Abstract
Human fatty acid synthase (FASN) is a 270-kDa cytosolic dimeric enzyme that is responsible for palmitate synthesis. FASN is slowly emerging and rediscovered as a marker for diagnosis and prognosis of human cancers. Recent studies showed that FASN is an oncogene and inhibition of FASN effectively and selectively kill cancer cells. With recent publications of the FASN crystal structure and the new development of FASN inhibitors, targeting FASN opens a new window of opportunity for metabolically combating cancers. In this article, we will review critically the recent progresses in understanding the structure, function, and the role of FASN in cancers and pharmacologically targeting FASN for human cancer treatment.
Keywords: Fatty acid synthase, inhibitors, drug resistance, cancer, prognosis, diagnosis
Introduction
Altered metabolism in human cancers has long been recognized. The first observation of increased anaerobic glycolysis in cancer cells was made by Otto Warburg, the so called “Warburg effect” [1]. The “Warburg effect” has now become a hallmark of the transformed phenotype of cancer cells, and is thought to provide growth advantages to these cells [2, 3]. One of the metabolic changes in cancer is the altered lipogenic pathway with increased de novo fatty acid synthesis [4].
Fatty acids serve as important substrates of metabolism for energy, essential building blocks of cellular membranes, intracellular second messengers, and anchorage for membrane proteins. Fatty acids exist either as components of triacylglycerol, phospholipids and cholesterol or in free forms. Free fatty acids include dietary ones and the ones derived from de novo synthesis catalyzed by fatty acid synthase (FASN) in lipogenic tissues such as liver, adipose tissue, lactating breast and cycling endometrium.
However, the altered lipogenic pathway in cancers did not become a focus of interest until 1994, when Kuhjada and colleagues identified the oncogenic antigen-519 (OA-519), a molecule found in tumor cells from breast cancer patients with markedly worsened prognosis, as fatty acid synthase (FASN) [5]. Human FASN is a 270-kDa cytosolic enzyme [6, 7]. It is also referred as the cytosolic type I FASN complex while type II fatty acid synthesis system exists in mammalian mitochondria, which resembles the prokaryotic type II FASN. It is believed that the type II system produces fatty acids that play important roles in the mitochondrial function [8]. The type I FASN has recently been shown to have oncogenic activity [9, 10] and its inhibition has been shown to effectively and selectively kill cancer cells, with minimal side effects to normal cells [11–17]. Thus, targeting type I FASN opens a new window of opportunity for metabolically combating cancers. In this review, we will focus on the cytosolic type I FASN protein and perform a critical review on the recent progresses in understanding the structure, function, and the role of FASN in cancers and pharmacological targeting FASN for human cancer treatment.
Structure and function of mammalian FASN
The de novo synthesis of fatty acids from glucose consists of the following key elements: 1) citrate lyase, which converts citrate to acetyl-CoA; 2) acetyl-CoA carboxylase, which carboxylates acetyl-CoA to malonyl-CoA and is the rate limiting enzyme for fatty acid synthesis; 3) nicotinamide adenine dinucleotide phosphate (NADPH) as a reducing equivalent and ATP as the energy source; and 4) FASN, the enzyme that condenses acetyl-CoA and malonyl-CoA to 16-carbon palmitate (Figure 1).
Figure 1.
De novo fatty acid synthesis. The de novo fatty acid synthesis pathway functions in both cancers and lipogenic tissues. In both cases, excess glucose goes through glycolysis and TCA cycle, and exits mitochondria as citrate which is then converted to acetyl-CoA by ATP citrate lyase. Carboxylation of acetyl-CoA to malonyl-CoA is catalyzed by acetyl-CoA carboxylase (ACC). FASN condenses one acetyl-CoA and seven malonyl-CoA into palmitate which can be then modified into various lipids such as phospholipids.
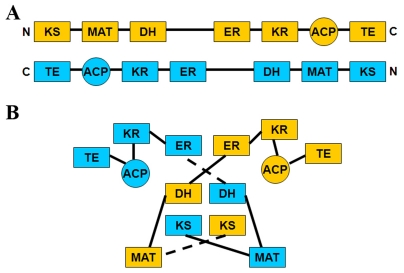
Mammalian FASN is a multifunctional polypeptide containing seven catalytic domains: (β-ketoacyl synthase (KS), malonyl/acetyltransferase (MAT), dehydrogenase (DH), enoyl reductase (ER), (β -ketoacyl reductase (KR), acyl carrier protein (ACP) and thioesterase (TE) [18] (see Figure 2A). In the conventional model of mammalian FASN, it was thought that FASN forms a fully extended head-to-tail homodimer (Figure 2A). However, results from mutant complementation [19, 20], chemical crossl-inking [21] and subunit interaction [22] studies were incompatible with this model. Therefore, a revised model was proposed, in which FASN forms an intertwined, X-shaped, head-to-head homodimer [23] (Figure 2B).
Figure 2.
Models of domain organization of FASN. (A) Conventional dimeric model of FASN. In this model, the two subunits in the homo-dimeric FASN are arranged in a fully extended head-to-tail organization. (B) Revised model of domain organization. In this revise model, FASN adopts an X-shaped dimeric form with each monomer in coiled structure to allow multiple intra- and inter-subunit interactions. KS = ketoacyl synthase; MAT = malonyl/acetyltransferase; DH = dehydrogenase; ER = enoyl reductase; KR=ketoacyl reductase, ACP = acyl carrier protein; TE = thio-esterase.
In the new model, each subunit in the dimeric FASN adopts a coiled conformation that allows multiple intra- and inter-subunit interactions between the functional domains, with the KS domain located in the central portion of the structure. This model was further supported by the results from cryo-electron microscopy and crystal structure studies [23–26]. The 3.2 Å crystal structure of FASN containing the MAT, KS, DH, ER and KR domains demonstrates that FASN assembles as an intertwined “X”-shaped dimer (Figure 3). The whole structure can be divided into two portions: the condensing portion including KS and MAT domains and the β-carbon modifying portion including DH, ER, and KR domains. In addition, two nonenzymatic domains, “pseudo-methyltransferase” (ψME) and “pseudo-ketoreductase” (ψKR) are located at the periphery of the modifying portion. The two subunits associate with each other mainly through hydrophobic interactions between the KD, ER and DH domains of the two subunits and have a buried surface area of 5400 Å2.
Figure 3.
Atomic structure of FASN. The overall structure of FASN dimer is X-shaped (viewed in perpendicular to its pseudo-2-fold axis). One subunit is colored by different shades of blue and green for different domains. The other subunit is in infrared colors ranging from magenta to orange. The two non-enzymatic domains, pseudo-ketoreductase (ΨKR) and pseudo-methyltransferase (ΨME), are colored in gray and black, respectively, for both subunits. This figure was created from FASN structure (PDF ID: 2VZ8)
The FASN-catalyzed synthesis of fatty acids involves three major steps: (1) initiation with the condensation of malonyl-CoA and acetyl-CoA catalyzed by MAT; (2) elongation, a repeating cycle of reduction and dehydration to add 2 carbons in each cycle to the elongating fatty acid chain catalyzed by KS, DH, ER, and KR; and (3) termination to release palmitate from ACP catalyzed by TE (Figure 4). Synthesis of one palmitate consumes 1 acetyl-CoA, 7 malonyl-CoA, 7 ATP, and 14 NADPH molecules.
Figure 4.
FASN-catalyzed palmitate synthesis. FASN-catalyzed palmitate synthesis involves three steps: initiation, elongation, and termination. The initiation step involves condensation of acetyl-CoA and malonyl-CoA catalyzed by the MAT domain. The elongation step of condensation of additional malonyl-CoA is catalyzed by KS, KR, DH, and ER domains. The final step of termination is catalyzed by the TE domain to release palmitate from FASN.
