Abstract
Recurrence of focal segmental glomerulosclerosis (FSGS) with nephrotic syndrome is relatively common after kidney transplantation in young recipients whose predialysis course consists of heavy proteinuria, hypertension and subacute loss of kidney function. The gene(s) mediating this effect remain unknown. We report an unusual circumstance where kidneys recovered from a deceased African American male donor with MYH9-related occult FSGS (risk variants in seven of eight MYH9 E1 haplotype single nucleotide polymorphisms) were transplanted into an African American male child with risk variants in four MYH9 E1 risk variants and a European American female teenager with two MYH9 E1 risk variants. Fulminant nephrotic syndrome rapidly developed in the African American recipient, whereas the European American had an uneventful posttransplant course. The kidney donor lacked significant proteinuria at the time of organ procurement. This scenario suggests that donor–recipient interactions in MYH9, as well as other gene–gene and gene–environment interactions, may lead to recurrent nephrotic syndrome after renal transplantation. The impact of transplanting kidneys from donors with multiple MYH9 risk alleles into recipients with similar genetic background at high risk for recurrent kidney disease needs to be determined.
Keywords: African Americans, focal segmental glomerulosclerosis, MYH9, nephrotic syndrome, pediatric recipient, renal transplant
Introduction
Polymorphisms or sequence variants in the MYH9 gene underlie 70% of idiopathic focal segmental glomerulosclerosis (FSGS) in African Americans, far more than polymorphisms in the α-actinin 4 (ACTN4) and transient receptor potential cation channel 6 (TRPC6) genes or podocin- (NPHS2), phospholipase C epsilon 1 (NPHS3), CD2-associated protein (CD2AP), Wilm's Tumor-1 (WT1) and mt DNA tRNA leucine nephrin (NPHS1)-associated glomerulosclerosis (1,2). Although MYH9 is strongly associated with FSGS and kidney disease in European Americans (odds ratio 7.7), as well as FSGS and Human Immunodeficiency Virus (HIV)-associated nephropathy (odds ratio 5.0) and the disease historically referred to as `hypertension-associated end-stage renal disease' (ESRD) in African Americans (odds ratio 3.4), risk variants in MYH9 are far less common in European Americans and ethnic differences in MYH9 genotype frequency explain much of the excess risk of non-diabetic forms of ESRD in African Americans (2–4). MYH9 was detected using `Mapping by Admixture Linkage Disequilibrium' (MALD). This is a form of genome-wide association that contrasts the frequencies of 1500 genetic markers that are spread throughout the genome and that differ markedly between African Americans and European Americans. African Americans are an admixed population containing approximately 80% African-derived and 20% European-derived gene variants. When MALD markers were tested for frequency differences in African American nephropathy cases versus healthy African American controls, excess African ancestry was detected in a peak on chromosome 22 in nephropathy cases and MYH9 was located under this association peak. Since kidney disease occurs more often in African Americans than European Americans, a peak with excess African ancestry was anticipated. At this time, MYH9 polymorphisms appear to contribute to approximately 43% of all cases of ESRD in African Americans, as well as underlie cases of kidney disease in European-derived individuals (5). Associations between MYH9 and complex kidney diseases are the strongest inherited causes of common human disease yet identified. The functional consequences of causative polymorphisms remain under study. African Americans develop FSGS significantly more often than European Americans and the disease often has a poor prognosis with high risk for disease progression (6). FSGS can recur rapidly after renal transplantation and circulating factors may be causative in some cases (7,8).
A `second hit', either a gene–environment or gene–gene interaction, is required to initiate kidney disease in MYH9 risk homozygotes due to the high frequency of risk alleles in African-derived populations. For example, the frequency of carrying 1 and 2 MYH9 E1 risk haplotypes in African Americans, respectively, is 80% and 64% in cases with non-diabetic ESRD, relative to 60% and 36% in the general community. First-degree relatives of African Americans with non-diabetic ESRD, often acting as living kidney donors, possess 1 and 2 MYH9 risk haplotypes at slightly higher frequencies than their ESRD relatives (similar frequencies are seen in parents and offspring of ESRD cases and slightly higher frequencies are observed in siblings).
