Abstract
Objective
The current study tests encephalogram (EEG) measures as a potential endophenotype for Attention-Deficit Hyperactivity Disorder (ADHD) by examining sibling and parent-offspring similarity, familial clustering with the disorder, and association with the dopamine receptor D4 (DRD4) candidate gene.
Method
The sample consists of 531 participants (191 parents and 340 children) from 132 multiplex families with ADHD who participated in a larger genetics study. All members of the families underwent extensive assessment including semi-structured diagnostic interviews and EEG recording.
Results
Strong sibling similarity and parent-offspring correlations in EEG power emerged, suggesting high trait heritability. Among children, increased theta power was observed among children with ADHD when compared to unaffected children and there were no differences according to ADHD subtype. Within the parent sample, ADHD diagnostic status and ADHD subtype group differences emerged in the theta, alpha and beta frequency bands. DRD4 effects for both parents and children were apparent in the beta frequency band and for children only in the theta frequency band.
Conclusions
This study suggests that EEG measures are a promising avenue of study in the search for putative endophenotypes for ADHD and that variability at the DRD4 gene may contribute to this endophenotype.
Keywords: electrophysiology, endophenotype, genetic, ADHD
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is one of the most common childhood psychiatric disorders and involves deficits of inattention and/or hyperactivity and impulsivity. Heritability is high (~.76) and the disorder runs in families as evidenced by increased odds ratios for all first degree family members of an affected child.1 The findings from molecular genetic studies, candidate gene, and genome-wide linkage/association studies have yielded promising leads2 but gene effects are small and heterogeneity large; this makes replication difficult and findings to date have been equivocal. The complexity of the ADHD phenotype coupled with the variability in genetic findings suggests that refining the ADHD phenotype by identifying endophenotypes3 may be a useful strategy. While the search for genetic variants that confer risk for ADHD moves forward, the identification of intermediate phenotypes or endophenotypes continues in parallel and the integration of these two approaches is likely to yield more definitive results.
Toward this end, we propose the use of electroencephalogram (EEG) measures as potential endophenotypes in ADHD because of its known high heritability and success in previous genetic investigations for psychiatric disorders.4, 5 When electrical activity and brain-wave forms are compared, greater similarity is observed among first-degree relatives than unrelated individuals6 with the greatest similarity seen among monozygotic twins.7 Heritability estimates for EEG power in the delta, theta, alpha and beta bands range between .7 to .9.8 A recent meta-analysis combining twin and family studies of EEG estimated the average heritability across 11 studies at 79% for alpha power.7 EEG has also been used successfully in previous genetic investigations of complex traits such as alcoholism. In the Collaborative Study of the Genetics of Alcoholism (COGA) multi-site family study of alcoholism, EEG beta power and the evoked related potential (ERP) P3 component were used as biological endophenotypes. Linkage analyses performed on the EEG and ERP data resulted in significant linkage for beta activity on chromosome 4 (MLS=5.01) and P3 amplitude on chromosome 6 (MLS=3.41).5 Follow-up association analyses between beta activity and GABA genes were significant, with a 3-SNP haplotype of the GABRA2 gene showing a very strong association with alcoholism.9 Taken together, the findings suggest that variation in the GABRA2 gene may influence susceptibility to alcoholism through its effects on cortical excitation and activation. This study demonstrates the power that electrophysiological measures may have to identify gene variants contributing to psychiatric or psychological traits because they offer a window on brain processes that may reflect neurophysiological pathways of gene to disease phenotypes.
High heritability of EEG patterns and the successful localization of genetic variants for psychiatric disorders using EEG endophenotypes, coupled with the strong association of EEG patterns with ADHD provide the rationale for the investigation of EEG as an endophenotype for ADHD. Numerous independent studies have reported EEG differences among children with ADHD compared to their non-ADHD peers.10, 11 Children with ADHD display fairly consistent EEG differences compared to non-ADHD controls, particularly with respect to increased theta (4–7 Hz) activity, which has also been observed among adolescents and adults with ADHD relative to controls.12 A recent meta-analysis of EEG studies in ADHD reported an effect size (ES) of 1.31 for increased theta power in a collective sample of 1498 people.13 Frontal midline theta activity is thought to originate from the anterior cingulate, a region that has been implicated in ADHD and is known to play an important role in attentional processes.14
Aside from the findings for theta, results for other frequency bands such as beta (definitions vary but generally in the 13–25 Hz range) and alpha (8–12 Hz) have been more variable in ADHD. While some studies have found decreased beta (indicative of heightened cortical activation) activity in frontal and central regions,10, 15 others have not.16 In adult populations, posterior (rather than frontal) beta appears to be decreased.12, 17 Similarly, studies have found mixed results for alpha power, with some studies showing it to be increased10, 17 in others it is decreased,18 and still other studies find it to be similar12 between those with ADHD and normal controls. Gender and age may play a role in these discrepant findings, as well as differences in methods such as sampling (i.e., community vs. clinical samples), diagnostic procedures, EEG data collection and processing.