Regulation of FASN expression
In normal adults, FASN is primarily expressed in hormone-sensitive cells and cells with high lipid metabolisms [27]. FASN expression in normal liver and adipose tissues is controlled mainly by nutritional signals. In a well-nourished individual, normal cells preferentially use circulating free fatty acids from diet. Thus, the de novo fatty acid synthesis is rarely needed and the FASN protein level is low. Carbohydrate ingestion, thyroid hormone, insulin, and glucocorticoid coordinately up-regulate while unsaturated fatty acids, cyclic-AMP, and glucagon down-regulate FASN expression [28]. In cycling endometrium, FASN expression is high in the proliferate phase and decreases in the secretory differentiation phase. Proliferative gland and stroma cells have high levels of FASN, as well as high levels of estrogen and progesterone receptors and Ki-67, indicating that FASN expression may be under the control by hormone and associate with proliferation [29]. In lactating breast tissues, FASN expression is up-regulated to produce milk fat [30].
In cancer cells and pre-neoplastic lesions, the expression of FASN has been found to be up-regulated [29, 31–45]. Because of FASN up-regulation, over 90% of the triacylglycerol in cancer cells are synthesized de novo despite the presence of high levels of circulating free fatty acids. Cancer cells are so dependent on de novo fatty acid synthesis that inhibition of lipogenesis targeting FASN induces apoptosis selectively in human cancer cells both in vitro and in vivo [46–49], with minimal effect on normal cells [17,50,51].
FASN expression in cancer cells is no longer responsive to the nutritional signals and its expression is regulated at multiple steps including gene amplification, transcription, translation and post-translational modifications. The increased FASN gene copy number has been found in prostate cancer cell line PC-3 and LNCaP, as well as in prostate adenocarcinoma and metastatic cancers [52]. The increased FASN staining in tumor tissues correlates with a 25% increase in gene copy number, whereas in benign tissues, only 1% of the cells with high FASN staining showed increased gene copy number. Thus, gene amplification in cancer cells may partly contribute to the increased FASN expression in prostate cancers.
Transcriptional regulation of FASN expression has been well-studied and is considered the major contributor to the increased FASN expression in cancer cells. Growth factors, hormones and their receptors have been shown to be the main factors that cause up-regulation of FASN transcription in cancer cells. Epidermal growth factor (EGF) can stimulate FASN expression through EGF receptor ERBB1 and ERBB2 [53, 54]. In breast and prostate cancer cells that have functional hormone receptors, FASN expression has been shown to be up-regulated at transcriptional level upon hormone treatment [55–57].
The effect of growth factors or hormones and their receptors on FASN expression involves complicated downstream signaling and crosstalk between multiple signal transduction pathways. The two well-studied major pathways that are possibly involved in regulating FASN expression are the mitogen-activated protein kinase (MAPK) and PI3K/AKT pathways. In H-ras transformed and immortalized human mammary epithelial cell line MCF10A1, FASN expression was significantly elevated upon EGF treatment [58]. Treatment of this cell line with MEK-1 inhibitor, U0126, blocked ERK activation and subsequently decreased FASN expression, while transient transfection of MCF10A1 cells with constitutively activated MEK-1 increased FASN expression. Similarly, MAPK inhibitors also decreased FASN promoter activity and FASN protein level in MCF7 and HCT116 cancer cells [58]. In another study, EGF was found to up-regulate the promoter activity of FASN and its expression while MAPK inhibitor abolished the EGF-stimulated FASN expression [59]. These observations suggest that MAPK pathway plays an important role in regulating and mediating EGF-stimulated FASN expression.
Multiple studies have demonstrated the relationship between PI3K/Akt activity and FASN expression. In the first study by Van de Sande et al. [60], it was found that the PI3K/Akt pathway was involved in FASN expression in the PTEN-null prostate cancer cell line LNCaP. Treatment with PI3K-specific inhibitor, LY294002 and transfection with PTEN both significantly decreased FASN expression level as well as FASN transcriptional activity. Co-transfection of constitutively active Akt with PTEN reversed the inhibitory effect of PTEN on FASN expression and its promoter activity. In human prostate tumors, an inverse correlation between FASN and PTEN expression has also been observed [61]. In several later studies of different cell lines, it was confirmed that PI3K inhibitors could reduce FASN expression in various cancer cell lines [58, 61–64]. PI3K/Akt pathway has also been suggested to mediate the induction of FASN expression by heregulin [63] and diogenin [64]. Hypoxia, which causes the generation of reactive oxygen species, can also up-regulate FASN expression thorough activation of Akt. Addition of H2O2 in several breast cancer cell lines increased FASN expression, which is in good agreement with the amount of ROS generated in these cell lines under hypoxic condition [65].
The relationship between PI3K/Akt pathway and FASN expression has also been observed in clinical studies of human cancer tissues. Following the initial observation that PI3K/Akt may regulate FASN expression in LNCaP cells, van de Sande et al. investigated this relationship in prostate cancer tissues and found that the increased FASN expression correlates with activation and nuclear localization of Akt [66]. In another study of more than 400 papillary thyroid carcinoma tissues, a significant correlation was also observed between FASN expression and PI3K/Akt activation using immunohistochemis-try [67]. Yet in a third study of more than 400 colorectal cancer tissues on a tissue array, a significant correlation between FASN expression and PI3K/Akt activation was also found [68]. Together, these observations suggest that PI3K/Akt pathway may play important roles in regulating FASN expression, not only in cultured cells but also in human cancer tissues. However, it is noteworthy that the findings of these correlation studies are also consistent with the possibility that FASN over-expression up-regulates PI3K/Akt activation (see discussion below).
The major transcription factor that is involved in regulating FASN transcription is sterol regulatory element binding protein 1 (SREBP-1). SREBP-1 is one of the two SREBP membrane bound transcription factors of the basic-helix-loop-helix-leucine zipper family that regulate fatty acid and cholesterol synthesis [69]. The membranebound SREBPs are activated and released from membranes by protease cleavage in response to fatty acid and cholesterol depletion. The active SREBPs then translocate into nucleus and activate gene transcription. It has been suggested that SREBP-1 is important in regulating fatty acid synthesis while SREBP-2 is for cholesterol synthesis [70, 71].
In the androgen responsive prostate cancer cell line LNCaP, it was reported that androgen increased mRNA and protein levels of SREBP precursors and the mature active SREBP as well as elevated FASN transcript and protein levels [72]. The increased FASN transcription appeared to depend on the presence of SREBP biding site in the FASN promoter sequence. Deletion of this site abrogated the transcriptional activation of FASN by androgen. It has also been shown that androgen not only increased expression and activation of SREBPs, but also the expression of SREBP-activating protein (SCAP), that helps transport SREBP from their synthesis site to the proteolytic activation site and, therefore, enhances the maturation of SREBP [73].
SREBP also appears to mediate the regulation of FASN expression by growth factors such as EGF [59]. Using LNCaP prostate cancer cell lines, it was found that EGF stimulated FASN expression and its promoter activity can be stimulated by EGF via its SREBP-binding site. Introduction of a dominant negative SREBP eliminated EGF-stimulated FASN expression. In another study, FASN, SREBP-1 and Ki-76 were found to co-localize in primary human colorectal carcinoma specimens [74]. Similarly, in human mammary epithelial cell line MCF-10A1 and cancer cell line MCF-7 as well as a panel of primary human breast cancer tissues, it was found that the coordinated elevation of FASN and SREBP-1 was under the control of EGF and its downstream PI3K/Akt and MAPK pathway [75].
A transcriptome analysis of Her2 (ERBB2) in breast cancer cells has revealed a molecular connection between FASN and Her2 through PI3K-Akt-dependent signaling [53]. In this study, the authors used DNA microarray to compare and identify genes induced by Her2 in mammary epithelial cell line with ectopic Her2 over-expression and breast cancer cell lines derived from patients with different level of Her2 expression. They found that Her2 over-expression activated FASN promoter and transcription as well as increased protein production and activity, while inhibitors of Her2, Herceptin and CI-1003, attenuated the effect of Her2 on FASN expression. PI3K activity was thought to be the mediator of the Her2 control on FASN expression because LY294002, a known PI3K inhibitor, abrogated Her2-induced FASN protein production in the Her2-over-expressing normal mammary epithelial and breast cancer cells. Thus, the transcription of FASN gene may be induced by Her2 via the PI3K pathway and possibly by the transcription factor, SREBP, as a downstream effector of Her2-PI3K pathway [53].
