Abstract
X-chromosome linked inhibitor of apoptosis, XIAP, is cellular caspase inhibitor and a key regulator of apoptosis. We and others have previously shown that XIAP expression is regulated primarily at the level of protein synthesis; the 5′ untranslated region (UTR) of XIAP mRNA contains an Internal Ribosome Entry Site (IRES) that supports cap-independent expression of XIAP protein during conditions of pathophysiological stress, such as serum deprivation or gamma irradiation. Here, we show that XIAP is encoded by two distinct mRNAs that differ in their 5′ UTRs. We further show that the dominant, shorter, 5′ UTR promotes a basal level of XIAP expression under normal growth conditions. In contrast, the less abundant longer 5′ UTR contains an IRES and supports cap-independent translation during stress. Our data suggest that the combination of alternate regulatory regions and distinct translational initiation modes is critical in maintaining XIAP levels in response to cellular stress and may represent a general mechanism of cellular adaptation.
INTRODUCTION
The X chromosome-linked inhibitor of apoptosis, XIAP (also known as BIRC4), is a key intrinsic regulator of apoptosis, primarily by virtue of its ability to bind to and inhibit both initiator and effector caspases (1). Dysregulation of XIAP has been shown to correlate with various human pathologies; loss of XIAP was shown to sensitize cells to inappropriate cell death, as is observed in X-linked lymphoproliferative syndrome (2), while overexpression of XIAP is seen in a number of human cancers and correlates with enhanced chemo- or radiation resistance (3–6). Indeed, inhibition of XIAP expression by small molecule inhibitors has shown therapeutic promise in clinical trials (7,8).
Although the cellular levels of XIAP are regulated by several independent mechanisms, the predominant regulation appears to be the control of XIAP mRNA translation (9). Translation of XIAP is mediated by a 162-nt Internal Ribosome Entry Site (IRES) element located within its 1.7-kb-long 5′ untranslated region that allows synthesis of XIAP protein during cellular stress and apoptosis (10–12). IRES elements have emerged as important regulators of selective mRNA translation, in particular under conditions of reduced global, cap-dependent translation, such as hypoxia, endoplasmic reticulum stress or serum deprivation (13). Such conditions are frequently experienced by cancer cells whose survival thus relies on IRES-dependent translation of key pro-angiogenic, hypoxia-response and survival mRNAs (13–15). In contrast to cap-dependent initiation of translation, which requires recognition of an mRNA by the cap-dependent initiation complex eIF4F, IRES-containing mRNAs are believed to recruit ribosomes directly to the vicinity of the start codon, thus bypassing the requirement for cap-binding and movement to the initiation codon (16). There are some cellular mRNAs, however, including those encoding vascular endothelial growth factor (VEGF)-A, HIF1α (17) and Bcl-2 (18), that appear to use a dual mechanism of translation initiation employing either the cap or an IRES.
Here, we show that translation of XIAP is controlled by alternative noncoding regions. The shorter, 323-nt 5′ UTR is present in the dominant XIAP mRNA variant that is responsible for the high level of XIAP expression observed under normal growth conditions. In contrast, the longer 1.7-kb 5′ leader is present in the less-abundant mRNA variant and contains an IRES element that mediates efficient translation of XIAP under conditions of reduced global protein synthesis. Our data suggest that the combination of alternate regulatory regions and distinct translational initiation modes is critical in maintaining XIAP levels in response to cellular stress.
MATERIALS AND METHODS
Cell culture, expression constructs and transfection
Human embryonic kidney (HEK293) cells were maintained in standard conditions in serum- and antibiotic-supplemented Dulbecco's modified Eagle's medium (DMEM). Reporter constructs monoCAT, mono-L-CAT, pBic and pBic-XIAP (here termed pBic-L) were previously described (10,19). The shorter 5′ UTR of XIAP was RT-PCR amplified from total RNA extracted from HEK293 cells using specific primers (see Supplementary Table for primer sequence information) and inserted into monocistronic or bicistronic reporter plasmids to create mono-S-CAT and pBic-S, respectively. Hairpin constructs (denoted by HP) were created by insertion of a double-stranded DNA fragment containing an internal PmeI site into the NheI site immediately 5′ of the UTR in each construct. The hairpin element was created by annealing oligonucleotides: 5′-CTAGCGTTTAAACCCGGCCGGCCGGCCGGCCGGCCAAAGGCCGGCCGGCCGGCCGGCCGG and 5′-CTAGCCGGCCGGCCGGCCGGCCGGCCTTTGGCCGGCCGGCCGGCCGGCCGGGTTTAAACG. The presence of the hairpin was verified by restriction digest and sequencing.