In a preliminary study, we recently reported that EEG measures collected within families that have two or more ADHD offspring demonstrate significant sibling similarity and familial clustering.19 Sibling correlations for EEG measures were moderate during baseline conditions and significantly higher for the cognitive activation condition. Children with a higher genetic loading for ADHD, as measured by parental affection status for ADHD, exhibited decreased alpha band activity in frontal and parietal regions while performing a cognitive activation task. This preliminary study suggests that EEG is a promising endophenotype in ADHD, but was conducted in a small sample of families (n=27) and therefore warrants more research.
The current study takes a step in that direction by investigating EEG within an independent sample of 132 multiplex families (i.e., 2 or more children) with ADHD. First, we examine familial clustering by examining sibling similarity and parent-offspring correlations of EEG spectral power. Second, we test for association of EEG power with ADHD symptoms among the children and their parents. We hypothesize that theta power will be significantly higher among children with ADHD compared to their non-ADHD siblings. Based on our prior findings, 18 we predict that alpha power will be significantly lower among parents with ADHD when compared to non-ADHD parents. Finally, we test one candidate gene that has been found associated with ADHD in previous studies1 with the EEG endophenotypes suggested from our investigations. We propose that the ADHD risk variant of the dopamine receptor D4 (DRD4) 48-bp (i.e., 7-repeat allele) will be associated with EEG endophenotypes such as increased theta power when compared to those without the 7-repeat allele.
Method
Participants
The sample consists of 531 participants from 132 multiplex families with ADHD who participated in a larger genetics study of ADHD. Of these, 191 parents and 340 children aged 5–18 years old were seen. There is no overlap of these 132 families with the previous study of 27 families. 19 Families were recruited from the community through radio and newspaper advertisements, community organizations (i.e. CHADD), local schools and primary care physicians. Demographic and clinical variables are presented in Table 1. In order to participate in the study, families were screened to have at least two affected children with ADHD. While the majority of families were multiplex (i.e., 2 or more children with ADHD; N=106), a small number of children (N=26) either did not meet criteria for ADHD (i.e., had ≤ 4 symptoms of inattention and hyperactivity-impulsivity) or had an unaffected sibling that was recruited to the study in addition to the affected sibling pair. After receiving verbal and written explanations of study requirements, all participants provided written informed consent/assent approved by the UCLA Institutional Review Board.
Table 1.
Clinical and demographic variables for child and parent samples
| Children | Parents | ||
|---|---|---|---|
| Sample size (N) | 340 | 191 | |
| Age in years (mean, SD) | 11.07 (3.4) | 44.3 (5.5) | |
| Gender, Males (N, %) | 195 (57%) | 96 (50%) | |
| DRD4 status -7R/ not 7 | 65/144 | 43/116 | |
|
Psychiatric diagnoses (lifetime) | |||
| ADHD | 304 (88%) | 80 (42%) | |
| Combined Type | 174 (51%) | 26 (14%) | |
| Inattentive type | 117 (34%) | 47 (25%) | |
| Hyperactive-Impulsive type | 13 (4%) | 7 (3%) | |
| Anxiety | 58 (17%) | 35 (18%) | |
| Mood | 50 (15%) | 82 (43%) | |
| ODD/CD | 134 (39%) | 21 (11%) | |
| Family level variables | |||
| N_families | 132 | ||
| SES (1=lowest, 5=highest) | 3.5 (1.8) | ||
| Ethnicity | |||
| Caucasian/non-Hispanic | 72% | ||
| Caucasian/Hispanic | 8% | ||
| African-American | 3% | ||
| Other, Mixed | 13% | ||
Note. DRD4=Dopamine Receptor D4 gene, 7R=7-repeat allele, ADHD=Attention-Deficit Hyperactivity Disorder, ODD=Oppositional Defiant Disorder,CD= Conduct Disorder, SES=Socioeconomic Status using Hollingshead scale
Procedure
All members of the families underwent extensive assessment including diagnostic interviews and EEG recording. Parents were interviewed directly using the Schedule for Affective Disorders and Schizophrenia (SADS-LAR)20 supplemented with the Behavioral Disorders supplement from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS-PL)21 to screen for the presence of ADHD and other psychiatric disorders. All children were evaluated based on an interview with the primary caretaker (usually mother) using the KSADS-PL and a direct interview with the child (if 8 years of age or older). All interviews were conducted by clinical psychologists or highly trained, master’s level interviewers with extensive experience in psychiatric diagnoses. ‘Best estimate’ diagnoses were determined after individual review of diagnoses, symptoms, and impairment level by senior clinicians (JJM, JTM). Inter-rater reliabilities were computed with a mean weighted kappa of 0.84 across all diagnoses with a greater than 5% occurrence in the sample. Subjects were excluded from participation if they were positive for any of the following: neurological disorder, head injury resulting in concussion, lifetime diagnoses of schizophrenia or autism, or estimated Full Scale IQ < 70. Subjects on stimulant medication were asked to discontinue use for 24 hours prior to their visit.