However, a later study by Yoon et al. showed that Her2 regulation of FASN expression might be at the step of translational control [76]. In this study, breast cancer cell lines SK-BR-3 and BT-474 with high expression of Her2 were compared with MCF7 and MDA-MB-231 that have low levels of Her2 expression and a correlation between the levels of FASN and Her2 was found. However, the total and the activated nuclear level of SREBP1 and SREBP2 did not correlate with FASN expression. Furthermore, ectopically over-expressing Her2 in MDA-MB-231 breast cancer cells induced an increase in the level of FASN protein but not the level of FASN mRNA. These findings indicate that Her2-induced FASN protein production in MDA-MB-231 cells is not at the transcriptional step via SREBP. Nevertheless, the PI3K inhibitor LY294002, blocked Her2-induced FASN expression, suggesting that the PI3K/Akt pathway is indeed involved in mediating Her2-induced FASN expression. It was further shown that the PI3K downstream target mTOR mediates the regulation of FASN expression by increasing the overall translation rates of FASN mRNA via activating elF4E and S6 ribosomal protein and that both the 5'- and 3'- UTRs of FASN are involved in its translational regulation.
Both above studies clearly showed that Her2 induces FASN expression via the PI3K/Akt pathway. However, the difference resides in the step, transcription or translation, at which FASN expression is up-regulated by Her2. This discrepancy between these two studies may be due to the different cell lines used and suggests that both transcriptional and translational regulations may be involved in FASN expression. These findings clearly indicate that the regulation of FASN expression is complicated.
Regulation of FASN expression at its post-translational stability/degradation step has also been suggested. In prostate cancer cells, FASN protein stability has been shown to be regulated by an ubiquitin-specific protease, USP2a [77]. Knockdown of USP2a reduced FASN expression. Microarray analysis from human prostate cancers has revealed a significant association between the genes in fatty acid metabolism and high USP2a expression [78].
In drug-selected breast cancer cell lines, it was found that FASN expression was further up-regulated compared to its parental cancer cell line [17]. The mechanism for this further up-regulationof FASN remains unknown. However, it has been observed that treatment with topoisomerase inhibitors doxorubicin (Adriamycin) and etopside increased FASN promoter activity in SK-Br3 breast cancer cells [79]. This druginduced transcriptional activation of FASN did not appear to be via SREBP-binding site in the FASN promoter sequence. In our recent studies, both mRNA and protein levels of FASN increased in the series of stepwise drug-selected MCF7/AdVp cell lines compared with drug sensitive MCF7 parental cells (Figure 5). However, there is an obvious discordance in the mRNA and protein expression level of FASN in these drug-selected cells lines. The mRNA level increased to the maximum level in the low resistant cell line MCF7/AdVp100 while FASN protein level increased to the maximum level only in the most resistant MCF7/AdVp3000 cells although both mRNA and protein levels dropped in the partially revertant MCF7/AdVpRev cells that have lost most of its drug resistance following extended growth of MCF7/AdVp3000 cells in the absence of selection pressure. Based on theses observations, it is reasonable to speculate that in drug resistant cell lines, FASN expression is controlled at multiple levels including transcriptional and post-transcriptional regulation.
Figure 5.
FASN expression in MCF7 and the stepwise-selected drug resistant and revertant cell lines. (A). Western blot analyses. 20 mg proteins, each from MCF7, its stepwise-selected MCF7/AdVplO, MCF7/AdVplOO, and MCF7/AdVp3000 cells as well as the revertant cell line MCF7/Rev, were separated by SDS-PAGE followed by western blot analyses using FASN antibody. GAPDH was used as a loading control. (B). Real time RT-PCR analyses. RNAs isolated from MCF7, its stepwise-selected MCF7/AdVplO, MCF7/AdVplOO, and MCF7/AdVp3000 cells as well as the revertant cell line MCF7/Rev were subjected to real time RT-PCR analysis using SYBR green. The relative level of FASN mRNA calculated in the fold change (2ΔΔCt) relative to that in MCF7 cells after normalization by internal control, GAPDH.
Role of FASN in tumorigenesis and cancer cell proliferation
Increased FASN expression level has been found in various human cancers of breast [31], colon [32], prostate [33], lung [34], bladder [35], ovary [36], stomach [37], endometrium [29], kidney [38], skin [39], pancreas [40], head and neck [41], tongue [42–44], and soft tissues [45]. In addition, increased FASN expression has been observed in ductal carcinoma in-situ (DCIS) [80] and lobular carcinoma in situ (LCIS) [81] of breast. Thus, FASN has been considered as a metabolic oncogene.
The first evidence that shows the oncogenic function of FASN was from in-vitro studies where transient over-expression of ectopic FASN increased the proliferation, survival, and anchorage-independent growth of an immortalized breast epithelial cell line HBL100 [10]. Immortalized human prostate epithelia cell lines (iPrECs) with ectopic FASN over-expression had an increased rate of proliferation and anchorage-independent growth in soft agar in vitro, similar as the breast epithelial cell line HBL100 [10]. Histological examination of the prostate section of FASN transgenic animals showed prostate lumens full of proliferating cells, indicating the prostate hyperplasia. Several older male mice showed enlarged prostate which blocks the bladder outflow. However, there were no invasive prostate carcinomas observed in these mice. The above findings suggest that FASN over-expression alone may not be sufficient to generate prostate tumors in vivo. Indeed, over-expression of androgen receptor together with FASN transformed iPrECs to form invasive tumors in immune deficient mice [9], suggesting that the oncogenic function of FASN in prostate epithelial cells may require the coordination of androgen receptors. It is also possible that the oncogenic function of FASN in mammary epithelial cells require estrogen receptor. Clearly, this possibility and the mechanism of coordination of FASN with hormones in tumorigenesis require further investigation.
In both the above studies, it was clearly demonstrated that ectopic over-expression of FASN caused significant increase in proliferation of the non-tumorigenic mammary and prostate epithelial cells. Inhibition of FASN by siRNA or chemical inhibitors also caused significant growth arrest of cancer cells [82–85]. It is, however, noteworthy that it has also been observed that altering the FASN level either by siRNA or ectopic over-expression did not affect the growth rate of MCF7 and MCF7-derivied drug resistant breast cancer cells [17]. Although the reason for the difference between these studies is not known, it is possible that the extent of FASN inhibition may be the culprit. Nevertheless, more studies are clearly needed to demonstrate the role of FASN in promoting cell proliferation.
The possible role of FASN in promoting cell proliferation may be via affecting cell cycle progression. It was observed that inhibiting FASN activity by C75 produced rapid and potent blockage of DNA replication and inhibits S phase progression [86]. In another study, it was found that inhibiting FASN expression or activity resulted in arrest in G1/S phase transition [83]. This arrest of G1/S phase transition was thought to be due to the effect of FASN on Rb pathway. Inhibiting FASN activity reduced phosphorylation of the Rb protein, a parameter that governs the interaction of this protein with E2F-1 and subsequent entry into S phase; up-regulated p27Kip1, which negatively regulates cyclin-dependent kinase activity; and down-regulated Skp2, a protein component of the E3 ubiquitin ligase that regulates degradation of p27Kip1[83]. This observation was later confirmed by a genome-wide analysis of FASN knockdown using siRNA [87]. In the genome-wide analysis, several other genes such as p21 that regulate cell cycle progression were also found to be up-regulated by FASN knockdown. This observation is consistent with an earlier study that a biphasic stress response was found with a transient accumulation in S and G2 at 4 and 8 hrs and a marked reduction in cyclin A- and B1-associated kinase activities, and then growth arrest in G1 and G2 with accumulation of p53 and p21 proteins at 16 and 24 hrs following FANS inhibition [84]. However, it was found later that the cell cycle arrest induced by FASN inhibition was independent of p53 in hepatoma cell lines, but may involve the p38 MAPK pathway [82]. Thus, the role of p53 in mediating FASN-inhibition-induced cell cycle arrest is debatable and certainly needs further investigation.