For DNA transfections, HEK293 cells were seeded 24 h prior to transfection at 3 × 105 cells per well, and transfected with 1.5 µg of construct DNA per well using Lipofectamine 2000 (Invitrogen). Cells were harvested 48 h later in either CAT Lysis buffer (Roche) for protein analysis, or in Lysis Buffer (Stratagene) for RNA analysis. For synthetic RNA transfections, HEK293 cells were seeded 24 h prior to transfection at 3 × 105 cells per well. Transient transfections of 1 µg synthetic RNA per well were performed using Lipofectamine™ 2000. Cells were harvested 4 h later as described above. For siRNA experiments, double-stranded siRNAs targeting the longer 5′ UTR or the shorter 5′ UTR were designed, and nontargeting siRNA was used as a negative control (Qiagen). As a positive control, SMARTpool siRNA targeting the 3′UTR of XIAP was used (Dharmacon). HEK293 cells were seeded as for DNA transfections and transfected with 20 nM siRNA, using DharmaFECT™ 1 (Dharmacon). Forty-eight hours later, cells were harvested in radioimmunoprecipitation assay (RIPA) buffer for protein analysis, or for RNA in Stratagene Lysis Buffer.
For serum deprivation experiments, 48 h after siRNA transfection the media was removed from the wells, and each well was carefully rinsed with warm phosphate-buffered saline (PBS). To the control wells, complete DMEM was added, while serum-free DMEM was added to the wells to be serum starved. For polysome profiling, this took place in 15-cm dishes. After 24 h, media was removed, and cells were harvested in RIPA buffer for protein quantification, or in CHX RNA Lysis Buffer [0.3 M NaCl, 15 mM MgCl2, 15 mM Tris pH 7.4, 1% Triton-X100, 100 U/ml RNAsin, 0.1 mg/ml Cycloheximide (CHX)] for polysome profiling.
Reporter activity assays
Cell lysates harvested in CAT lysis buffer (Roche) were tested in three different assays, depending on the reporter construct used. In lysates from cells transfected with bicistronic or monocistronic DNA constructs, the levels of neomycin phosphotransferase II (NEO) and chloramphenicol acetyltransferase (CAT) were determined. Lysates from cells transfected with bicistronic DNA were also tested for levels of βgalactosidase (βgal), while in the lysates from cells transfected with reporter RNA, only the levels of CAT were determined. βGal activity was assessed using an ONPG colorimetric assay as described (20). CAT and NEO expression were quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (CAT, Roche; NEO, Agdia). To correct for transfection efficiency differences between the constructs, the expression of CAT and βgal reporter genes was normalized to the levels of NEO, which is expressed from the same plasmid but from an independent promoter. If monocistronic plasmids were used, the amount of CAT reporter protein produced was corrected to NEO protein, and CAT and NEO RNA levels. Spurious splicing and potential cryptic promoter activity that could arise with the pBGAL/XIAP/CAT bicistronic vector has been excluded as previously addressed (21).
In vitro transcription
DNA templates for the synthesis of the reporter RNAs were generated from a corresponding 5′UTR-containing monocistronic plasmid by PCR. The 5′ primers incorporated the T7 promoter sequence to allow for RNA transcription; the reverse primer included the 3′- end of the CAT gene, as well as 31 T’s, which were added to the end of the polymerase chain reaction (PCR) product to provide a polyA tail. The PCR products were purified by agarose gel electrophoresis. In vitro transcription and capping were performed with the mMessage mMachine kit (Ambion), as per the manufacturer’s protocol, and the newly synthesized RNA was purified using a Megaclear column (Ambion).
Quantitative reverse transcriptase-PCR
To measure relative mRNA expression, total RNA was isolated from HEK293 cells following indicated treatment (Absolutely RNA miniprep, Stratagene) and cDNA generated (First-Strand cDNA synthesis kit, GE Biosciences). Quantitative PCR was performed on a Stratagene Mx3005P™ real-time thermocycler using 1 µg of total RNA together with SYBRGreen (Qiagen) and gene-specific primers for XIAP coding region, shorter 5′ UTR, longer 5′ UTR, CAT, NEO, βgal, GAPDH and β-actin (see Supplementary Table for primer sequence information). Relative expression levels were determined using the standard curve method. Controls lacking reverse transcriptase (RT) demonstrated no significant genomic DNA amplification (>10 cycle difference). Semi-quantitative PCR was performed using Taq polymerase (Invitrogen) in DNA Engine Dyad® Peltier Thermal cycler (MJ Research) and PCR products were resolved on a 1.5% agarose gel, visualized with EtBr, and quantified using Odyssey densitometry software (Licor). The RT-PCR efficiency controls were done by performing PCR directly on construct DNA (mono-L-CAT and mono-S-CAT), or on cDNA obtained from synthetic longer and shorter 5′ UTR-containing RNAs.
Western blot analysis
Cells were lysed in RIPA buffer for 30 min at 4°C, followed by centrifugation at 10 000 g to remove debris. Equal amounts of protein were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to PVDF membranes using a semi-dry transfer protocol and probed with antibodies to XIAP [rabbit αRIAP3, (22)], GAPDH (BD Laboratories), β-actin (Sigma), rpS6 and P-rpS6 (Cell Signalling Technology). Membranes were then incubated with species-specific horseradish peroxidase-conjugated secondary antibody (GE Biosciences) followed by detection with ECL Plus substrate (GE Biosciences) and were quantified using Odyssey densitometry software (Licor).