Electrophysiologic methods
EEG recording was carried out using 40 Ag/AgCl surface electrodes that were embedded in an electrode cap in an extended International 10/20 location system (ElectroCap, Eaton, Ohio) and was referenced to linked ears. Impedance was below 10 kOhms and EEG signal was recorded using MANSCAN (Sam Technology, San Francisco, CA) hardware and software. EEG data were sampled at a rate of 256 samples per second. Eye movements were monitored by electrodes placed on the outer canthus of each eye for horizontal movements and by electrodes above the eye for vertical eye movements. EEG recording for all subjects consisted of 2 baseline conditions (3 minutes each: eyes open [EO] and eyes closed [EC]) and a cognitive activation condition. During the cognitive activation condition, a continuous performance test (CPT) was administered as a measure of response inhibition and sustained attention. The CPT is a 14-minute computerized task during which subjects sat approximately 27–36 inches away from the 19” presentation screen and were asked to press the space bar when any letter except the target letter “X” appeared. Task specific are as follows: 360 trials, 36 presentations of the inhibition target (X), targets (including ‘go’ targets: A,B,C,D,F,I,L,O,T) were presented in randomized order for 250-msec in Arial 80-point font with variable inter-trial interval of 750-, 1750-, and 3750-msec). Continuous EEG data were reviewed off-line by a technician experienced in EEG and all segments containing eye, head movement or muscle artifact were removed from further analysis. Using a fast fourier transform, EEG power (µV2) was averaged for each condition and exported in the following bandwidths: Theta (4–7Hz), Alpha (8–11Hz), and Beta1 (12–15Hz), Beta 2 (16–20Hz). A subset of electrodes was used and grouped as follows: anterior frontal (AF3, AF4, AFz), frontal (F3, F4, Fz), central (C3, C4, Cz), parietal (P3, P4, Pz). Relative power for each frequency band was calculated and log transformed to assume a normal distribution. EEG technicians were blind to ADHD diagnostic status.
Genotyping
The dopamine receptor D4 (DRD4) 48-bp VNTR was genotyped according to standard protocols.22 Genotypes were not available for the whole sample due to a number of factors, primarily a genotyping lag where some of the families have not yet been genotyped. Other factors include: insufficient or degraded DNA, poor call rate, etc. The final number of genotypes available was 209 children and 159 parents. There were no differences between those genotyped and not genotyped for ADHD subtype distributions, age, sex, and frequency of co-morbid diagnoses (data not shown). Since the DRD4-7-repeat allele is thought to be the ‘risk’ allele for ADHD, subjects were stratified according to whether they possessed at least one copy of the 7-repeat allele (7R) or not (not 7).
Data analytic plan
Statistical analyses were run in SPSS 15.0 and SAS 9.1. To correct for multiple comparisons, we employed a False Discovery Rate (FDR)23 to maintain the family-wise experimental error at p ≤ .05. Proc Mult-test was used to generate FDR adjusted p-values (denoted as FDR p), which are presented in addition to raw p-values (denoted as p); the threshold for statistical significance was set at FDR p ≤ .05. Because age has significant effects on EEG power, it was used as a covariate in all analyses. To assess sibling correlations, Pearson correlations were calculated for the two eldest siblings. If there were more than two children in the family, only the two eldest children were used for this analysis. Parent-offspring correlations were calculated by taking the mean EEG relative power for both parents and using it in a correlation with the oldest child with ADHD.