Approximately 5% of African American MYH9 E1 risk homozygotes develop FSGS, while 12% of HIV-infected African Americans homozygous for MYH9 risk alleles develop HIVAN. This 140% increase in risk results from environmental exposure to HIV. Additional exposures appear likely to serve as triggering events for MYH9-associated nephropathy, and non-HIV viral infections appear likely in immunocompromised kidney transplant recipients. Whether gene–gene interactions also contribute is under study. Hyperfiltration resulting from the presence of a solitary kidney recently exposed to cold ischemia could serve as an environmental trigger for MYH9-associated nephropathy. Since variables degrees of glomerulosclerosis and interstitial fibrosis are seen in patients with chronic allograft nephropathy, the role of MYH9 gene polymorphisms on long-term allograft function remain to be explored, especially in the presence of viral co-infection (9). MYH9 polymorphisms also have the potential to contribute to the poorer allograft survival of African American donor kidneys when transplanted into African American and European American recipients.
Herein we report transplantation of two kidneys with occult FSGS from a deceased donor African American male who had risk variants in seven of eight MYH9 E1 risk haplotype single nucleotide polymorphisms (SNPs) into two pediatric recipients, one African American and one European American. Massive proteinuria with nephrotic syndrome developed within 48 h of transplantation in the African American recipient who had four risk variants in the MYH9 E1 haplotype. The European American recipient with two MYH9 risk variants in E1 is currently free of renal disease. This case suggests that donor MYH9 risk variants may interact with recipient genetic predisposition to impact risk for renal disease after kidney transplantation. It further suggests that donor MYH9 genotypes may need to be considered when transplanting patients who have MYH9 risk alleles and are at risk for developing recurrence of non-diabetic forms of kidney disease.
Clinical Course of the Kidney Donor and Recipients
Kidney donor
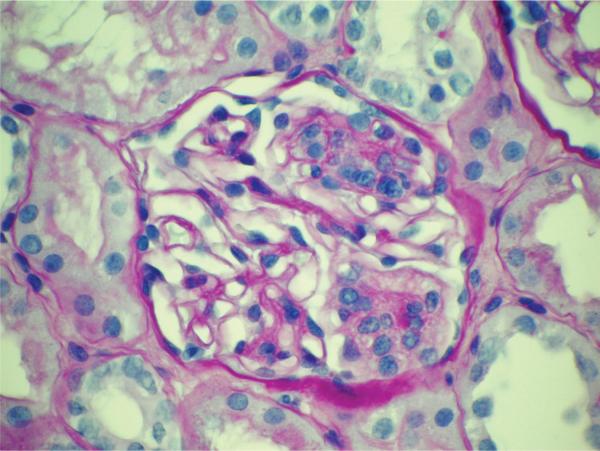
A previously healthy 18-year-old African American male collapsed with ventricular tachycardia during a sporting event. After prolonged resuscitation at the scene, the patient was transferred to the Intensive Care Unit. Acute kidney injury was present on admission, CPK levels peaked at 25 156 U/L and initial serum creatinine and albumin concentrations were 1.3 mg/dL and 3.5 g/dL, respectively. Four days later, terminal serum creatinine concentrations ranged from 0.8 to 1.0 mg/dL and urinalysis revealed specific gravity (SG) 1.026, 30 mg/dL protein, 12 RBC and 9 WBC per high power field. Anoxic brain injury resulting in brain death was confirmed and organs were procured for donation after obtaining family consent. The two kidneys were transplanted into different pediatric recipients at the same institution. An intraoperative postreperfusion biopsy was obtained from the kidney transplanted to the African American male; this revealed in addition to the anticipated acute tubular injury, presence of FSGS of the cellular variant (Figure 1).
Figure 1.
Glomerulus from allograft postreperfusion biopsy in kidney recipient 1 reveals the presence of segmental glomerulosclerosis consistent with FSGS, cellular variant (PAS stain)
Kidney recipient 1
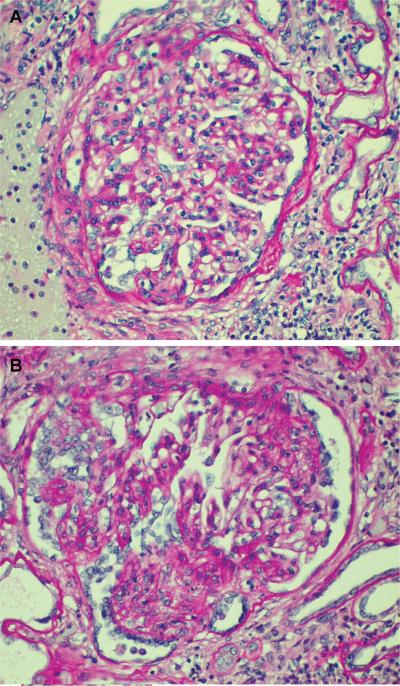
A 5-year-old African American male child presented with idiopathic nephrotic syndrome at the age of 3. There was no family history of kidney disease. After an initial response to steroid treatment; the nephrotic syndrome recurred and an initial renal biopsy was interpreted as consistent with FSGS based upon light microscopic examination, with inadequate tissue for electron and immunofluorescence microscopy. Bilateral nephrectomies were subsequently performed due to severe hypoalbuminemia. Pathologic examination of the native kidneys revealed that this child actually had end-stage immune complex-mediated glomerulonephritis with the residual preserved glomeruli displaying various degrees of focal segmental scarring (Figure 2A and B). Immunofluorescence microscopy revealed capillary wall and mesangial deposits of IgG (3+), IgA (3+), IgM (3+), C3 (3+) and Kappa light chain (3+). Electron microscopy revealed massive mesangial and capillary wall electron-dense immune complex-type deposits.