Polysome profiling
HEK293 cells from three 15-cm plates per condition were lysed in cold polysome lysis buffer (15 mM Tris–HCl, pH 7.4, 15 mM MgCl2, 300 mM NaCl, 1% (v/v) Triton X-100, 0.1 mg/ml cycloheximide, 100U /ml RNasin). Equal OD254 units were loaded onto 10–50% linear sucrose gradients and centrifuged at 39 000 r.p.m. for 90 min at 4°C. Gradients were fractionated from the top (Densi-Flow, Labconco) and RNA/protein was monitored at 254 nm using a HPLC system (Åkta Explorer, GE Biosciences). One-milliliter fractions were collected and flash-frozen in liquid nitrogen. After thawing on ice, the fractions were spiked with 10 μl of a 10 ng/μl solution of in vitro transcribed CAT RNA, to ensure technical consistency in RNA extraction. RNA was isolated from individual fractions by proteinase K digestion followed by phenol/chloroform extraction and was recovered by ethanol precipitation. Equal volumes of RNA from each fraction were used to generate cDNA using oligo-dT primers and a reverse transcription kit (First-Strand cDNA synthesis kit, GE Biosciences). PCR primers specific for XIAP isoforms, CAT, GAPDH or β-actin (Supplementary Table) were used to amplify messages using a quantitative PCR as described above.
Statistical analysis
Data are expressed as mean ± standard deviation. Unless otherwise stated, all results reported here were obtained through minimum three independent experimental replications. For all reporter assays, each independent replicate consisted of biological triplicates. Unpaired t-test was used to determine data significance.
RESULTS
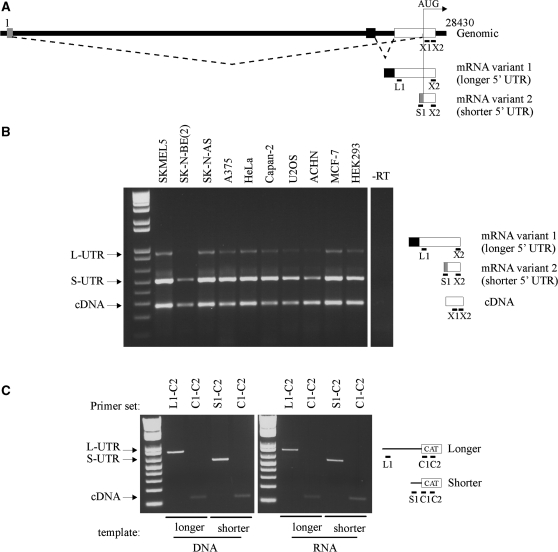
The XIAP 5′ UTR is encoded by two exons and is ∼1.7 kb long (23). The 5′ UTR contains an IRES element that is required for the expression of XIAP protein during various pathophysiological conditions (6,10–12). Recently, it has been suggested that splicing occurs within the XIAP 5′UTR creating an alternative 5′ UTR that lacks an IRES (24–26) (Figure 1A). We used semi-quantitative multiplex PCR to analyze the expression pattern and abundance of both XIAP mRNA isoforms in a panel of 10 different cell lines (Figure 1B). Both mRNA isoforms could be detected in all cell lines tested. We noted that the relative intensity of the bands representing each transcript was fairly consistent over the cell line panel. Interestingly, the band representing the shorter 5′ UTR variant was always much more intense (∼10-fold) than the band representing the longer 5′ UTR variant, suggesting that the shorter 5′ UTR is more abundant than the longer UTR variant. To eliminate the possibility that this discrepancy is due to different efficiencies of the PCR primers, or the inability of RT to efficiently transcribe the structured IRES-containing 5′ UTR (19), we performed control PCR reactions on either DNA templates, or on chimeric, in vitro synthesized RNA templates in which the XIAP coding region was replaced with the sequence of the chloramphenicol acetyl transferase (CAT) gene. In all cases, the relative intensities of shorter and longer 5′ UTR variants were virtually identical (Figure 1C). We therefore conclude that the relative transcript abundance indicated by semi-quantitative PCR is correct; the longer XIAP 5′ UTR variant is present at much lower levels than the shorter variant.
Figure 1.
The two variants of the human XIAP mRNA are present in a number of cell lines. (A) Schematic of the genomic arrangement of XIAP, at Xq25. The numbering of the genomic sequence is arbitrary, where 1 is the first nucleotide of the shorter 5′ UTR. Exons are indicated as boxes; an arrow (AUG) indicates the location of the start codon. The splicing is indicated by dotted lines. Positions of PCR primers are indicated by black bars below the exons. (B) Total RNA was extracted from indicated cell lines, reverse-transcribed with a random hexamer RT primers, and XIAP coding region (cDNA), longer (L) and shorter (S) 5′ UTRs were amplified in a multiplex PCR reaction using specific forward primers, and a common reverse primer, as described in ‘Material and Methods’ section. (C) The efficiency of PCR primers was tested as in (B), using monocistronic DNA constructs containing either the longer or the shorter 5′ UTR as a template (left), or using equal amounts of cDNA template reverse-transcribed from synthetic monocistronic RNAs containing either the longer or the shorter 5′ UTR. In place of the XIAP coding region primers, primers specific to the CAT coding sequence were used.