To test the clustering of EEG activity with ADHD symptoms, a repeated measures analysis of variance (RMANOVA) with Greenhouse-Geiser correction when appropriate was run separately for children and parents. In order to assess EEG differences by ADHD diagnostic status and ADHD subtype, the between-subjects variable incorporated ADHD diagnosis and subtype and had three levels (non-ADHD, ADHD Predominantly Inattentive type [ADHD-PI], ADHD Combined type [ADHD-CT]). EEG recording condition (EC, EO, CPT) was used as the within-subjects variable. Separate RMANOVAs were run once for each frequency band (theta, alpha, beta1, beta2) within each brain region (anterior frontal, frontal, central, parietal) for both adults and children separately. There are two results of interest from these analyses; the first is a within-subjects (WS) interaction effect between recording condition and ADHD group that tests whether the pattern of EEG activation across the three recording conditions (EC, EO, CPT) is different by ADHD group status. A positive WS finding suggests that the non-ADHD, ADHD-PI, or ADHD-CT groups have different profiles of activation across the three recording conditions. The second result of interest is a between-subjects (BS) result that indicates that there is a significant effect of ADHD group collapsed across condition. A significant BS finding suggests that the three groups (non-ADHD, ADHD-PI, ADHD-CT) differ in their mean EEG relative power over all three recording conditions. The ADHD-Hyperactive-Impulsive group was deleted due to the small number of cases with this subtype (children N=13, adults N=7). Significant effects of diagnostic status or ADHD subtype were followed with post-hoc Tukey HSD to test pairwise differences between groups. Candidate gene (DRD4) effects were also tested using repeated measures ANOVAs and run separately for children and parents, controlling for age.
Results
Estimates of familiality using sibling correlations
Sibling and parent-offspring correlations for EEG spectral power in frontal, central and parietal regions in each of the three conditions, EO, EC, and CPT are presented Table 2. Overall, there is a high degree of sibling similarity in EEG power, however, these data indicate differential levels of sibling correlation according to region and frequency band. Siblings are most similar at rest (EC) in the lower frequencies (theta power r2 range: .36–59, p < .001) and (alpha power range: .42–49, p < .001) and most similar under cognitive activation conditions (EO & CPT) in the beta 1 frequency band (range: .45–61, p < .001). Parent-offspring correlations were more variable, but strongest and significantly correlated in the alpha frequency band during EO and CPT conditions (range: .39–52, p < .001).
Table 2.
Parent-Offspring and Sibling Correlations for EEG frequency bands during baseline and cognitive activation conditions.
| Region | Band | Sibling Correlations | Parent-Offspring | ||||
|---|---|---|---|---|---|---|---|
| EC | EO | CPT | EC | EO | CPT | ||
| frontal | theta | 0.36** | 0.25* | 0.30** | 0.15 | 0.23 | 0.34** |
| central | theta | 0.38*** | 0.31** | 0.19 | 0.15 | 0.12 | 0.22 |
| parietal | theta | 0.59*** | 0.34** | 0.22* | 0.11 | 0.15 | 0.13 |
| frontal | alpha | 0.49*** | 0.30** | 0.30** | 0.34** | 0.56*** | 0.50*** |
| central | alpha | 0.42*** | 0.31** | 0.15 | 0.28** | 0.48*** | 0.47*** |
| parietal | alpha | 0.48*** | 0.33** | 0.24* | 0.15 | 0.47*** | 0.46*** |
| frontal | beta1 | 0.57*** | 0.56*** | 0.51*** | 0.34** | 0.15 | 0.15 |
| central | beta1 | 0.55*** | 0.61*** | 0.61*** | 0.20 | 0.18 | 0.13 |
| parietal | beta1 | 0.45*** | 0.53*** | 0.56*** | 0.06 | 0.26* | 0.11 |
| frontal | beta2 | 0.28** | 0.13 | 0.34** | 0.29* | 0.29* | 0.16 |
| central | beta2 | 0.44*** | 0.35** | 0.52*** | 0.36** | 0.30* | 0.23 |
| parietal | beta2 | 0.52*** | 0.36** | 0.54*** | 0.41*** | 0.30* | 0.38** |
Note: Significant correlations in bold. False-discovery rate (FDR) adjusted p-values are indicated as follows:
p ≤ .05,
p ≤ .01,
p ≤ .001,
no asterisk denotes that the correlation became non-significant when using FDR adjustment. Frequency bands were defined as Theta (4–7 Hz), Alpha (8–12 Hz), Beta1 (12–16 Hz), Beta2 (16–20Hz).