Figure 2. Glomeruli from kidney recipient 1 nephrectomy specimen performed 2 months prior to transplantation reveal.
(A) a relatively preserved glomerulus displaying moderate mesangial hypercellularity and segmental adhesions to Bowman's capsule and (B) glomerulus with advanced segmental tuft scarring and hypertrophy/hyperplasia of podocytes (PAS stain).
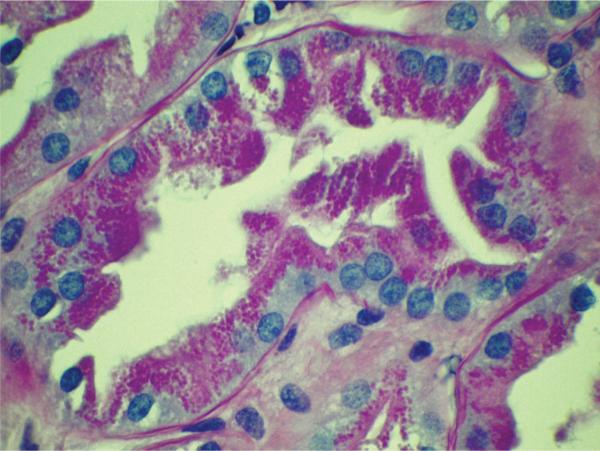
This individual received a 1AB, 1DR HLA-matched deceased donor transplant after 2 months on hemodialysis. There was immediate graft function, good urine output and a nadir serum creatinine concentration of 0.4 mg/dL after rabbit anti-thymocyte globulin (Thymoglobulin®, Genzyme, Cambridge, MA) induction therapy (3 mg/kg at time of transplant, then 1.5 mg/kg/dose for four subsequent doses), followed by mycophenolate mofetil, tacrolimus and prednisone maintenance therapy. Urine output fell to 3 mL/h after 48 h and the urine protein:creatinine ratio (UPCR) ranged from 29.5 to 124.8 g/g over the ensuing 2 days. Renal biopsy on posttransplant day 4 revealed, in addition to persistent acute tubular injury, the presence of protein reabsorption droplets in better preserved proximal tubular profiles consistent with heavy proteinuria (Figure 3). Given the short posttransplant interval there was no evidence of rejection; further, glomeruli revealed an absence of immunoglobulin or complement component (C3 and C1q) deposits. Twenty plasmapheresis treatments over a 7-week period and two doses of rituximab (Rituxan®, Genentech Inc., San Francisco, CA) 375 mg/m2 were administered to treat presumed FSGS in the donor kidney. Nephrotic-range proteinuria persists 3 months after transplantation with current UPCR 11 g/g and serum albumin 1.4 g/dL, despite preserved renal function (serum creatinine concentration 0.3 mg/dL).
Figure 3.
Tubule from allograft biopsy of kidney recipient 1 performed 4 days after transplantation reveals lining epithelium engorged with protein reabsorption droplets (PAS stain)
Kidney recipient 2
A 15-year-old European American female teenager with a history of surgically repaired meningomyelocele, neurogenic bladder, bilateral hydronephrosis, hypertension and chronic pyelonephritis required chronic hemodialysis at the age of 14. After 15 months on hemodialysis, a 0AB, 1DR HLA-matched deceased donor kidney transplant was performed. Immunosuppression consisted of alemtuzumab (30 mg at the time of transplant) and pulsed steroid induction therapy (daily steroids were discontinued on day 6), followed by mycophenolate mofetil and tacrolimus maintenance therapy. Although UPCR was 1.30 g/g on posttransplant day 3, at 3 months the serum creatinine concentration is 0.4 mg/dL, serum albumin 3.9 g/dL and urinalysis reveals trace albumin (urine SG 1.013). The patient performs bladder catheterization every 2 h during the day and Foley catheter drainage at night.
