypertensive—with and without hyperaldosteronism.
Study Impact: The positive relationship between hyperaldosteronism and severity of obstructive sleep apnea (OSA) observed in the current analysis supports the hypothesis that aldosterone excess contributes to the development of OSA. The results highlight the increased likelihood of hyperaldosteronism and OSA coexisting in patients with resistant hypertension and may explain, at least in part, the high prevalence of OSA in patients with resistant hypertension.
Hyperaldosteronism is common in patients with resistant hypertension. Approximately 20% of patients with resistant hypertension have biochemical criteria consistent with primary aldosteronism.15–18 In patients with resistant hypertension in whom we had diagnosed hyperaldosteronism, we observed that many had been previously diagnosed with OSA. We, therefore, hypothesized that the 2 diseases may be mechanistically related, that is one contributing to the other. Our center noted increased aldosterone excretion in patients with resistant hypertension who had symptoms of OSA.19 We then showed that a significant correlation exists between plasma aldosterone concentration (PAC) and OSA severity in patients with resistant hypertension but not in normotensive control subjects.14 Although we cannot directly infer causality from these studies, these results are consistent with the hypothesis that aldosterone excess may contribute to worsening severity of OSA. To gain further insight into the association among resistant hypertension, OSA, and hyperaldosteronism, we evaluated the relationship between aldosterone levels and OSA severity in patients with resistant hypertension with and without hyperaldosteronism.
METHODS
Study Participants
The study was approved by the University of Alabama at Birmingham Institutional Review Board, and all subjects provided written informed consent prior to study participation. We prospectively enrolled 109 consecutive patients referred to the University of Alabama at Birmingham Hypertension Clinic for evaluation of resistant hypertension over a 6-year period (June 2002 to June 2008). The first 76 subjects of this current analysis were included in our prior evaluation relating OSA severity to PAC.14
Resistant hypertension was defined as a blood pressure that remained above target values despite the concurrent use of 3 antihypertensive agents, prescribed at optimal dose amounts.12 All patients had been on a stable antihypertensive regimen for at least 4 weeks prior to evaluation. No antihypertensive medications were discontinued, except for spironolactone, eplerenone, amiloride, or triamterene, for which hydrochlorothiazide was substituted for at least 6 weeks prior to evaluation. Serum potassium levels were corrected as necessary with oral supplementation to maintain a serum level of greater than 3.5 mmol/L. Secondary causes of hypertension other than hyperaldosteronism, such as renovascular disease, pheochromocytoma, and Cushing syndrome, were excluded by laboratory analysis or suitable imaging, as clinically indicated. Patients with a history of recent myocardial infarction (within 6 months prior to study), congestive heart failure, chronic kidney disease (creatinine clearance [Cr Cl] < 60 mL/min per 1.73m2), or long-term corticosteroid therapy were excluded from enrollment.
Blood Pressure Measurements
Seated clinic blood pressure was measured manually using a mercury sphygmomanometer and an appropriate-sized cuff after 5 minutes of rest, according to standard guidelines. All patients underwent 24-hour ambulatory blood pressure monitoring (Spacelabs model 90207, Spacelabs Healthcare, Issaqhah, WA, or Suntech Medical model 0413, Morrisville, NC). The monitor recorded systolic and diastolic blood pressure every 20 minutes during the daytime (06:00 to 22:00) and every 30 minutes at night (22:00 to 06:00). The ambulatory data were included in the analysis if the monitoring period was at least 20 hours and there were no period of 2 hours or greater without measurements.
Biochemical Evaluation
All subjects underwent biochemical evaluation in an ambulatory outpatient setting. Initial evaluation included an early morning (07:00-09:00) plasma renin activity (PRA) and PAC and a 24-hour urine collection for sodium (UNa), Cl Cr, and aldosterone (UAldo) on the subjects' ad-lib diet.