The shorter and longer XIAP 5′ UTR variants support distinct modes of translation
The existence of two XIAP mRNA isoforms that differ in their 5′ UTRs suggests that the distinct UTRs might play a role in the translational control of XIAP expression. To verify this hypothesis, we generated a set of reporter constructs; each XIAP 5′ UTR variant was cloned into monocistronic (monoCAT) and bicistronic (pBic) reporter plasmids (Figures 2 and 3). Transient transfections with these constructs were carried out in HEK293 cells, and the amount of each reporter gene product present was determined using enzyme activity assays (for βgal) and ELISAs for CAT and neomycin phosphotransferase II (NEO) gene products. The monocistronic assays reflect the sum of cap-dependent and IRES-dependent translation, while the bicistronic assays provide information about cap-independent, IRES-dependent translation.
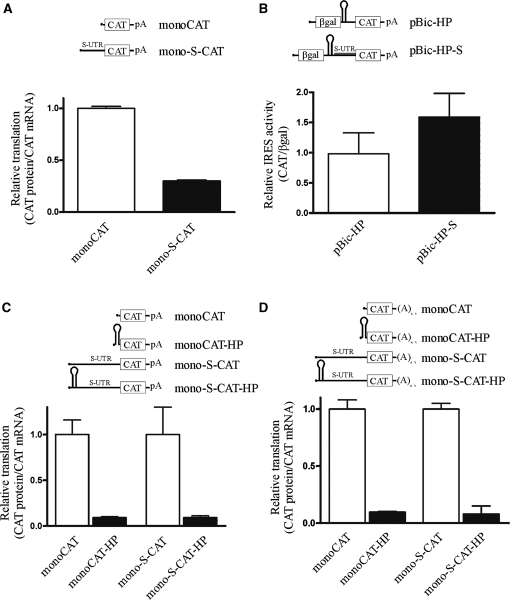
Figure 2.
The longer XIAP 5′ UTR supports cap-independent translation. (A) HEK293 cells were transfected with indicated monocistronic constructs and assayed for reporter protein and mRNA levels. Relative translation is expressed as a ratio of CAT protein to CAT mRNA; expression of monoCAT was set to 1. (B) HEK293 cells were assayed following transient transfection of an indicated bicistronic DNA reporter construct. IRES activity is expressed as the ratio of CAT expression over β-gal activity; expression of pBic-HP was set to 1. (C) HEK293 cells were transfected with indicated monocistronic constructs and assayed for reporter protein and mRNA levels. Relative translation is expressed as a ratio of CAT protein to CAT mRNA; expression of monoCAT was set to 1. (D) HEK293 cells were transfected with indicated synthetic RNA constructs and assayed for reporter protein and mRNA levels. Relative translation is expressed as a ratio of CAT protein to CAT mRNA; expression of monoCAT was set to 1.
Figure 3.
The shorter XIAP 5′ UTR does not support cap-independent translation. (A) HEK293 cells were transfected with indicated monocistronic constructs and assayed for reporter protein and mRNA levels. Relative translation is expressed as a ratio of CAT protein to CAT mRNA; expression of monoCAT was set to 1. (B) HEK293 cells were assayed following transient transfection of an indicated bicistronic DNA reporter construct. IRES activity is expressed as the ratio of CAT expression over β-gal activity; expression of pBic-HP was set to 1. (C) HEK293 cells were transfected with indicated monocistronic constructs and assayed for reporter protein and mRNA levels. Relative translation is expressed as a ratio of CAT protein to CAT mRNA; expression of monoCAT was set to 1. (D) HEK293 cells were transfected with indicated synthetic RNA constructs and assayed for reporter protein and mRNA levels. Relative translation is expressed as a ratio of CAT protein to CAT mRNA; expression of monoCAT was set to 1.
As reported previously (10), the longer XIAP 5′UTR does not repress translation of a downstream cistron in a monocistronic context (Figure 2A). When expressed in the context of a bicistronic DNA reporter, the longer 5′ UTR exhibited more than 50-fold increase in CAT translation, compared to a reporter plasmid lacking the 5′ UTR (Figure 2B). Since the validity of XIAP IRES in this assay has been questioned (24,25,27), we performed two additional experiments to validate XIAP 5′ UTR as a bona fide IRES. First, we used a monocistronic construct that incorporates a strong hairpin structure (ΔG = −38 kcal/mol) immediately upstream of the UTR. This hairpin is quite efficient at impeding cap-dependent translation, as evidenced by the dramatic reduction in translation of the empty control vector following hairpin insertion (Figure 2C). However, the insertion of a hairpin in the longer XIAP UTR-containing vector had no effect on the expression of CAT reporter gene. This indicates that nearly all translation driven by this UTR is IRES-mediated. Second, we confirmed the IRES activity of the longer UTR by direct RNA transfection. The capped and polyadenylated reporter RNA was in vitro transcribed from the DNA template. To create transcripts restricted in their capacity for cap-dependent translation, a strong hairpin structure was included in the 5′ end of each RNA. Following transfection of these synthetic transcripts into HEK293 cells, CAT protein and RNA levels were quantified. The addition of a hairpin strongly repressed CAT expression from the control transcript (Figure 2D). In contrast, the transfection of the RNA containing the longer XIAP UTR showed only a small reduction in CAT translation with hairpin inclusion (Figure 2D). These data confirm the capacity of the longer 5′ UTR to support IRES-dependent translation initiation. These results, along with previously published validation of XIAP IRES (21) also definitively exclude confounding of apparent XIAP IRES activity by cryptic promoter activity or splicing.