EO=Eyes open, EC= Eyes Closed, CPT= continuous performance task condition, EEG = Electroencephalogram
Association of EEG measures with ADHD affection status and subtype within families
Repeated-measures ANOVAs using ADHD affection status and subtype (Non-ADHD, ADHD-Inattentive Type or ADHD-Combined Type) were run separately within each brain region for children and parents. Among the children, significant between-subjects effects on theta power emerged in the anterior frontal [F(2, 169)=3.49, p=.03, FDR p=.12], frontal F(2,169)=4.34, p=.01, FDR p=.04], central [F(2, 169)=4.38, p=.01, FDR p=.04], and parietal [F(2, 169)=3.51, p=.03, FDR p=.12] regions, with non-ADHD children having lower theta power than children with ADHD-PI or ADHD-CT. There were no significant differences between the two ADHD subtypes as shown in Figure 1. Findings for anterior frontal and parietal theta power become non-significant trends with FDR adjustment. None of WS or BS effects for EEG power in other frequency bands reached statistical significance. These analyses were re-run using ADHD status (since there are no differences by ADHD subtype) and only one child per family (either the eldest affected sib or the unaffected sibling when available) to ensure these effects were not the result of multiple children per family (data non-independence). Results were in the same direction with the ADHD group (N=75) exhibiting increased theta power when compared to the non-ADHD group (N=23) (Frontal: F(1, 95)=12.4, p=.001, FDR p=.02; Central: F(1, 95)=7.94, p=.006, FDR p=.03; Parietal F(1,95)=5.07, p=.03, FDR p=.09).
Figure 1. Familial clustering of EEG measures with ADHD affection status and subtype.
ADHD = Attention-Deficit / Hyperactivity Disorder, CT = Combined type, EC=eyes closed, EO=eyes open, CPT=Continuous performance test, Hz=hertz, EEG = Electroencephalogram. *between subjects effect=p≤.05, **between subjects effect=p≤.01, ## within subjects effect=p≤.01. The between-subjects effect suggests that the mean EEG power across the three conditions was lower according to ADHD diagnostic status and subtype. The within-subjects effect indicates that the ADHD-Inattentive group had a different profile of EEG activation across recording condition compared to the ADHD-CT and non-ADHD groups. Error bars represent the standard error.
Among adults, significant differences emerged in the theta, alpha and beta2 frequency bands. Similar to children, significant between-subjects effects emerged in frontal and central theta power among the parents. Theta power was significantly elevated for parents with ADHD-PI compared to the ADHD-CT and non-ADHD groups in the anterior frontal (F (2, 126)=5.16, p=.01, FDR p=.04) and frontal (F (2, 126)=5.16, p=.01, FDR p=.04) region, with a trend toward significance in the central (F (2, 126)=2.8, p=.07, FDR p=.10) region (see Figure 1). In addition, significant between-subjects effects in the beta2 frequency band emerged with ADHD-CT parents exhibiting significantly increased beta2 power when compared to the non-ADHD group in the anterior frontal (F(2,125)=3.64,p=.02, FDR p=.05), frontal (F(2,125)= 4.91, p=.01, FDR p=.04), central (F(2,124)=4.33, p=.02, FDR p=.04), and parietal (F(2, 125)= 3.90, p=.02, FDR p=.04). The ADHD-PI group did not differ significantly from either the non-ADHD or ADHD-CT groups. Finally, significant within-subjects and between-subjects effects emerged in the alpha band suggesting that there were different profiles of EEG activation across recording conditions by ADHD group as well as different levels of alpha power by ADHD group status. Within-subjects effects were primarily in the frontal regions (anterior frontal: F(4, 338)=4.13, p=.01, FDR p=.04; frontal F(4, 338)=3.55, p=.01, FDR p=.04) and indicate that the ADHD-PI group exhibited a different pattern of EEG activation where alpha power does not attenuate when going from EC to EO as it does for the ADHD-CT and non-ADHD groups. Between-subjects effects emerged globally for alpha power (anterior frontal: F(2, 126)=4.08, p=.02, FDR p=.04; frontal F(2, 126)=4.27, p=.02, FDR p=.04; central F(2, 126)=4.21, p=.02, FDR p=.04; parietal F(2, 126)=3.63, p=.03, FDR p =.05), indicating that adults with ADHD-CT exhibit reduced levels of alpha power compared to the non-ADHD and ADHD-PI groups.