Based on the biochemical results, patients were characterized according to aldosterone status. High-aldosterone status (H-Aldo) was defined as a suppressed PRA (< 1.0 ng·mL-1·h-1) and elevated UAldo (≥ 12 μg/24h). Normal-aldosterone status (N-Aldo) was defined as UAldo less than 12 μg/24h, the absence of suppressed PRA (≥ 1.0 ng·mL-1·h-1), or both. A cutoff value of UAldo of 12 μg/24h or greater was used to separate high and normal aldosterone.20,21
PAC (reference range, 4.0 to 31.0 ng/dL; to convert to picomoles per liter, multiply by 27.74), PRA (reference range 1.31 to 3.95 ng·mL-1·h-1) and UAldo (reference range 2 to 16 μg/24h) were measured at commercial laboratories (Quest Diagnostics; Atlanta, GA, or Mayo Medical Laboratories, Rochester, MN) using standard techniques. The aldosterone to renin ratio (ARR) was calculated as PAC divided by PRA.
OSA Screening
All subjects with resistant hypertension underwent full-night, attended, diagnostic polysomnography. Polysomnography evaluation included airflow monitoring with thermocouple and/or nasal pressure, respiratory effort using piezo belts at the chest and abdominal positions, oxygen saturation using pulse oximetry, heart rate using a 2-lead electrocardiogram, electroencephalogram (C4-A1, C3-A2, O2-A1, O1-A2), submental and tibial electromyograms, and bilateral electrooculograms. Apnea was defined as a cessation in airflow of at least 10 seconds. Hypopnea was defined as a reduction in the amplitude of airflow at least 30% for at least 10 second, followed by either a decrease in oxygen saturation of 4%, or signs of physiologic arousal (at least 3 s of α activity). The apnea-hypopnea index (AHI) was calculated as the total number of apneas plus hypopneas divided by the hours of sleep. Sleep was staged according to criteria of Rechtschaffen and Kales.22 The percentage of sleep time spent with an oxygen saturation less than 90%, the hypoxic index (HI), was also determined. Sleep stage and nocturnal events were scored by a registered polysomnographic technologist. The scoring of all studies was confirmed by an American Board of Sleep Medicine Diplomat blinded to the biochemical assessment. OSA was defined as an AHI of at least 5 per hour.22 Patients with significant central sleep apnea (> 5% of events) were excluded. No patient had Cheyne-Stokes respiration.
Statistical Evaluation
Values are reported as mean ± SD or median (range), where applicable. Variables were assessed for normality by Kolmogorov-Smirnov test. Values between groups were compared by t-test, Kruskal-Wallis (KW) test, χ2 test, or Fisher exact test, where appropriate. Correlation between AHI with PAC, UAldo, PRA, UNa, blood pressure, neck circumference, and body mass index (BMI) was evaluated by Pearson r or Spearman ρ correlation, where applicable. Two-tailed p values are provided for descriptive purposes, with p < 0.05 considered significant. Data analysis was carried out using JMP version 8.0 (SAS Institute Inc; Cary, NC).
RESULTS
Patient Characteristics
In total, 109 patients (50% men) with resistant hypertension were evaluated (Table 1). Thirty-one (28%) of the patients were classified as H-Aldo and 78 (72%) as N-Aldo. OSA was diagnosed in 84 (77%) patients: 26 (84%) of H-Aldo and 58 (74%) of N-Aldo patients. OSA and hyperaldosteronism were coexistent in 24% of study patients.
Table 1.