Currently, it is not clear how FASN inhibition induces cell cycle arrest at G1/S checkpoint. However, it is possible that FASN inhibition significantly reduces the synthesis of phospholipids [88, 89], which are major components of cellular membranes, and phospholipids biosynthesis is highest in S phase in preparation for cell division [90]. Thus, shortage in phospholipids due to reduced FASN expression may cause arrest at G1/S checkpoint or inhibit S phase progression. Nevertheless, supplementation of palmitate, the end product of FASN, to culture did not appear to affect cell cycle distribution [91]. Ectopic over-expression of human FASN in MCF7 cells also did not appear to affect cell cycle distribution (Figure 6). Hence, whether FASN really plays any role in cell cycle regulation requires further detailed investigation.
Figure 6.
Cell cycle analysis of FASN over-expressing MCF7 cells and vector transfected control MCF7 cells. 5x105 cells were harvested, labeled with propidium iodide and analyzed by flow cytometry analysis for stage of cell cycle, G0/G1, S, G2/M.
Role of FASN in prognosis and drug resistance
As discussed above, FASN was initially identified as an independent prognostic molecule in breast cancer cells from patients with markedly worsened prognosis [5, 92]. Breast cancers with high level of FASN staining were 4 times more likely to recur and metastasize than the ones with no staining [92]. Further studies of breast cancer samples indicated that patients with high FASN expression showed significantly shorter disease free survival and overall survival, even in patients with very early stage of breast cancer [31, 93]. It is now clear that increased FASN expression associates with cancer progression, higher risk of recurrence and shorter survival in many other types of cancers including prostate cancer [94], ovarian neoplasms [36], squamous cell carcinoma of lung [34], melanoma [39], nephroblastoma [38], renal cell carcinoma [95], soft tissue sarcoma [45], endometrium carcinoma [96], head and neck squamous cell carcinoma [41], pancreatic carcinoma [40], squamous cell carcinoma of the tongue [44], and colorectal carcinoma [97].
In-vitro studies with cancer cell lines also showed that FASN over-expression may cause resistance of cancer cells to treatments and, thus, contribute to clinical poor prognosis. In a recent study, FASN was found to be over-expressed in Adriamycin-selected breast cancer cell line with multidrug resistance phenotype and its expression increases with the level of resistance [17]. FASN over-expression in the drug selected breast cancer cell line has been demonstrated to contribute to the multidrug resistance phenotype of this cell line possibly by over-producing palmitate. The observed gradual increase in FASN expression in the series of stepwise-selected drug resistant breast cancer cell lines suggests that tumor cells with elevated FASN expression in a clinical setting may be selected following anticancer drug treatment which in turn causes relapse of the disease and eventual failure of treatments.
In an earlier study, Menendez et al reported that inhibiting FASN activity synergistically enhanced the cytotoxicity of docetaxel, in the Her2-over-expressing breast cancer cell lines [98]. Inhibiting FASN expression or activity also sensitized cancer cells to vinorelbine [48], paclitaxel [99], 5-fluorouracil [100], Herceptin [101, 102], and TRAIL [103]. Because the Her2-over-expressing cancer cell lines were used in most of these studies, it was thought that Her2 may play an important role in the drug resistance observed. Indeed, inhibiting FASN expression and activity reduced Her2 expression [98]. Thus, it is possible that inhibiting FASN down-regulates Her2 which in turn causes sensitization of the cancer cells to the anticancer drugs tested in these studies. On the other hand, it was thought that the DNA damage-inducible transcript 4 (DDIT4), a stress-response gene that negatively regulates the mTOR pathway, may mediate the role of FASN in TRAIL-induced apoptosis [101]. It is also noteworthy that in the study of MCF7-derived cells by Liu et al. [17] it was found that FASN over-expression caused resistance only to DNA-damaging anticancer drugs but not to paclitaxel and vinca alkaloid vinblastine. Further studies are needed to resolve the differences between these studies.
Both in-vitro studies and clinical data indicated that elevated FASN expression confers cancer cell resistance to anti-cancer therapies, which may be the reason of shorter survival of patients with high FASN expression. Although the detailed mechanism of drug resistance induced by FASN over-expression is currently unknown, the findings from the past studies suggest that FASN may regulate survival, apoptosis, and DNA repair pathways (see discussion below).
Mechanism of FASN action in cancers
As discussed above, several signal transduction pathways may mediate the function of FASN in tumorigenesis and resistance to drug treatments. Although the detailed mechanism of FASN action in signal transduction pathways remains to be determined, various hypotheses have been proposed.
Fatty acids synthesized by FASN in cancer cells are not only used for cellular membrane construction, but also involved in the production of lipid signaling molecules, anchorage of membrane proteins, and modulate cellular responses to anticancer drugs. It is possible that the increased de novo synthesis of palmitate by FASN over-expression plays an important role in mediating FASN effect on Her1/Her2 activation. Recently, it was shown that palmitoylation of Wnt-1 by enforced expression of ectopic FASN in immortalized human prostate epithelia cells (iPrECs) stabilized and activated β-catenin and resulted in increased oncogenicity of the iPrECs cells [104]. It has also been found that supplementation of palmitate to primary mouse embryonic fibroblast (MEF) and primary osteoblasts compromised the normal response of these cells to DNA damages, favoring the mutated cells to survive and leading to tumorigenesis [91]. Supplementation of palmitate to cancer cells also increased the ability of these cells to resist DNA damaging anticancer drugs Adriamycin and mitoxantrone [17].
Palmitoylation helps to locate the palmitoylated proteins to specific regions of plasma membranes, a detergent-resistant membrane micro domain (lipid raft) for their proper functions [105]. Many proteins involved in signal transduction, apoptosis, membrane transport, and cell adhesion are associated with lipid raft [106–108]. Her2 is one example of the signaling proteins that co-localize with lipid rafts [101]. It has been shown that FASN mainly affects the synthesis of phospholipids that incorporate into lipid raft, but has a less effect on the synthesis of non-raft associated lipids [88]. Elevated FASN expression may also contribute to the increased ratio of saturated to unsaturated fatty acids and, thus, affect the structure and function of membrane lipids [109]. Changes in lipid rafts and membrane structures due to FASN over-expression likely affect the signaling proteins residing in the raft to enhance cancer cell survival and progression.
Recently, it was found that knocking down FASN expression using siRNA induced apoptosis by activating caspase-8 in tumor cells and inhibition of FASN sensitized cancer cells to TRAIL treatment [103]. It was also found that over-expressing ectopic FASN blocked caspase-8 activation-induced by Adriamycin (unpublished observation). These findings suggest that FASN may function by regulating the apoptosis pathway upstream of caspase-8 activation. One important mediator may be ceramide lipid molecules. Inhibition of FASN in several breast cancer cell lines by siRNA treatment induced cancer cell apoptosis by up-regulating ceramide synthesis. The increased ceramide level is thought to be the result of malonyl-CoA accumulation due to FASN inhibition, which in turn inhibits carnitine palmitoyltransferase (CPT-1) [110]. It was also found that several proapoptotic genes including BNIP3, TRAIL and DAPK2 were induced following FASN inhibition and these genes may play a role in mediating FASN inhibition-induced apoptosis [110]. We recently found that FASN over-expression in MCF-7 breast cancer cells decreased ceramide generation-induced by Adriamycin and, thus, inhibited the drug-induced apoptosis (unpublished observation). However, a recent study showed that FASN over-expression in prostate epithelial cells protects these cells from camptothecin induced apoptosis by inhibiting caspase-9 activation, but did not protect cells from anti-Fas ligand-induced apoptosis which activates caspase-8 [9]. The reason for the difference between this and the other studies on the role of FASN in caspase activation is currently unknown. However, the normal prostate epithelial cells may differ from breast cancer cells in the mechanism of FASN regulation of apoptosis.