MYH9 Genotype Results
We selected 16 strongly associated SNPs from our prior MYH9 association analyses in African Americans with FSGS and non-diabetic forms of ESRD. In particular, four SNPs in the E1 haplotype provide a reliable profile for determining susceptibility to MYH9-associated FSGS and risk assessment can be based on their alleles. Genotyping was performed on the Sequenom Mass Array (San Diego, CA) and results are displayed in Table 1. One maternal and one paternal variant of each SNP are inherited for a total of eight variants in the four MYH9 E1 SNPs. The African American kidney donor with occult FSGS had risk variants in seven of the eight SNPs comprising the MYH9 E1 haplotype. In contrast, the European American recipient had two risk variants and the African American recipient who developed recurrent nephrotic syndrome had four risk variants in the MYH9 E1 haplotype.
Table 1.
MYH9 E1 SNP genotypes in kidney donor and recipients
| SNP rs # | Risk variant | African American kidney donor | European American kidney recipient | African American kidney recipient |
|---|---|---|---|---|
| 4821480 | G | GG | TT | GT |
| 2032487 | C | CT | TT | CT |
| 4821481 | C | CC | TT | TC |
| 3752462 | T | TT | TT | TC |
| Total risk alleles (maximum 8) | 7 | 2 | 4 |
E1 haplotype: rs4821480; rs2032487; rs4821481; rs3752462 (risk variants G;C;C;T).
Discussion
Herein we report an interesting scenario where two kidneys from an African American male donor with occult FSGS, minimal proteinuria and a normal serum albumin concentration were transplanted into recipients of different ethnic backgrounds with different underlying etiologies of kidney disease. The kidney transplanted into the African American male child rapidly developed nephrotic-range proteinuria that was unresponsive to plasmapheresis and rituximab, while its counterpart has had an uneventful course in a European American female teenager. While it has long been recognized that African Americans are at higher risk for developing FSGS than European Americans, the genetic and environmental causes of this disparity remain unknown. The degrees of HLA matching and immunosuppressive regimens were similar in the two kidney recipients, although their underlying causes of ESRD were not the same. Different etiologies of kidney disease may translate into variable graft function and transplant efficiencies. Although this case does not prove that donor and/or recipient MYH9 genotypes are major factors producing susceptibility to recurrent nephrotic syndrome, it suggests that donor genetic susceptibility, in concert with that of the recipient, may prove to be important.
These kidneys were transplanted at a time when the donor had trace proteinuria in a highly concentrated urine sample (SG 1.026) and normal serum albumin and creatinine concentrations. Although the European American recipient initially excreted approximately 1.3 g of urinary protein per day (potentially from acute kidney injury and the single remaining native kidney), there has been subsequent resolution of proteinuria with normal renal function 3 months after transplantation. Diminution of proteinuria could relate to reduced perfusion to the single native kidney after transplantation. The European American recipient of this genetically `at risk' kidney (seven of eight MYH9 E1 variants were risk alleles) had only two of eight MYH9 E1 risk alleles. In contrast, the African American recipient with prior immune complex-mediated glomerulonephritis and without native kidneys received a kidney from the same donor. This kidney rapidly progressed from having trace protein-uria to severe nephrosis within 48 h. Coincidentally, the African American recipient of this kidney had four of eight MYH9 E1 risk alleles.
This case suggests that quiescent FSGS can lead to massive nephrotic level proteinuria after transplantation of kidneys into a new genetic environment. This is not a case of recurrent FSGS, as the recipient initially had immune-complex mediated glomerulonephritis. It remains possible that the nephrotic syndrome in the African American child was due to immune-complex disease and not FSGS in the donor kidney. This is less likely since no immune complexes were seen and recurrent immune complex-mediated glomerulopathies that are associated with the nephrotic syndrome typically do not manifest nephrotic level proteinuria this early after transplantation.
Treatment options for recurrent FSGS in pediatric transplantation include plasmapheresis with or without calcineurin inhibition (cyclosporine and tacrolimus), calcineurin inhibition alone, cytotoxic agents (cyclophosphamide), and immunoabsorption with protein A columns followed by intravenous immunoglobulin. Most of these treatment approaches resulted from non-blinded trials. There is a lack of prospective, randomized controlled trial data available to establish optimal therapies for this aggressive disorder. This is unfortunate since up to 50% of pediatric recipients with FSGS may develop recurrent disease (10). Although calcineurin inhibitors, steroids and plasmapheresis were tried in this case of presumed `donor kidney-associated FSGS', the nephrotic syndrome has been refractory to treatment.