Demographic and biochemical values for all of the subjects and for subjects according to aldosterone status
| Parameter | All subjects |
Aldosterone level |
|
|---|---|---|---|
| Total n = 109a | High n = 31 | Normal n = 78 | |
| Men | 50.5 | 61.3 | 46.1 |
| African American | 47.2 | 45.2 | 48.1 |
| Age, y | 55.9 ± 9.1 | 56.4 ± 7.8 | 55.7 ± 9.7 |
| BMI, kg/m2 | 33.3 ± 6.3 | 34.1 ± 5.5 | 33.0 ± 6.6 |
| Neck, cm | 41.5 ± 4.3 | 42.0 ± 3.8 | 41.3 ± 4.5 |
| Duration of HTN, y | 17.0 ± 10.2 | 18.3 ± 10.8 | 16.5 ± 10.0 |
| Medicines, no. | 4.3 ± 1.0 | 4.3 ± 1.0 | 4.3 ± 1.0 |
| Serum potassium, mmol/L | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.4 |
| PAC, ng/dL | 11.9 ± 7.1 | 16.3 ± 8.1 | 10.2 ± 5.9b |
| PRA, ng/mL/h | 2.4 ± 3.8 | 0.6 ± 0.1 | 3.1 ± 4.2b |
| ARR | 14.4 ± 16.3 | 28.8 ± 21.2 | 8.6 ± 9.1b |
| UAldo, μg/24h | 13.0 ± 9.0 | 19.1 ± 7.2 | 10.5 ± 8.5b |
| UNa, mmol/24h | 186.2 ± 99.3 | 200.5 ± 105.6 | 180.7 ± 97.0 |
| CrCl, mL/min | 116.0 ± 43.8 | 127.0 ± 43.4 | 111.7 ± 43.5 |
Data are shown as mean ± SD, except men and African American race, which are percentages. BMI refers to body mass index; HTN, hypertension; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, aldosterone renin ratio; UAldo, 24-hour urinary aldosterone excretion; UNa, 24-hour urinary sodium; Cr Cl, creatinine clearance.
n = 109, except race, serum potassium, UNa (108); BMI (107); neck, CrCl (104), duration of HTN (101).
p < 0.0001 compared to High-Aldosterone group.
Patients took an average of 4.3 ± 1.0 antihypertensive medications, which included a diuretic (91%), a β-receptor antagonist (76%), a calcium channel blocker (75%), an angiotensin-converting enzyme inhibitor (61%) or an angiotensin receptor blocker (58%), a α-receptor–blocking agent (9%), and other antihypertensive medications (42%). There were no significant differences in the number or type of prescribed antihypertensive medications between the H-Aldo and N-Aldo groups. There was no significant statistical difference in BMI, sex, age, and Cr Cl between the H-Aldo and N-Aldo groups. There were no statistically significant differences noted in diastolic blood pressure or heart rate (at any time measure) between the H-Aldo and N-Aldo groups. However, all systolic blood pressure measures including office and ambulatory blood pressure monitoring values (daytime, nighttime, and 24-h) were significantly higher in the H-Aldo compared with the N-Aldo group (p < 0.005).
Relationship Between OSA and Aldosterone Concentration
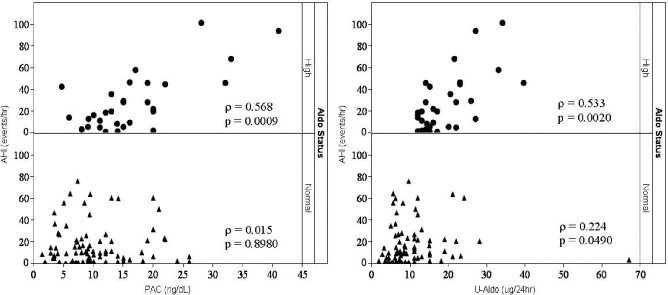
Severity of OSA was greater in the H-Aldo versus The N-Aldo group. Median AHI and HI were significantly higher in the H-Aldo compared with the N-Aldo group (19.9 vs 10.0 events/h, KW p = 0.0491; and 7.0% vs 3.0%, KW p = 0.0041, respectively)(Table 2). Mean oxygen saturation (Mean O2) was lower in the H-Aldo compared with the N-Aldo group (93.2% vs 94.8%, KW p = 0.0185). PAC and UAldo were positively and significantly correlated with AHI in the H-Aldo group (Spearman ρ = 0.568, p = 0.0009; ρ = 0.533, p = 0.002, respectively). Although to a lesser degree, UAldo also correlated with AHI in the N-Aldo group (ρ = 0.224, p = 0.049). There was no significant correlation between PAC and AHI in the N-Aldo group (ρ = 0.015, p = 0.898) (Figure 1).