Effects of the Dopamine receptor D4 gene (DRD4) on EEG
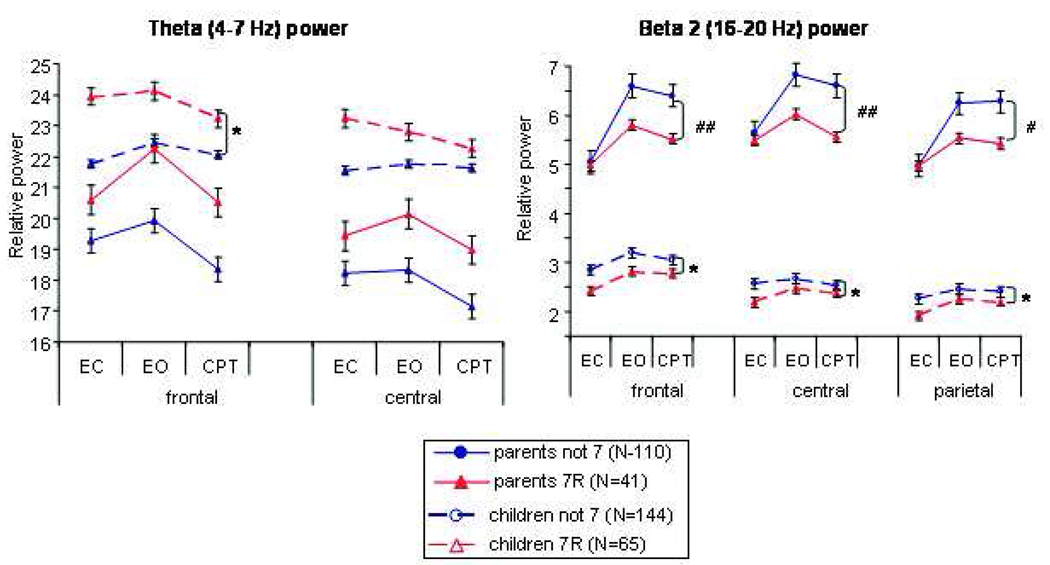
The DRD4 gene was significantly associated with cortical activity in the beta2 frequency band among both parent and child samples (see Figure 2) and the theta band within the child sample only. DRD4 effects on all other frequency bands were not significant. Within the child sample, the within-subjects analyses were not significant but two significant between-subjects effects emerged, suggesting DRD4-7-repeat allele (7R) carriers had significantly increased frontal theta power (frontal: F(1,206)=5.77, p=.02, FDR p= .05; central: F(1,206)=3.83, p=.05, FDR=.10; parietal F(1,206)=1.83, p=.18, FDR p= .45) and reduced global beta2 power (frontal: F(1,206)=5.79, p=.02, FDR p= .05; central: F(1,206)=5.82, p=.02, FDR=.05; parietal F(1,206)=6.35, p=.01, FDR p= .04) when compared to those children who do not have at least one copy of the 7-allele (not 7). The significant between group effect suggests that the DRD4-7 repeat allele is associated with EEG variability among children with ADHD. Among parents, DRD4 also had significant global (i.e., over the whole head) effects in the beta2 frequency band, however, it was the within-subjects and not the between-subjects effect that was significant (frontal: F(2, 312)=4.46, p=.01, FDR p=.04; central: F(2, 312)=6.03, p=.003, FDR p=.02; parietal: F(2, 304)=3.77, p=.03, FDR p=.07). The significant within-subjects effect suggests that DRD4-7R carriers had similar beta2 power in the eyes closed condition, but exhibited reduced beta2 power in the eyes open and CPT conditions compared to the DRD4 not-7 group. DRD4 appeared to have similar effects on theta power among adults, however, the results for the between-subjects effect were non-significant trends (frontal: F(1, 156)=2.6, p=.10, FDR p=.11; central: F(1, 156)=1.9, p=.16, FDR p=.16; parietal: F(1, 152) <1, p=.49, FDR p=.49).
Figure 2. DRD4 effects on EEG power among children and parents.
EC=eyes closed, EO=eyes open, CPT=continuous performance test, Hz=hertz, EEG = Electroencephalogram, DRD4 = Dopamine receptor D4. *between-subjects effect p<.05; # within-subjects effect p<.05; ## within subjects effect p≤.01. The between-subjects effect suggests that the mean EEG power across the three conditions was lower for the DRD4-7 group compared to the DRD4-not 7 group. A within-subjects effect indicates that DRD4-7 group had similar EEG power in the EC condition, but reduced beta power in the EO and CPT conditions. Error bars represent the standard error.
Discussion
The purpose of the present study is to examine the familial clustering of EEG phenotypes within a large sample of multiplex families with ADHD to guide and refine the phenotype for subsequent genetics studies of ADHD. The findings presented here suggest that EEG is a promising avenue of study in the search for putative endophenotypes for ADHD. First, the familiality of EEG was demonstrated through significant sibling and parent-offspring correlation of EEG measures, particularly in the alpha and beta frequency bands. Second, EEG demonstrates familial clustering with ADHD status and symptoms among families. Theta is primarily implicated among children whereas theta, alpha and beta power differed according to ADHD status and type among parents. Finally, EEG measures reflecting cortical activation in the theta and beta2 frequency bands are significantly associated with the DRD4-7-repeat allele, which accounts for variability in the EEG phenotype among multiplex families with ADHD. While these results do not implicate one single EEG metric (i.e., frequency band or cortical region) as the endophenotype, they suggest that EEG is strongly familial within multiplex families with ADHD and that further phenotype refinement using multivariate methods (such as latent class analysis) may be helpful.