PI3K/Akt survival pathway is known to be important for cancer cell survival and resistance to chemo-and radiation therapy [111–113]. As discussed above, activation of the PI3K/Akt pathway can increase FASN expression in human cancers for cell survival both in vivo and in vitro. However, PI3K/Akt pathway may also play an important role in mediating FASN function in a feed forward loop. It has been found that inhibition of FASN activity caused a decrease in the level of phosphorylated-Akt, which preceded the induction of apoptosis both in vitro and in vivo [14, 15, 62, 99]. It has also been observed that inhibition of the PI3K/Akt pathway by LY294002 sensitized human ovarian and breast cancer cells to FASN inhibitor-induced apoptosis [62, 114], indicating that Akt could serve as a downstream mediator of FASN in cell survival and protects cancer cells against FASN inhibitor and other drug-induced apoptosis.
FASN has also been suggested to play an important role in regulating gene expression. A genomic profiling analysis of a breast cancer cell line MDA-MB-435 following FASN knockdown using siRNA showed that FASN likely regulates the expression of genes of a variety of biological processes including cell proliferation, DNA replication and transcription, as well as apoptosis [87]. Although the mechanism of this regulation is currently unknown, it is possible that several signal transduction pathways that are under FASN control may mediate the FASN regulation of gene expression. In addition to regulating genes at their transcriptional level, FASN also appears to play a role in regulating gene expression at translational level. It has been found recently that knocking down FASN expression or treatment with FASN inhibitor Orlistat inhibited phosphorylation of translation initiation factor, elF4E, which regulates the synthesis of many growth-controlling proteins [103].
FASN inhibitors
Cancer cells are so dependent on de novo fatty acid synthesis that inhibition of lipogenesis targeting FASN induces apoptosis selectively in human cancer cells both in vitro and in vivo [46–48, 98], with minimal effect on normal cells [17, 50, 51]. The differential expression of FASN, together with the different responses to FASN inhibition between cancer and normal cells, makes FASN a suitable target for cancer treatment with good therapeutic index. Indeed, pharmacological inhibitors of FASN have been identified and shown to block tumor cell proliferation, elicit tumor cell death, and prevent tumor growth in animal models. These studies confirmed the potential use of FASN inhibitors as novel antitumor therapeutics (Table 1). These pharmacological inhibitors are discussed in more detail below.
Table 1.
FASN inhibitors and their acting sites in FASN
| Name | FASN Domain targets | References |
|---|---|---|
| Cerulenin | KS | [115, 116] |
| C75 | KS, ER, and TE | [127] |
| C93 | KS | [141] |
| Orlistat and β-lacton derivative | TE | [16, 158–160] |
| 5-(furan-2-ylmethylene) pyrimidine 2,4,6-trione | TE | [161] |
| Tea polyphenol (EGCG and ECG) | KR | [150] |
| EGCG hydrolytic product | MAT | [155] |
| Flavonoid | KR | [156] |
| Triclosan | ER | [157] |
Cerulenin
Cerulenin [(2R.3S), 2-3-epoxy-4-oxo-7,10-trans.transdodecadienamide], isolated from the culture filtrate of the fungus Cephalosporum caerulens, is the first known FASN inhibitor that inhibits the biosynthesis of fatty acids and steroids [115, 116]. Cerulenin is a potent non-competitive irreversible inhibitor [117–119] of all known types of FASN, from bacteria to yeast to mammal, and is originally used as an antifun-gal antibiotic. The crystal structure of a fungal FASN in complex with cerulenin revealed that it covalently binds to a cysteine residue in the active site of the KS domain and causes significant conformational changes [120]. Cerulenin treatment significantly decreased fatty acid synthesis in cancer cells [89] and induced selective cytotoxicity in various types of cancer cells [13, 121, 122], delayed the disease progression in an ovarian cancer xenograft model [11], as well as suppressed liver metastasis in a colon cancer xenograft model [123]. However, cerulenin has a limited clinical relevance because of its highly reactive epoxy group that may interact with other cellular processes besides FASN-catalyzed lipid synthesis, including palmitoyla-tion, proteolysis, and antigen processing [124–126].
C75
To increase the potential applicability of cerulenin, an analogue, C75 (trans-4-carboxy-5-octyl-3-methylenebutyrolactone), has been synthe-sized by eliminating the reactive epoxy group [127]. Different from cerulenin which acts as a non-competitive inhibitor of the KS domain only, C75 acts as a competitive irreversible inhibitor of FASN on the KS domain, as well as the ER and TE domains [128] although the reason for this difference is currently unknown. In addition, C75 appears to be easily inactivated by DTT in solutions [128], suggesting that its efficacy in vivo may be affected by endogenous thiols such as glutathione. Nevertheless, C75 has been shown to have significant antitumor effects on cancer cell lines of human breast [12], prostate [13], mesothelioma [129], and ovary [62], as well as renal carcinoma in xenograft animal model [95].
Although both cerulenin and C75 have apparent antitumor effect, they both also have a side-effect in inducing profound weight loss which is far from ideal for cancer patients undergoing chemotherapy. This side effect is apparently due to their activity in increasing fatty acid oxidation through direct activation of CPT-1 [130–136]. They also reduce food intake by blocking the production of hypothalamic neuropeptide-Y [135, 137, 138]. These side effects clearly hinder the further clinical development of cerulenin and C75. A newer generation of cerulenin derivative, C93, was rationally designed to have FASN inhibitory effect without parallel stimulation of fatty acid oxidation [139] and was shown to inhibit tumor growth in vivo in a preclinical model of lung cancer, without causing anorexia or weight loss [15, 140, 141]. C93 may prove to be an interesting lead for further development into a clinical useful FASN inhibitor.
Olistat
Orlistat (tetrahydrolipstatin) is a reduced form of the natural product lipstatin and currently marketed as the first US Food and Drug Administration (FDA) approved over-the-counter antiobesity medication. Orlistat works primarily on pancreatic and gastric lipase within the gastrointestinal (G1) tracts [142] by blocking hydrolysis of triglycerides and, thus, uptake of fatty acids from the diet [117, 143]. In 2004, Orlistat was found to also inhibit FASN in an activity-based screening for inhibitors of serine hydrolases in prostate cancer cells and it acts as a tight-binding irreversible inhibitor of the TE domain of FASN [16]. Several follow-up studies has shown that Orlistat exhibits antitumor effects toward melanoma, breast and prostate cancer cells in vitro and in vivo by inhibiting FASN activity, with no adverse effect on normal cells [16, 17, 50, 83, 102, 103, 144–146]. Orlistat could also sensitize drug resistant breast cancer cells to Adriamycin and mitoxantrone via inhibiting FASN [17], validating FASN as a chemosensitization target. Orlistat treatment also appears to induce endoplasmic reticulum stress and apoptosis of tumor cells [145] and inhibit endothelial cell proliferation and angiogenesis [50] although the detailed mechanisms of these actions are currently unknown. Nevertheless, because Orlistat has a very poor solubility and oral bioavailability, its potential in clinical application for systematic use in cancer chemotherapy is very limited.
One important aspect of Orlistat is that a co-crystal structure of Orlistat and the TE domain has been solved [147] which shows that Orlistat binds to the active sites of TE dimers in two forms: a covalent bounded acyl-enzyme intermediate and a hydrolyzed product (Figure 7). The intermediate is a Ser2308 adduct that interacts with the protein surface of the TE mainly by hydrophobic interactions (Figure 7A). The Orlistat-binding pocket in TE domain can be divided into three portions: a cavity that holds the N-formyl-L-leucine moiety of Orlistat, the specificity channel where the 16-carbon palmitate core binds and the short-chain pocket that fits in the hexanoyl tail. The electrostatic field presented by Glu2431 and Arg2428 and the constriction caused by Ala2363 and Tyr2424 could be the cause of the preference for 16-carbon-containing substrates. The oxyanion is stabilized by the main chain nitrogen atoms of lle2250 and Tyr2309 and a hydrogen bond with Glu2251. The interaction between the hexanoyl tail and His2481 may help prevent the activation of a water molecule that would result in the immediate hydrolysis of the intermediate. The hydrolyzed product of Orlistat has the C1 atom shifted out of the oxyanion hole and the hexanoyl tail shifted out of the original short-chain pocket (Figure 7B). The palmitate core also shifted about two carbon units toward the distal chamber of the cavity. These findings together with a molecular docking study [148] provide useful information for structure-based drug design targeting FASN in finding better inhibitors targeting the TE activity of FASN.