In contrast to the longer 5′ UTR, the shorter XIAP 5′ UTR exhibited completely different behavior in the above-described set of experiments. The presence of the shorter 5′ UTR in the context of the monocistronic reporter construct markedly reduced translation of the CAT gene (Figure 3A). When placed in the bicistronic DNA reporter, the shorter 5′ UTR did not exhibit any appreciable IRES activity (Figure 3B). Similarly, the shorter 5′ UTR did not support CAT translation if it contained a strong hairpin structure either in DNA transfection (Figure 3C) or RNA transfection (Figure 3D) experiments. These results indicate that although cap-dependent translation of the shorter 5′ UTR of XIAP is inefficient, there is no cap-independent translation being supported by this 5′ UTR. Furthermore, these data are in accordance with our hypothesis that the two distinct 5′ UTRs of XIAP play a role in the translational control of the XIAP protein expression.
The longer XIAP 5′ UTR mediates an increase in XIAP protein levels during serum deprivation
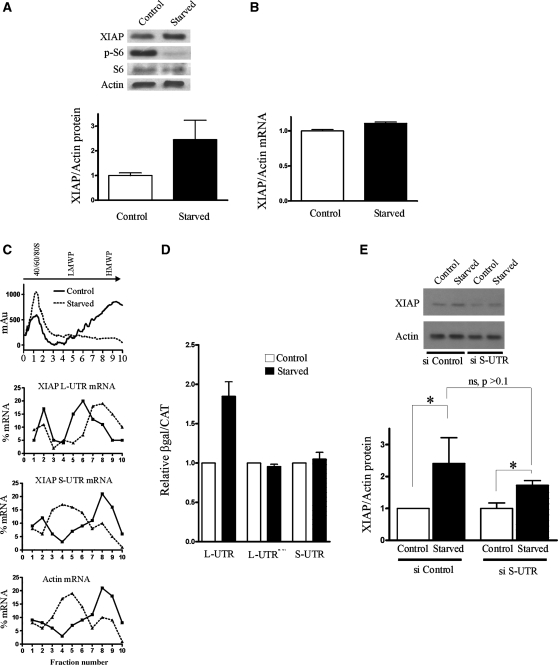
IRES-dependent translation is believed to predominate during cellular stress, when cap-dependent translation is attenuated (13). We therefore reasoned that the longer 5′ UTR of XIAP will mediate preferential translation of XIAP mRNA during stress. When HEK293 cells were deprived of serum for 24 h, this resulted in a marked increase in XIAP protein levels (Figure 4A), without a concomitant increase in XIAP mRNA levels (Figure 4B). We then employed polysomal profiling to examine the association of both XIAP mRNA isoforms with translating ribosomes. As expected, serum deprivation resulted in a marked loss of heavy polysomes and an increase in monosomes with a concomitant redistribution of β-actin mRNA (Figure 4C), indicating a repression of global protein synthesis. Significantly, RT-PCR amplification of longer 5′ UTR XIAP isoform from individual fractions showed that this XIAP isoform is recruited into the heavier polysomes following serum deprivation, confirming that the translation of the longer 5′ UTR XIAP mRNA is enhanced during repression of cap-dependent translation. In contrast, the distribution of shorter 5′ UTR XIAP isoform following serum deprivation changed from heavy to light polysomes indicating that this mRNA isoform is no longer efficiently translated. We wished to further verify that the enhanced translation is indeed mediated by the IRES element present in the longer 5′ UTR. HEK293 cells were thus transfected with the bicistronic reporter constructs harboring either the longer or the shorter XIAP 5′ UTR and the reporter activity was determined following serum deprivation. Indeed, the translation mediated by the longer 5′ UTR, but not the shorter 5′ UTR, was increased following serum deprivation (Figure 4D). Furthermore, mutation of the XIAP IRES that renders it nonactive (10) prevented the serum deprivation-mediated increase in CAT expression. In addition, the integrity of bicistronic reporter mRNA was not affected by serum deprivation (Supplementary Figure S2).
Figure 4.
The longer XIAP 5′ UTR mediates translational up-regulation of XIAP during cellular stress. (A) HEK293 cells were grown in serum-depleted growth media for 24 h, and the levels of XIAP protein and mRNA were determined by (A) western blotting and (B) qPCR. (C) Polysomal profiling of HEK293 cells. The RNA profile (indicated by absorbance at 250 nm) is shown on top for control and serum-deprived cells. Individual fractions were probed for the presence of longer and shorter XIAP 5′ UTR variants and β-actin by qPCR, which is shown as the percent distribution of specific mRNAs relative to internal CAT RNA control across the gradient. LMWP, low-molecular-weight polysomes; HMWP, high-molecular-weight polysomes. Data from a representative polysomal profiling experiment are shown. (D) HEK293 cells were transiently transfected with the indicated bicistronic reporter DNA constructs and the reporter activity was assayed after 24 h of serum-deprivation. IRES activity is expressed as the ratio of CAT expression over β-gal activity; IRES activity of each reporter plasmid in control cells was set to 1. (E) HEK293 cells were transiently transfected with siRNA targeting the shorter XIAP 5′ UTR variant, or a non-targeting control siRNA, and the expression of XIAP protein was determined by western blotting following 24 h of serum-deprivation. XIAP levels are expressed as the ratio of XIAP to β-actin, and this ratio was set to 1 in control, nonstarved cells. Densitometric analysis is shown below the representative western blot (n = 3, P < 0.05, mean ± SD).