As originally conceptualized by Gottesman & Shields,24 an endophenotype is an internal, biological phenomena that lies along the same etiologic pathway between genotype and disorder, but is closer to the gene action and expression. Several criteria for evaluating whether an endophenotype such as heritability, association with disease state, and candidate gene effects have been proposed.3 The current results, coupled with a previous investigation using an independent sample of families recorded on different EEG hardware and software,19 suggest that EEG measures demonstrate strong familial clustering among multiplex families with ADHD. Previous studies have implicated cognitive processes such as inhibition, 25 working memory,26 as well as brain imaging phenotypes such as structural and volumetric differences in prefrontal, striatal and cerebellar regions. We suggest that integrated panels of phenotypes that span multiple biological (e.g., structural and functional MRI, EEG/ERP), cognitive and behavioral levels will comprise more powerful phenotypes for future genetic studies.
The current results are largely consistent with prior studies of EEG in ADHD in that significant differences between children with and without ADHD emerged in the theta frequency band. It should be noted that the non-ADHD group in this study consists entirely of unaffected siblings of probands with ADHD, which may have reduced the magnitude of difference between the groups. Within the framework of the endophenotype model, unaffected siblings share some genes that may be responsible for variation in the putative endophenotype resulting in a deficit that is in the middle of their affected siblings and healthy controls. Since we re-ran the analysis using one child per family and the results remained significant, these findings suggest that increased theta power among children with ADHD is particularly robust, even when compared to unaffected siblings. The lack of ADHD subtype differences within the child sample may reflect the widely observed instability of ADHD subtypes in childhood27. EEG differences were more fine grained within the parent sample and showed strong patterns not only by ADHD diagnosis, but also by ADHD subtype. EEG differences between adults with and without ADHD have not been well studied, but results have been variable in the theta and alpha frequency bands.12, 18 Previous studies have not examined ADHD subtype differences within adult samples and this may have contributed to variability in findings if the composition of the adult ADHD groups differed in terms of ADHD subtype. We also did not examine the theta/beta ratio, which has been shown to significantly discriminate ADHD and non-ADHD populations13; further research on this marker is needed.
Finally, the association between DRD4 7-repeat allele and beta2 power was found among both child and parent samples. The differences were between-subjects for children and within-subjects for adults, suggesting that the candidate gene association is subject to developmental variation in brain functioning. Among children, the 7R group had reduced beta2 power which likely reflects reduced cortical activation across baseline and cognitive activation conditions when compared to children who did not have the DRD4 7-repeat allele. Parents, on the other hand, were highly similar in the eyes-closed resting condition, but the DRD4 7R group had reduced cortical activation upon opening their eyes and performing the CPT task relative to the DRD4 not 7 group. If smaller regional brain structure is associated with reduced activation in that region, the current results are generally consistent with previous findings where DRD4 7-carriers had smaller dorsolateral prefrontal cortex28, but conflicts with previous studies that found smaller fronto-striatal volumes among DRD4-4 carriers, 29 and no difference between the DRD4 alleles.30 The relationship between brain volume and functional activation, however, has not been well studied and requires further research. These results suggest that variability in the DRD4 gene may contribute to this brain structure and function and further studies are needed to determine the direction of the effect as well as mechanism through which the DRD4 affects brain function and structure.
The primary limitation of the current study is possible lack of generalizability of findings because the current sample is comprised of multiplex families with ADHD, which are thought to have a higher genetic loading for ADHD. Thus, the findings reported here may not generalize to singleton families where there is only a single child with ADHD. As noted previously, many of the findings are consistent with the larger ADHD EEG literature and thus seem to be less impacted by the characteristics of the particular sample. In addition, we have run a large number of statistical tests that may have lead to false positives (Type 1 error). We have calculated and presented a False Discovery Rate (FDR) to give a p-value adjusted for experiment wise error. Any FDR-adjusted p-values that are statistically non-significant should be interpreted with caution.
In conclusion, this paper reports significant familial clustering of EEG phenotypes collected within the largest study of multiplex families with ADHD to date. Coupled with a previous, independent study, the results suggest that EEG is a promising avenue of study in the search for endophenotypes for ADHD. While theta, alpha and beta 2 power are promising EEG endophenotypes, multivariate modeling using all the frequency bands simultaneously to identify latent subgroups within our family sample may be a more promising approach. Although EEG appears to be a promising metric for identifying genetically homogeneous subgroups, we are not suggesting or endorsing the use of EEG for clinical diagnosis of ADHD.