Figure 7.
Binding mode of Orlistat in the TE domain of FASN. Only the molecular surface of the TE domain where Orlistat binds is shown. The binding of Orlistat in the TE domain exists as a serine adduct intermediate (pink ball and stick, panel A) and hydrolyzed product (gray ball and stick, panel B). The serine intermediate is also superimposed to show the shifts and changes in the hydrolyzed product in panel B. This figure was generated from data in PDB (ID: 2PX6).
Polyphenols/flavonoids
Recently, many plant-derived natural compounds have been explored as FASN inhibitors. Green tea polyphenols and plant-derived flavonoids were mostly studied and shown to have promising antitumor effect related to their FASN inhibitory activity. Green tea polyphenols, epigal-locatechin gallate (EGCG) and epicatechin gal late (ECG), competitively inhibited the KR activity of FASN [149, 150]. EGCG can induce selective apoptosis in human breast and prostate cancer cells [151–153]. EGCG suppresses FASN expression through down-regulating EFG receptor and downstream PI3K/Akt pathway [63, 152]. The galloyl-moiety of the green tea polyphenol is critical for their effect on FASN inhibition, as ungallated polyphoenol showed no inhibitory effect on FASN [149]. EGCG had no stimulatory effect on fatty acid oxidation and does not induce weight loss in experimental animals as cerulenin and C75 [134]. Unfortu-nately, the potency of EGCG in inhibiting FASN is low with IC50 of 52 μM [154]. Recently, it was found that heating EGCG in acid could increase EGCG potency by 350 fold [155]. It was thought that EGCG underwent a series of reactions in acidic conditions that resulted in a smaller product of 211 Dalton which trimerizes to form a more active FASN inhibitor. Different from the original EGCG, this new trimeric product inhibits FASN in a competitive fashion at the MAT domain.
Consumption of food rich in flavonoids has been shown to decrease the incidence of various types of cancers. Flavonoids, such as luteoin, have been shown to inhibit FASN in-vitro and produce cytotoxic effects in breast, prostate and hepatocellular carcinoma cells [156]. The exact FASN domains inhibited by flavonoid are unknown. However, the structural similarity between flavonoids to EGCG suggests that they may also target the KR domain of FASN.
Triclosan
Triclosan (2,4,4-trichloro-2-hydroxydiphenyl ether) is a commonly used antibiotic in soaps, mouthwashes and oral dentifrices due to its ability to inhibit the type II enoyl-reductases in bacteria. This antibiotic has also been demonstrated to inhibit the enoyl-reductase activity of mammalian FASN and cause growth arrest and reduce cell viability in MCF-7 and SKBr-3 breast cancer cells in culture [157]. Triclosan showed little toxicity in experimental animals.
Other inhibitors
As discussed above, the FDA-approved anti-obesity drug, Orlistat, is poorly soluble with low bioavailability. To improve this drug, more (β-lactone type FASN inhibitors have been synthesized based on the structure of Orlistat [158–160]. Some of these compounds showed improved potency in inhibiting FASN activity and inducing tumor cell death, and some showed improved solubility compared with Orlistat [159]. In addition, from a high throughput screening of 36,500 compounds, 18 compounds were identified to have a novel 5-(furan-2-ylmethylene) pyrimidine 2,4,6-trione pharmacophore [161]. This class of compounds competitively inhibited the TE activity of human FASN, de-novo fatty acid synthesis, and induced FASN-dependent death of MDA-MB-435 breast cancer cells. These inhibitors of TE activity of FASN may prove to be useful clinically in future studies.
Taken together, the known FASN inhibitors have distinct inhibition mechanisms and the active site of FASN being inhibited include the KS, KR, ER, and the TE domain. Apart from their different working mechanisms, these inhibitors were all proven to be effective at eliciting anti-tumor effects, suggesting that any one of the six catalytic domains of FASN may be a promising target for drug discovery to inhibit FASN for cancer chemotherapy. As discussed above, the type II FASN in mitochondria consists of several dissociated individual enzymes resembling the bacterial type II FASN [162–164]. Although the mitochondrial type II FASN accounts for only a few percentage of the overall cellular fatty acid production, it provides the octanoyl group for the endogenous synthesis of lipoic acid. Defective mitochondrial type II FASN has been shown to cause a lethal syndrome of metabolic acidosis and renal tubular acidosis in infant [165] and cell death of human embryonic kidney 203T cells in culture [166]. Thus, future studies in drug discovery targeting FASN may need consideration in avoiding the domains that have high resemblance to the type II FASN in mitochondria.
Conclusion and future perspectives
As a large protein with a complex structure and multiple catalytic domains, FASN is slowly emerging and re-discovered as an important metabolic enzyme and potentially a target in human cancers. Numerous clinical and basic studies have shown that FASN over-expression confers cancer cells distinct growth advantages and the function of FASN in normal cells is limited only to the lipid-producing organs. Elevated FASN expression appears to be an early event in the process of tumorigenesis, and in response to chemotherapy and it is under regulation by several signaling pathways. Elevated FASN in cancer cells also appears to modulate lipid raft domains and various biological processes which in turn promote cell survival and/or prevent apoptosis induced by chemotherapeutic agents. However, the detailed mechanism on how FASN regulates these various biological processes is currently unknown. One hypothesis is the overproduction of palmitate which functions as a secondary messenger that relays signals to various biological signal transduction pathways for cell survival. This hypothesis is waiting to be tested.
Although it is now known that FASN may be a proto-oncogene and its over-expression promotes tumorigenesis and survival, how FASN is up-regulated in the first place in normal or preneoplastic cells to prime tumorigenesis is currently unclear. Future studies directed to understanding what up-regulates FASN may help reveal the secret regarding this issue. Findings from studies using cancer cells may contribute to this endeavor. However, studies using normal cells may be more fruitful.
The differences in the FASN expression level between normal and cancer cells, together with the specific cytotoxicity of FASN inhibition in cancer cells, as well as the role of FASN in chemotherapeutic resistance led to the exploration of FASN as a therapeutic target for cancer treatment. With the available crystal structure of FASN as well as co-crystal structures of FASN with its known inhibitors, more inhibitors of FASN are expected to emerge and some of these inhibitors may get tested in clinical settings in the foreseeable future. However, caution should be taken when targeting various catalytic domains of FASN for drug discovery to eliminate their potential effect on the mitochondrial type II FASN.
One other important area to watch for is the development of FASN as a diagnostic marker. Elevated FASN levels have been detected in the blood of patients with breast, prostate, colon, and ovarian cancers compared with normal subjects using ELISA [167], suggesting that FASN may be used as a diagnosis marker for cancers. This line of research may offer an important approach for early diagnosis of human cancers.
Acknowledgement
This work was supported in part by National Institutes of Health grant R01 CA 113384 (JTZ). HL and XW were recipients of the Department of Defense Predoctoral Fellowships W81XWH-06-1 -0490 and W81XWH-10-1-0057, respectively.