Table 2.
Polysomnography parameters for all subjects and for subjects according to aldosterone status
| All subjects |
Aldosterone Status |
|||||
|---|---|---|---|---|---|---|
| Total n = 109 |
High n = 31 |
Normal n = 78 |
||||
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | |
| AHI, events/h | 20.4 ± 21.7 | 11.5 (0.2-101.6) | 27.6 ± 26.2 | 19.9 (0.9-101.6) | 17.5 ± 19.0a | 10.0 (0.2-75.9) |
| HI, % | 7.4 ± 11.1 | 3.3 (0.0-54.7) | 10.3 ± 12.1 | 7.0 (0.1-51.7) | 6.2 ± 10.6b | 3.0 (0.0-54.7) |
| Mean SaO2, % | 94.6 ± 2.2 | 94.4 (89.6-99.8) | 93.8 ± 2.3 | 93.5 (89.6-99.3) | 95.0 ± 2.1c | 95.0 (89.6-99.8) |
| SaO2 nadir, % | 79.8 ± 8.77 | 82.1 (47.5-92.0) | 78.6 ± 10.1 | 82.9 (47.5-91.0) | 80.3 ± 8.2 | 81.8 (61.3-92.0) |
AHI refers to apnea-hypopnea index; HI, hypoxic index, defined as an SaO2 < 90%; SaO2, oxygen saturation.
p < 0.05 compared with the high-aldosterone group;
p < 0.01 compared with the high-aldosterone group;
p < 0.05 compared with the high-aldosterone group.
Figure 1.
Correlation between apnea-hypopnea index (AHI), plasma-aldosterone concentration (PAC), and 24-h urinary aldosterone excretion (UAldo) in high (H-Aldo) and normal (N-Aldo) aldosterone subjects
PAC and UAldo were positively and significantly correlated with AHI in the H-Aldo group (Spearman's ρ = 0.568, p = 0.0009; Spearman's ρ = 0.533, p = 0.0020, respectively). To a lesser degree, UAldo was correlated with AHI in the N-Aldo subjects (Spearman's ρ = 0.224, p = 0.0490). PAC was not significantly correlated with AHI in the N-Aldo group (Spearman's ρ = 0.015, p = 0.8980).
Relationship Among OSA, Aldosterone, and Sex
OSA was more common in male versus female patients (91% vs 63% respectively, p = 0.0005). Overall, men presented with significantly higher median AHI compared to women (20.3 vs 8.1 events/h, KW p < 0.0001). The sex difference in terms of severity of OSA was most pronounced in the H-Aldo subjects. In H-Aldo subjects, the median AHI was 29.8 in men versus 11.4 events/h in women (KW p = 0.0106), compared to 16.2 versus 6.9 events/h in the N-Aldo men versus women (KW p = 0.0018). An independent interaction between sex and OSA severity was tested for, but was not statistically present.
DISCUSSION
Previous studies from our center have noted an association among resistant hypertension, OSA, and aldosterone. In patients with resistant hypertension, aldosterone excretion is increased in patients with symptoms of OSA.19 We also have noted that PAC is significantly correlated with OSA severity.14 This current analysis extends these findings by determining the prevalence and severity of OSA according to aldosterone status in patients with resistant hypertension. OSA is extremely common in patients with resistant hypertension regardless of aldosterone status, but the severity is significantly worse in the H-Aldo patients. Furthermore, the severity of OSA is strongly correlated with the degree of aldosterone excess in the H-Aldo but not the N-Aldo patients.