We wished to demonstrate that the longer 5′ UTR XIAP mRNA isoform indeed gives rise to XIAP protein during serum deprivation. Since both the longer and the shorter 5′ UTR isoforms encode the same XIAP protein, the antibodies directed against XIAP protein are unable to distinguish which mRNA is used to produce the protein. We therefore wished to employ an RNA interference approach. We designed siRNAs specifically targeting either the longer or the shorter 5′ UTR variant of XIAP mRNA, and tested their efficacy and specificity on both transfected reporter mRNAs as well as endogenous XIAP mRNA (Supplementary Figure S1). While the siRNA targeting the shorter isoform was consistently able to reduce the levels of XIAP mRNA and protein, none of the nine siRNAs targeting the longer 5′ UTR variant was able to do so (data not shown). This result is perhaps not unexpected, given the relative abundance of the endogenous longer and shorter variants (Figure 1B). We therefore used only the shorter 5′ UTR targeting siRNA in subsequent experiments. HEK293 cells were transiently transfected with either the control or the shorter 5′ UTR-targeting siRNAs, and the levels of XIAP protein were determined following serum deprivation (Figure 4E). We observed that XIAP protein levels were reduced upon transfection with the shorter 5′ UTR-targeting siRNA, confirming that the shorter 5′ UTR isoform is the major XIAP mRNA isoform. However, serum deprivation elicited induction of XIAP expression even in cells with reduced levels of the shorter 5′ UTR variant, suggesting that it is the longer 5′ UTR variant that mediates XIAP mRNA translation upon serum deprivation. Together, our data strongly suggest that the longer 5′ UTR of XIAP harbors a stress-inducible IRES element that mediates preferential translation of XIAP mRNA during cellular stress.
DISCUSSION
We describe here characterization of two alternative isoforms of XIAP mRNA that differ in their 5′ UTRs and impose distinct translational regulation on XIAP protein expression during cellular stress. The two 5′ UTRs share the 33 nt immediately upstream of the initiation AUG codon; the remaining sequence is encoded by three different exons. The EST support for the existence of both isoforms has been provided previously (25); however, the confirmation of the existence of these isoforms in cells, and their potential effect on the translation of XIAP mRNA was lacking. We demonstrate that the shorter, 323 nt 5′ UTR is the predominant isoform of the XIAP mRNA. Interestingly, when cloned upstream of a reporter gene, this 5′ UTR attenuates translation of the downstream gene. This is likely a consequence of the high GC content (66.5%) and a high degree of predicted secondary structure that would be expected to have an inhibitory effect on recruitment or movement of the ribosome to the initiation codon, and thus translation (28). However, the translation supported by this 5′ UTR is cap-dependent, as evidenced by almost complete inhibition of translation when a strong hairpin structure was placed in front of this UTR. In addition, this 5′ UTR did not exhibit any IRES activity when tested either in a bicistronic DNA reporter construct, or by direct RNA transfection. The siRNA-mediated targeting of the shorter 5′UTR XIAP isoform provided evidence that despite the rather inefficient translation, the shorter 5′ UTR isoform is the mRNA that produces the majority of XIAP protein under normal growth conditions. This is likely a result of the high abundance of this mRNA isoform, which would compensate for the inefficient translation.
The longer, 1.7 kb XIAP 5′ UTR isoform is significantly less abundant than the shorter isoform. Based on our RT-PCR data in various cell lines, we estimate that the longer 5′ UTR isoform represents ∼10% of the total cellular XIAP mRNA pool. In contrast to the shorter 5′ UTR, the longer 5′ UTR exhibits efficient cap-independent translation. Recently, the validity of XIAP IRES has been challenged, based on the fact that the longer 5′ UTR mediates spurious splicing in the context of a bicistronic DNA reporter (24–26,27). Some of the spurious splicing was later attributed to the use of the dual luciferase reporter system (21). Our present data provide additional and unequivocal evidence that the longer XIAP 5′ UTR is a bona fide IRES. In addition, our data help to understand the confounding issue of spurious splicing exhibited by this 5′ UTR, which was reported by above-mentioned laboratories. The endogenous XIAP coding sequence contains a 3′ splice acceptor sequence that is located 33 nt upstream of the initiation AUG codon, and the alternative use of this site gives rise to two distinct 5′ UTRs. Since the longer 5′ UTR contains the full, unprocessed 3′ splice site, this site may be used when placed in the appropriate context of the bicistronic DNA, as reported (24,25,21). Interestingly, this spurious splicing of the reporter gene was never 100% efficient; rather, a ratio of spliced versus unspliced mRNA similar to that observed with the endogenous XIAP mRNA was reported irrespective of the reporter system used (24,27,21). To bypass this confounding effect, we have assessed XIAP IRES activity by direct RNA transfections, or through the use of monocistronic DNA constructs in which the cap-dependent translation was blocked by a strong hairpin. In both cases, the longer 5′ UTR, but not the shorter 5′ UTR supported efficient translation of the reporter gene confirming that XIAP IRES is a true IRES.