References
- [1].Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- [2].Shaw RJ., Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [3].Bui T, Thompson CB. Cancer's sweet tooth. Cancer Cell. 2006;9:419–420. doi: 10.1016/j.ccr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [4].Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- [5].Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, Pasternack GR. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003;42:289–317. doi: 10.1016/s0163-7827(02)00067-x. [DOI] [PubMed] [Google Scholar]
- [7].Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- [8].Zhang L, Joshi AK, Smith S, Cloning expression, characterization, and interaction of two components of a human mitochondrial fatty acid synthase. Malonyltransferase and acyl carrier protein. J Biol Chem. 2003;278:40067–40074. doi: 10.1074/jbc.M306121200. [DOI] [PubMed] [Google Scholar]
- [9].Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescan-dolo E, Shin E, Fiore C, Xie W, Kung AL, Febbo PG, Subramanian A, Mucci L, Ma J, Signoretti S, Stampfer M, Hahn WC, Finn S, Loda M. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101:519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA. Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif. 2008;41:59–85. doi: 10.1111/j.1365-2184.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–1193. [PubMed] [Google Scholar]
- [12].Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xeno-grafts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- [13].Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- [14].Alii PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24:39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- [15].Orita H, Coulter J, Tully E, Kuhajda FP, Gab-rielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clin Cancer Res. 2008;14:2458–2464. doi: 10.1158/1078-0432.CCR-07-4177. [DOI] [PubMed] [Google Scholar]
- [16].Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- [17].Liu H, Liu Y, Zhang JT. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther. 2008;7:263–270. doi: 10.1158/1535-7163.MCT-07-0445. [DOI] [PubMed] [Google Scholar]
- [18].Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. Faseb J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- [19].Joshi AK, Rangan VS, Smith S. Differential affinity labeling of the two subunits of the homodimeric animal fatty acid synthase allows isolation of heterodimers consisting of subunits that have been independently modified. J Biol Chem. 1998;273:4937–4943. doi: 10.1074/jbc.273.9.4937. [DOI] [PubMed] [Google Scholar]
- [20].Rangan VS, Joshi AK, Smith S. Mapping the functional topology of the animal fatty acid synthase by mutant complementation in vitro. Biochemistry. 2001;40:10792–10799. doi: 10.1021/bi015535z. [DOI] [PubMed] [Google Scholar]
- [21].Witkowski A, Joshi AK, Rangan VS, Falick AM, Witkowska HE, Smith S. Dibromopropanone cross-linking of the phosphopantetheine and active-site cysteine thiols of the animal fatty acid synthase can occur both inter- and intra-subunit. Reevaluation of the side-by-side, anti-parallel subunit model. J Biol Chem. 1999;274:11557–11563. doi: 10.1074/jbc.274.17.11557. [DOI] [PubMed] [Google Scholar]
- [22].Joshi AK, Rangan VS, Witkowski A, Smith S. Engineering of an active animal fatty acid synthase dimer with only one competent subunit. Chem Biol. 2003;10:169–173. doi: 10.1016/s1074-5521(03)00023-1. [DOI] [PubMed] [Google Scholar]
- [23].Asturias FJ, Chadick JZ, Cheung IK, Stark H, Witkowski A, Joshi AK, Smith S. Structure and molecular organization of mammalian fatty acid synthase. Nat Struct Mol Biol. 2005;12:225–232. doi: 10.1038/nsmb899. [DOI] [PubMed] [Google Scholar]
- [24].Maier T, Jenni S, Ban N. Architecture of mammalian fatty acid synthase at 4.5 A resolution. Science. 2006;311:1258–1262. doi: 10.1126/science.1123248. [DOI] [PubMed] [Google Scholar]
- [25].Maier T, Leibundgut M, Ban N. The crystal structure of a mammalian fatty acid synthase. Science. 2008;321:1315–1322. doi: 10.1126/science.1161269. [DOI] [PubMed] [Google Scholar]
- [26].Pappenberger G, Benz J, Gsell B, Hennig M, Ruf A, Stihle M, Thoma R, Rudolph MG. Structure of the human fatty acid synthase KS-MAT didomain as a framework for inhibitor design. J Mol Biol. 2010;397:508–519. doi: 10.1016/j.jmb.2010.01.066. [DOI] [PubMed] [Google Scholar]
- [27].Kusakabe T, Maeda M, Hoshi N, Sugino T, Wa-tanabe K, Fukuda T, Suzuki T. Fatty acid synthase is expressed mainly in adult hormonesensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48:613–622. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- [28].Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr. 1998;18:331–351. doi: 10.1146/annurev.nutr.18.1.331. [DOI] [PubMed] [Google Scholar]
- [29].Pizer ES, Lax SF, Kuhajda FP, Pasternack GR, Kurman RJ. Fatty acid synthase expression in endometrial carcinoma: correlation with cell proliferation and hormone receptors. Cancer. 1998;83:528–537. [PubMed] [Google Scholar]
- [30].Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis. Breast Cancer Res. 2007;9:204. doi: 10.1186/bcr1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474–482. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- [32].Visca P, Alo PL, Del Nonno F, Botti C, Trombetta G, Marandino F, Filippi S, Di Tondo U, Don-norso RP. Immunohistochemical expression of fatty acid synthase, apoptotic-regulating genes, proliferating factors, and ras protein product in colorectal adenomas, carcinomas, and adjacent nonneoplastic mucosa. Clin Cancer Res. 1999;5:4111–4118. [PubMed] [Google Scholar]
- [33].Epstein Jl, Carmichael M, Partin AW. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology. 1995;45:81–86. doi: 10.1016/s0090-4295(95)96904-7. [DOI] [PubMed] [Google Scholar]
- [34].Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC, Grizzle WE. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- [35].Visca P, Sebastiani V, Pizer ES, Botti C, De Carli P, Filippi S, Monaco S, Alo PL. Immunohistochemical expression and prognostic significance of FAS and GLUT1 in bladder carcinoma. Anticancer Res. 2003;23:335–339. [PubMed] [Google Scholar]
- [36].Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- [37].Kusakabe T, Nashimoto A, Honma K, Suzuki T. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40:71–79. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- [38].Camassei FD, Jenkner A, Rava L, Bosman C, Francalanci P, Donfrancesco A, Alo PL, Boldrini R. Expression of the lipogenic enzyme fatty acid synthase (FAS) as a predictor of poor outcome in nephroblastoma: an interinstitutional study. Med Pediatr Oncol. 2003;40:302–308. doi: 10.1002/mpo.10274. [DOI] [PubMed] [Google Scholar]
- [39].Innocenzi D, Alo PL, Balzani A, Sebastiani V, Silipo V, La Torre G, Ricciardi G, Bosman C, Calvieri S. Fatty acid synthase expression in melanoma. J Cutan Pathol. 2003;30:23–28. doi: 10.1034/j.1600-0560.2003.300104.x. [DOI] [PubMed] [Google Scholar]
- [40].Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27:2523–2527. [PubMed] [Google Scholar]
- [41].Silva SD, Agostini M, Nishimoto IN, Coletta RD, Alves FA, Lopes MA, Kowalski LP, Graner E. Expression of fatty acid synthase, ErbB2 and Ki-67 in head and neck squamous cell carcinoma. A clinicopathological study. Oral Oncol. 2004;40:688–696. doi: 10.1016/j.oraloncology.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [42].Krontiras H, Roye GD, Beenken SE, Myers RB, Mayo MS, Peters GE, Grizzle WE. Fatty acid synthase expression is increased in neoplastic lesions of the oral tongue. Head Neck. 1999;21:325–329. doi: 10.1002/(sici)1097-0347(199907)21:4<325::aid-hed6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [43].Silva SD, Cunha IW, Rangel AL, Jorge J, Zecchin KG, Agostini M, Kowalski LP, Coletta RD, Graner E. Differential expression of fatty acid synthase (FAS) and ErbB2 in nonmalignant and malignant oral keratinocytes. Virchows Arch. 2008;453:57–67. doi: 10.1007/s00428-008-0626-5. [DOI] [PubMed] [Google Scholar]
- [44].Silva SD, Perez DE, Nishimoto IN, Alves FA, Pinto CA, Kowalski LP, Graner E. Fatty acid synthase expression in squamous cell carcinoma of the tongue: clinicopathological findings. Oral Dis. 2008;14:376–382. doi: 10.1111/j.1601-0825.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- [45].Takahiro T, Shinichi K, Toshimitsu S. Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin Cancer Res. 2003;9:2204–2212. [PubMed] [Google Scholar]