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development (CD40275 to SKL), the National Institutes of Neurological Disease and Stroke (NS054124 to SKL) and National Institutes of Mental Health (MH058277 to SLS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. McGough consults and receives research support from Eli Lilly & Company, and Shire Pharmaceuticals. Dr. McCracken consults and receives research support from Bristol Myers Squibb, Aspect, Eli Lilly & Company, Wyeth, Sanofi-Aventis, and Pfizer. Drs. Loo, Hale, Shrestha, Nelson, and Smalley, and Mr. Hanada and Mr. Macion report no biomedical financial interests or potential conflicts of interest.
References
- 1.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Neale BM, Lasky-Su J, Anney R, Franke B, Zhou K, Maller JB, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1337–1344. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 4.Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, et al. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5(2):155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 5.Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, et al. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61(1–2):229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 6.Stassen HH, Bomben G, Hell D. Familial brain wave patterns: study of a 12-sib family. Psychiatric Genetics. 1998;8(3):141–153. doi: 10.1097/00041444-199800830-00003. [DOI] [PubMed] [Google Scholar]
- 7.van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61(1–2):111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 8.Zietsch BP, Hansen JL, Hansell NK, Geffen GM, Martin NG, Wright MJ. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol Psychol. 2007;75(2):154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996;40(10):951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- 11.Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR. EEG evidence for a new conceptualisation of attention deficit hyperactivity disorder. Clin Neurophysiol. 2002;113(7):1036–1044. doi: 10.1016/s1388-2457(02)00115-3. [DOI] [PubMed] [Google Scholar]
- 12.Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry. 1999;46(12):1690–1697. doi: 10.1016/s0006-3223(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 13.Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23(5):440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- 14.Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10(9):829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- 15.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: differences in two subtypes of attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2001;112(5):815–826. doi: 10.1016/s1388-2457(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuperman S, Johnson B, Arndt S, Lindgren S, Wolraich M. Quantitative EEG differences in a nonclinical sample of children with ADHD and undifferentiated ADD. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(8):1009–1017. doi: 10.1097/00004583-199608000-00011. [see comments] [DOI] [PubMed] [Google Scholar]
- 17.Koehler S, Lauer P, Schreppel T, Jacob C, Heine M, Boreatti-Hummer A, et al. Increased EEG power density in alpha and theta bands in adult ADHD patients. J Neural Transm. 2009;116(1):97–104. doi: 10.1007/s00702-008-0157-x. [DOI] [PubMed] [Google Scholar]
- 18.Loo SK, Hale TS, Macion J, Hanada G, McGough JJ, McCracken JT, et al. Cortical activity patterns in ADHD during arousal, activation and sustained attention. Neuropsychologia. 2009;47(10):2114–2119. doi: 10.1016/j.neuropsychologia.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo SK, Smalley SL. Preliminary report of familial clustering of EEG measures in ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(1):107–109. doi: 10.1002/ajmg.b.30575. [DOI] [PubMed] [Google Scholar]
- 20.Fyer AJ, Mannuzza SM, Klein D, Endicott J. Schedule for Affected Disorders and Schizophrenia - Lifetime Version Modified for the Study of Anxiety Disorders (1985), Updated for DSM-IV (SADS LA-IV) New York, New York State Psychiatric Institute: Anxiety Family Genetics Unit; 1995. [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Kustanovich V, Ishii J, Crawford L, Yang M, McGough JJ, McCracken JT, et al. Transmission disequilibrium testing of dopamine-related candidate gene polymorphisms in ADHD: confirmation of association of ADHD with DRD4 and DRD5. Mol Psychiatry. 2004;9(7):711–717. doi: 10.1038/sj.mp.4001466. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Goos LM, Crosbie J, Payne S, Schachar R. Validation and extension of the endophenotype model in ADHD patterns of inheritance in a family study of inhibitory control. Am J Psychiatry. 2009;166(6):711–717. doi: 10.1176/appi.ajp.2009.08040621. [DOI] [PubMed] [Google Scholar]
- 26.Loo SK, Rich EC, Ishii J, McGough J, McCracken J, Nelson S, et al. Cognitive functioning in affected sibling pairs with ADHD: familial clustering and dopamine genes. J Child Psychol Psychiatry. 2008;49(9):950–957. doi: 10.1111/j.1469-7610.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 27.Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley SL, McGough JJ, et al. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1605–1613. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- 28.Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 29.Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry. 2005;10(7):678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- 30.Castellanos FX, Lau E, Tayebi N, Lee P, Long RE, Giedd JN, et al. Lack of an association between a dopamine-4 receptor polymorphism and attention-deficit/hyperactivity disorder: genetic and brain morphometric analyses. Mol Psychiatry. 1998;3(5):431–434. doi: 10.1038/sj.mp.4000430. [DOI] [PubMed] [Google Scholar]