The characterization of the 5′UTR of each XIAP mRNA variant led to an interesting hypothesis. As these two isoforms appear to utilize exclusive mechanisms of translation initiation, it is likely that they are used under distinct cellular conditions to regulate XIAP expression. Indeed, we observed that serum starvation resulted in the recruitment of the longer 5′ UTR isoform into heavy polyribosomes, indicating that this isoform is preferentially translated during serum starvation. Importantly, serum starvation leads to increased levels of XIAP protein, and our data confirm that it is the IRES element within the longer 5′ UTR that is responsible for this translational increase. These data are in agreement with the strong in vivo evidence for cap-independent translation of XIAP in cellular stress (6,11,12).
IRES-mediated initiation of translation has emerged as an important mechanism that allows fine-tuning of the cellular stress response (13). It is not clear, however, precisely how the IRES mechanism operates, or how strict the distinction is between cap- and IRES-dependent translation of a given mRNA. Unlike some uncapped viral RNAs that use IRESes to recruit ribosomes, cellular mRNAs are naturally capped and could therefore use both cap-dependent and IRES-dependent modes of recruitment. Indeed, some cellular mRNAs, such as those encoding VEGF-A, HIF1α (17) and Bcl-2 (18) appear to use both the cap-structure and IRES elements as ribosomal recruitment sites. The data presented here for the XIAP mRNA suggest a third mechanism: two distinct 5′ UTRs, which are generated by alternative splicing or alternative transcription start sites, that support either cap-dependent (shorter 5′ UTR) or IRES-dependent (longer 5′ UTR) translation. While we have not observed changes in the ratio of the two XIAP mRNA isoforms, either in control or in serum-deprived cells, it will be informative to investigate if the splicing of the XIAP 5′ UTR is affected by cellular factors, or is regulated in response to a particular cellular event. Splicing factor hnRNP A1 is known to affect the choice of alternative splice sites and is a candidate for affecting alternative splicing in the XIAP mRNA. We have reported previously that hnRNP A1 regulates XIAP translation during osmotic shock; the cytoplasmic form of hnRNP A1 binds to and represses XIAP IRES activity (29). One of the binding sites for hnRNP A1 within the XIAP IRES overlaps the alternative 3′ splice acceptor site and it is therefore possible that in addition to regulating XIAP IRES function directly, hnRNP A1 may influence the usage of the 3′ splice site and thus the relative abundance of the two XIAP mRNA isoforms.
Interestingly, Baranick et al. (25) described possible 3′ splice sites in a number of IRES-containing mRNAs that may give rise to alternative 5′ UTRs. It is tempting to speculate that the expression of proteins encoded by these mRNAs will be regulated by a manner analogous to XIAP. There is some evidence in the published literature that this indeed may be the case. Several proteins expressed via mRNAs containing IRES elements are also subject to regulation by alternate noncoding regions. For example, the mRNA encoding the angiogenic factor, fibroblast growth factor-1 (FGF-1), exists as four mRNA isoforms; two of these are capable of driving high amounts of IRES-mediated translation initiation, while the remaining two use the cap-dependent mode of initiation (30). Similarly, the neurotrophin receptor TrkB (31), and the transcription factor AML1/RUNX1, are expressed as alternative transcripts, one of which utilizes an IRES for its translation (32). Recently, mRNAs with alternatively spliced 5′ UTRs were found to regulate expression of the cold-inducible RNA binding protein CIRP (33). Interestingly, the longest 5′ UTR of CIRP mRNA harbors an IRES; while the activity of this IRES is not inducible by hypothermia, the expression levels of this transcript increase in response to hypothermia resulting in an enhanced expression of CIRP. These examples suggest that the combination of alternate regulatory regions and distinct modes of translational regulation, such as we report here for XIAP, likely represent a more general mechanism of regulation of expression of genes involved in cellular stress response and adaptation.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
The Canadian Institutes for Health Research (CIHR; MOP 89737); CIHR Master’s Award and Ontario Graduate Scholarship (to A.R.); Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (to L.E.J.). Funding for open access charge: Canadian Institutes for Health Research.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Nehal Thakor, Steve Lewis and Stephen Baird for creative discussions, Dr. Baird for his help with EST databases, and Dr. Vincent Mauro for critical reading of the manuscript. This work forms part of the M.Sc. dissertation of A.R. M.H. is the CHEO Volunteer Association Endowed Scholar.