Polymorphisms in podocin (11) and nephrin (12) have been assessed in transplant recipients, not donors, as potential causes for recurrent FSGS. In this case, we propose that interactions between donor and recipient genetic and environmental background could have led to rapid development of nephrotic syndrome in a transplanted kidney affected with FSGS. Since the MYH9 gene encodes a motor protein that actively moves actin filaments within the podocyte, its function is likely essential to maintain normal podocyte cytoskeletal architecture and prevent proteinuria. Hence, removal of circulating factors with plasmapheresis may prove to be less important in MYH9-associated forms of FSGS. In the future it will be important to test for MYH9-gene interactions as potential causes of clinically evident FSGS after transplantation, particularly with NPHS1, NPHS2, NPHS3, ACTN4, TRPC6, WT1 and CD2AP. It would also be interesting to screen NPHS1 and NPHS2 for the presence of sequence variants in donors and recipients.
This case raises two important issues. First, studies are urgently needed to assess the effect of transplanting kidneys from donors harboring MYH9 risk variants into subjects with FSGS and other non-diabetic etiologies of ESRD. Second, most individuals inheriting MYH9 risk alleles do not develop nephropathy and additional triggering events appear necessary to initiate kidney disease (4). Human immunodeficiency virus infection markedly increases the risk for developing clinical nephropathy in African Americans who are homozygous for MYH9 risk haplotypes. The effect of donor–recipient MYH9 genotype, as well as other gene–gene and gene–environment interactions, may prove to be important contributors to long-term outcome after renal transplantation. It is conceivable that differential donor–recipient genetic susceptibility may necessitate individualized treatments. This may be especially important in African Americans where MYH9 manifests impressive association with non-diabetic forms of nephropathy including FSGS, HIV-associated nephropathy and the disease that has historically labeled as `hypertension-associated ESRD'.
Acknowledgments
Funding bodies: NIH grants RO1 DK 070941 (BIF) and RO1 DK 084149 (BIF).
Other funding bodies: None.
Footnotes
Do any of the articles, author, hold the position of investigator fellow or employee with NIH or HHMI: null.
References
- 1.Reidy K, Kaskel FJ. Pathophysiology of focal segmental glomerulosclerosis. Pediatr Nephrol. 2007;22:350–354. doi: 10.1007/s00467-006-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with non-diabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pattaro C, Aulchenko YS, Isaacs A, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int. 2009 Apr 22; doi: 10.1038/ki.2009.135. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Winn MP, Alkhunaizi AM, Bennett WM, et al. Focal segmental glomerulosclerosis: A need for caution in live-related renal transplantation. Am J Kidney Dis. 1999;33:970–974. doi: 10.1016/s0272-6386(99)70435-x. [DOI] [PubMed] [Google Scholar]
- 7.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 8.Crosson JT. Focal segmental glomerulosclerosis and renal transplantation. Transplant Proc. 2007;39:737–743. doi: 10.1016/j.transproceed.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Barzon L, Murer L, Pacenti M, et al. Investigation of intrarenal viral infections in kidney transplant recipients unveils an association between parvovirus B19 and chronic allograft injury. J Infect Dis. 2009;199:372–380. doi: 10.1086/596053. [DOI] [PubMed] [Google Scholar]
- 10.Weber S, Tonshoff B. Recurrence of focal-segmental glomerulosclerosis in children after renal transplantation: Clinical and genetic aspects. Transplantation. 2005;80:S128–S134. doi: 10.1097/01.tp.0000187110.25512.82. [DOI] [PubMed] [Google Scholar]
- 11.Bertelli R, Ginevri F, Caridi G, et al. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am J Kidney Dis. 2003;41:1314–1321. doi: 10.1016/s0272-6386(03)00364-0. [DOI] [PubMed] [Google Scholar]
- 12.Furue T, Hattori M, Tsukaguchi H, et al. Clinical features and mutational survey of NPHS2 (podocin) in Japanese children with focal segmental glomerulosclerosis who underwent renal transplantation. Pediatr Transplant. 2008;12:341–346. doi: 10.1111/j.1399-3046.2007.00752.x. [DOI] [PubMed] [Google Scholar]