Inappropriate sodium and fluid retention characterize hyperaldosteronism, including in patients with resistant hypertension.7 Displacing fluid from the lower extremities to the neck results in upper airway narrowing, suggesting that upper airway edema may contribute importantly to the pathogenesis of OSA.23 A recent study of patients with diastolic heart failure, systemic hypertension, and OSA showed that diuretic treatment significantly improved upper airway caliber and OSA, further implicating pharyngeal edema as a cause of upper airway collapse during sleep.24 In addition, volumetric analysis techniques with magnetic resonance imaging demonstrate that the volume of the tongue and lateral walls independently increase risk of OSA.25 We hypothesize that intravascular fluid retention related to hyperaldosteronism and resultant pharyngeal edema contribute to the greater severity of OSA observed in patients with resistant hypertension and hyperaldosteronism. This hypothesis is supported by the findings of a recent study from our center showing that patients with resistant hypertension displayed evidence of intravascular volume expansion when compared with normotensive control subjects.17
Impairment of ventilatory-control mechanisms also contribute to modulation of pharyngeal collapsibility during sleep.26 This suggests a role for central nervous system input, which may be influenced by humoral factors27 in addition to central and peripheral chemoreceptor activation.28–29 In animal models, aldosterone acts centrally to increase brain renin-angiotensin system activity, oxidative stress, and sympathetic drive.30 This aldosterone-induced activation of central receptors may result in dysregulation of normal central breathing mechanisms and potentially contribute to the association between excess aldosterone and severity of sleep apnea.
OSA may lead to increased aldosterone release via stimulation of the renin-angiotensin system, as suggested by a previous study that demonstrated that blood pressure reduction following continuous positive airway pressure treatment correlated with reduction in PRA and angiotensin II levels.31 Furthermore, recent data suggest an interaction between angiotensin converting enzyme gene polymorphisms and OSA as a cause of hypertension.32 Thus, it is possible that OSA stimulates aldosterone secretion, which in turn leads to excess fluid retention, upper airway narrowing, and worsening of OSA. Such an occurrence could then result in a vicious cycle of OSA-induced aldosteronism and aldosterone-induced worsening of OSA. Confirmation of either or both components of this possible cycle requires further investigation.
Strengths of the current study include its prospective design, large sample size, and assessment of OSA severity by attended fully diagnostic polysomnography. Potential limitations include the observational nature of the study and the fact that biochemical evaluation were conducted while patients were being maintained on their prescribed antihypertensive medication (except spironolactone, eplerenone, amiloride, or triamterene), although prior studies indicated that although some classes of agents predictably increase PRA (i.e., acetylcholine esterase inhibitor inhibitors, angiotensin receptor blockers, and diuretics), most do not significantly alter aldosterone levels.33,34
In conclusion, we confirm that OSA is highly prevalent in patients with resistant hypertension and further characterize the emerging relationship among resistant hypertension, OSA, and aldosterone. In patients with resistant hypertension, the severity of OSA is greater in those patients with hyperaldosteronism and correlates with the degree of aldosterone excess. The findings of a high prevalence of OSA (77%), hyperaldosteronism (28%), and coexistent disease (24%) in patients with resistant hypertension emphasizes the clinical importance of considering the likelihood of patients presenting with resistant hypertension of having OSA, hyperaldosteronism, or both.
Perspectives
The positive relationship between hyperaldosteronism and severity of OSA observed in the current analysis supports the hypothesis that aldosterone excess contributes to the development of OSA. The results highlight the increased likelihood of hyperaldosteronism and OSA coexisting in patients with resistant hypertension and may explain, at least in part, the high prevalence of OSA in patients with resistant hypertension.
DISCLOSURE STATEMENT
This was not an industry support study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank David Moore, RPSGT, and Melissa Butler, RPSGT, for assistance in conducting the overnight sleep studies.
Funding/Support: David A. Calhoun: NHLBI SCCOR P50 HL077100, and RO1-HL79040; Krishna K. Gaddam: T32 HL007457.