REFERENCES
- 1.Holcik M, Korneluk RG. XIAP, the guardian angel. Nat. Rev. Mol. Cell. Biol. 2001;2:550–556. doi: 10.1038/35080103. [DOI] [PubMed] [Google Scholar]
- 2.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 3.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin. Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 4.Pardo OE, Lesay A, Arcaro A, Lopes R, Ng BL, Warne PH, McNeish IA, Tetley TD, Lemoine NR, Mehmet H, et al. Fibroblast growth factor 2-mediated translational control of IAPs blocks mitochondrial release of Smac/DIABLO and Apoptosis in small cell lung cancer cells. Mol. Cell. Biol. 2003;23:7600–7610. doi: 10.1128/MCB.23.21.7600-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol. Cell. Biol. 2004;24:7003–7014. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19:4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- 7.Dean E, Jodrell D, Connolly K, Danson S, Jolivet J, Durkin J, Morris S, Jowle D, Ward T, Cummings J, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J. Clin. Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 8.Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, Altman JK, Karp JE, Kassis J, Hedley DW, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J. Clin. Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcik M. Translational upregulation of the X-linked inhibitor of Apoptosis. Ann. NY Acad. Sci. 2003;1010:249–258. doi: 10.1196/annals.1299.043. [DOI] [PubMed] [Google Scholar]
- 10.Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat. Cell. Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- 11.Yoon A, Peng G, Brandenburg Y, Zollo O, Xu W, Rego E, Ruggero D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 12.Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell. 2009;15:363–375. doi: 10.1016/j.ccr.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell. Biol. 2005;6:318327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 14.Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, Schneider RJ. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, Hochman T, Formenti SC, Schneider RJ. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell. Biol. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 16.Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 2001;15:1593–1612. doi: 10.1101/gad.891101. [DOI] [PubMed] [Google Scholar]
- 17.Lang KJD, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol. Biol. Cell. 2002;13:1792–1801. doi: 10.1091/mbc.02-02-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem. 2004;279:29066–29074. doi: 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- 19.Baird SD, Lewis SM, Turcotte M, Holcik M. A search for structurally similar cellular internal ribosome entry sites. Nucleic Acids Res. 2007;35:4664–4677. doi: 10.1093/nar/gkm483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacGregor GR, Nolan GP, Fiering S, Roederer M, Herzenberg LA. In: Methods in Molecular Biology. Murray EJ, Walker JM, editors. Vol. 7. Clifton, NJ: Humana Press Inc.; 1991. pp. 217–235. [DOI] [PubMed] [Google Scholar]
- 21.Holcik M, Graber T, Lewis SM, Lefebvre CA, Lacasse E, Baird S. Spurious splicing within the XIAP 5′ UTR occurs in the Rluc/Fluc but not the {beta}gal/CAT bicistronic reporter system. RNA. 2005;11:1605–1609. doi: 10.1261/rna.2158605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcik M, Lefebvre CA, Hicks K, Korneluk RG. Cloning and characterization of the rat homologues of the inhibitor of Apoptosis protein 1, 2, and 3 genes. BMC Genomics. 2002;3:5–10. doi: 10.1186/1471-2164-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagace M, Xuan JY, Young SS, McRoberts C, Maier J, Rajcan-Separovic E, Korneluk RG. Genomic organization of the x-linked inhibitor of apoptosis and identification of a novel testis-specific transcript. Genomics. 2001;77:181–188. doi: 10.1006/geno.2001.6635. [DOI] [PubMed] [Google Scholar]
- 24.Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA. 2004;10:720–730. doi: 10.1261/rna.5225204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranick BT, Lemp NA, Nagashima J, Hiraoka K, Kasahara N, Logg CR. Splicing mediates the activity of four putative cellular internal ribosome entry sites. Proc. Natl Acad. Sci. USA. 2008;105:4733–4738. doi: 10.1073/pnas.0710650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bert AG, Grepin R, Vadas MA, Goodall GJ. Assessing IRES activity in the HIF-1alpha and other cellular 5′ UTRs. RNA. 2006;12:1074–1083. doi: 10.1261/rna.2320506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saffran HA, Smiley JR. The XIAP IRES activates 3′ cistron expression by inducing production of monocistronic mRNA in the betagal/CAT bicistronic reporter system. RNA. 2009;15:1980–1985. doi: 10.1261/rna.1557809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak M. The scanning model for translation: an update. J. Cell. Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis SM, Veyrier A, Hosszu Ungureanu N, Bonnal S, Vagner S, Holcik M. Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation. Mol. Biol. Cell. 2007;18:1302–1311. doi: 10.1091/mbc.E06-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martineau Y, Le Bec C, Monbrun L, Allo V, Chiu IM, Danos O, Moine H, Prats H, Prats AC. Internal ribosome entry site structural motifs conserved among mammalian fibroblast growth factor 1 alternatively spliced mRNAs. Mol. Cell. Biol. 2004;24:7622–7635. doi: 10.1128/MCB.24.17.7622-7635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobson T, Minic A, Nielsen K, Amiott E, Krushel L. Internal initiation of translation of the TrkB mRNA is mediated by multiple regions within the 5′ leader. Nucleic Acids Res. 2005;33:2929–2941. doi: 10.1093/nar/gki605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozner A, Goldenberg D, Negreanu V, Le SY, Elroy-Stein O, Levanon D, Groner Y. Transcription-coupled translation control of AML1/RUNX1 is mediated by cap- and internal ribosome entry site-dependent mechanisms. Mol. Cell. Biol. 2000;20:2297–2307. doi: 10.1128/mcb.20.7.2297-2307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Fageeh MB, Smales CM. Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs. RNA. 2009;15:1164–1176. doi: 10.1261/rna.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.