ABBREVIATIONS
- ACE
- angiotensin-converting enzyme
- AHI
- apnea-hypopnea index
- ARB
- angiotensin receptor blocker
- ARR
- aldosterone to renin ratio
- BMI
- body mass index
- CPAP
- continuous positive airway pressure
- Cr Cl
- creatinine clearance
- H-Aldo
- high-aldosterone status
- HI
- hypoxic index
- HTN
- hypertension
- Mean O2
- mean oxygen saturation
- N-Aldo
- normal-aldosterone status
- OSA
- obstructive sleep apnea
- PAC
- plasma aldosterone concentration
- PRA
- plasma renin activity
- UAldo
- 24-hour urinary aldosterone excretion
- UNa
- 24-hour urinary sodium
REFERENCES
- 1.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea. Implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Kuniyoshi FHS, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–6. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Lon-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, White DP, Amin R, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee; Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep Apnea and Cardiovascular Disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing Council. Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Kales A, Bixler EO, Cadieux RJ, et al. Sleep apnea in hypertensive population. Lancet. 1984;2:1005–8. doi: 10.1016/s0140-6736(84)91107-3. [DOI] [PubMed] [Google Scholar]
- 10.Lavie P, Ben-Yosef R, Rubin AE. Prevalence of sleep apnea syndrome among patients with essential hypertension. Am Heart J. 1984;108:373–6. doi: 10.1016/0002-8703(84)90628-8. [DOI] [PubMed] [Google Scholar]
- 11.Williams AJ, Houston D, Finberg S, Lam C, Kinney JL, Santiago S. Sleep apnea syndrome and essential hypertension. Am J Cardiol. 1985;55:1019–22. doi: 10.1016/0002-9149(85)90738-6. [DOI] [PubMed] [Google Scholar]
- 12.Calhoun DA, Jones D, Textor S, et al. Resistant Hypertension: Diagnosis, Evaluation, and Treatment: A Scientific Statement From the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 13.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–9. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 15.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–26. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17:349–52. doi: 10.1038/sj.jhh.1001554. [DOI] [PubMed] [Google Scholar]
- 17.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, et al. Characterization of resistant hypertension. Association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159–64. doi: 10.1001/archinte.168.11.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–6. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112–7. doi: 10.1378/chest.125.1.112. [DOI] [PubMed] [Google Scholar]
- 20.Mattsson C, Young WF., Jr Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006;2:198–208. doi: 10.1038/ncpneph0151. [DOI] [PubMed] [Google Scholar]
- 21.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:3266–81. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human sleep. Los Angeles: Brain Information Service/ Brain Research Institute, UCLA; 1968. [Google Scholar]
- 23.Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–72. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–6. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 25.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 26.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea. Pathophysiology and diagnosis. Chest. 2007;132:325–7. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolk R, Johnson BD, Somers VK. Leptin and the ventilatory response to exercise in heart failure. J Am Coll Cardiol. 2003;42:1644–9. doi: 10.1016/j.jacc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol. 1989;67:2095–100. doi: 10.1152/jappl.1989.67.5.2095. [DOI] [PubMed] [Google Scholar]
- 29.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–6. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–74. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 31.Møller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–80. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 32.Boström KB, Hedner J, Melander O, et al. Interaction between the angiotensin-converting enzyme gene insertion/deletion polymorphism and obstructive sleep apnoea as a mechanism for hypertension. J Hypertens. 2007;25:779–83. doi: 10.1097/HJH.0b013e328017f6d5. [DOI] [PubMed] [Google Scholar]
- 33.Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis. 2001;37:669–705. doi: 10.1016/s0272-6386(01)80117-7. [DOI] [PubMed] [Google Scholar]
- 34.Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma rennin activity ratio in primary aldosteronism. Hypertension. 2002;40:897–902. doi: 10.1161/01.hyp.0000038478.59760.41. [DOI] [PubMed] [Google Scholar]