- [46].De Schrijver E, Brusselmans K, Heyns W, Verho-even G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- [47].Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV, Kuhajda FP. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330–7337. [PubMed] [Google Scholar]
- [48].Menendez JA, Colomer R, Lupu R. Inhibition of tumor-associated fatty acid synthase activity enhances vinorelbine (Navelbine)-induced cytotoxicity and apoptotic cell death in human breast cancer cells. Oncol Rep. 2004;12:411–422. [PubMed] [Google Scholar]
- [49].Menendez JA, Mehmi I, Atlas E, Colomer R, Lupu R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53-p21WAFl/CIPl, ERK1/2 MAPK, p27KIPl, BRCA1, and NF-kappaB. IntJ Oncol. 2004;24:591–608. [PubMed] [Google Scholar]
- [50].Browne CD, Hindmarsh EJ, Smith JW. Inhibi-tion of endothelial cell proliferation and angiogenesis by orlistat, a fatty acid synthase inhibitor. Faseb J. 2006;20:2027–2035. doi: 10.1096/fj.05-5404com. [DOI] [PubMed] [Google Scholar]
- [51].Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–5294. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- [52].Shah US, Dhir R, Gollin SM, Chandran UR, Lewis D, Acquafondata M, Pflug BR. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum Pathol. 2006;37:401–409. doi: 10.1016/j.humpath.2005.11.022. [DOI] [PubMed] [Google Scholar]
- [53].Kumar-Sinha C, Ignatoski KW, Lippman ME, Ethier SP, Chinnaiyan AM. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003;63:132–139. [PubMed] [Google Scholar]
- [54].Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics. 2005;4:1686–1696. doi: 10.1074/mcp.M400221-MCP200. [DOI] [PubMed] [Google Scholar]
- [55].Chalbos D, Chambon M, Ailhaud G, Roche-fort H. Fatty acid synthetase and its mRNA are induced by progestins in breast cancer cells. J Biol Chem. 1987;262:9923–9926. [PubMed] [Google Scholar]
- [56].Swinnen JV, Esquenet M, Goossens K, Heyns W, Verhoeven G. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Res. 1997;57:1086–1090. [PubMed] [Google Scholar]
- [57].Menendez JA, Oza BP, Colomer R, Lupu R. The estrogenic activity of synthetic progestins used in oral contraceptives enhances fatty acid synthase-dependent breast cancer cell proliferation and survival. Int J Oncol. 2005;26:1507, 1515. [PubMed] [Google Scholar]
- [58].Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res. 2002;279:80–90. doi: 10.1006/excr.2002.5600. [DOI] [PubMed] [Google Scholar]
- [59].Swinnen JV, Heemers H, Deboel L, Foufelle F, Heyns W, Verhoeven G. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene. 2000;19:5173–5181. doi: 10.1038/sj.onc.1203889. [DOI] [PubMed] [Google Scholar]
- [60].Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Role of the phos-phatidylinositol 3'-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 2002;62:642–646. [PubMed] [Google Scholar]
- [61].Bandyopadhyay S, Pai SK, Watabe M, Gross SC, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Markwell SJ, Wang Y, Huggenvik J, Pauza ME, liizumi M, Watabe K. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24:5389–5395. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- [62].Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A, Testa JR. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005 doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- [63].Pan MH, Lin CC, Lin JK, Chen WJ. Tea poly-phenol (-)-epigallocatechin 3-gallate suppresses heregulin-beta 1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J Agric Food Chem. 2007;55:5030–5037. doi: 10.1021/jf070316r. [DOI] [PubMed] [Google Scholar]
- [64].Chiang CT, Way TD, Tsai SJ, Lin JK. Dios-genin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007;581:5735–5742. doi: 10.1016/j.febslet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- [65].Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, liizumi M, Liu W, Ericsson J, Watabe K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- [66].Van de Sande T, Roskams T, Lerut E, Joniau S, Van Poppel H, Verhoeven G, Swinnen JV. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol. 2005;206:214–219. doi: 10.1002/path.1760. [DOI] [PubMed] [Google Scholar]
- [67].Uddin S, Siraj AK, Al-Rasheed M, Ahmed M, Bu R, Myers JN, Al-Nuaim A, Al-Sobhi S, Al-Dayel F, Bavi P, Hussain AR, Al-Kuraya KS. Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. J Clin Endocrinol Metab. 2008;93:4088–4097. doi: 10.1210/jc.2008-0503. [DOI] [PubMed] [Google Scholar]
- [68].Uddin S, Hussain AR, Ahmed M, Abubaker J, Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Bavi P, Al-Kuraya KS. High prevalence of fatty acid synthase expression in colorectal cancers in Middle Eastern patients and its potential role as a therapeutic target. Am J Gastroenterol. 2009;104:1790–1801. doi: 10.1038/ajg.2009.230. [DOI] [PubMed] [Google Scholar]
- [69].Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- [70].Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pai JT, Guryev O, Brown MS, Goldstein JL. Differential stimulation of cholesterol and unsaturated fatty acid biosynthesis in cells expressing individual nuclear sterol regulatory element-binding proteins. J Biol Chem. 1998;273:26138–26148. doi: 10.1074/jbc.273.40.26138. [DOI] [PubMed] [Google Scholar]
- [72].Swinnen JV, Ulrix W, Heyns W, Verhoeven G. Coordinate regulation of lipogenic gene expression by androgens: evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc Natl Acad Sci U S A. 1997;94:12975–12980. doi: 10.1073/pnas.94.24.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Heemers H, Maes B, Foufelle F, Heyns W, Verhoeven G, Swinnen JV. Androgens stimulate lipogenic gene expression in prostate cancer cells by activation of the sterol regulatory element-binding protein cleavage activating protein/sterol regulatory element-binding protein pathway. Mol Endocrinol. 2001;15:1817–1828. doi: 10.1210/mend.15.10.0703. [DOI] [PubMed] [Google Scholar]
- [74].Li JN, Mahmoud MA, Han WF, Ripple M, Pizer ES. Sterol regulatory element-binding protein-1 participates in the regulation of fatty acid synthase expression in colorectal neoplasia. Exp Cell Res. 2000;261:159–165. doi: 10.1006/excr.2000.5054. [DOI] [PubMed] [Google Scholar]
- [75].Yang YA, Morin PJ, Han WF, Chen T, Bornman DM, Gabrielson EW, Pizer ES. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res. 2003;282:132–137. doi: 10.1016/s0014-4827(02)00023-x. [DOI] [PubMed] [Google Scholar]
- [76].Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122–26131. doi: 10.1074/jbc.M702854200. [DOI] [PubMed] [Google Scholar]
- [77].Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein U, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/s1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- [78].Priolo C, Tang D, Brahamandan M, Benassi B, Sicinska E, Ogino S, Farsetti A, Porrello A, Finn S, Zimmermann J, Febbo P, Loda M. The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 2006;66:8625–8632. doi: 10.1158/0008-5472.CAN-06-1374. [DOI] [PubMed] [Google Scholar]
- [79].Menendez JA, Vellon L, Lupu R. DNA topoi-somerase llalpha (TOP2A) inhibitors up-regulate fatty acid synthase gene expression in SK-Br3 breast cancer cells: in vitro evidence for a ‘functional amplicon’ involving FAS, Her-2/neu and TOP2A genes. Int J Mol Med. 2006;18:1081–1087. [PubMed] [Google Scholar]
- [80].Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–2120. [PubMed] [Google Scholar]
- [81].Esslimani-Sahla M, Thezenas S, Simony-Lafontaine J, Kramar A, Lavaill R, Chalbos D, Rochefort H. Increased expression of fatty acid synthase and progesterone receptor in early steps of human mammary carcinogenesis. Int J Cancer. 2007;120:224–229. doi: 10.1002/ijc.22202. [DOI] [PubMed] [Google Scholar]
- [82].Gao Y, Lin LP, Zhu CH, Chen Y, Hou YT, Ding J. Growth arrest induced by C75, A fatty acid synthase inhibitor, was partially modulated by p38 MAPK but not by p53 in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:978–985. doi: 10.4161/cbt.5.8.2883. [DOI] [PubMed] [Google Scholar]
- [83].Knowles LM, Axelrod F, Browne CD, Smith JW. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J Biol Chem. 2004;279:30540–30545. doi: 10.1074/jbc.M405061200. [DOI] [PubMed] [Google Scholar]
- [84].Li JN, Gorospe M, Chrest FJ, Kumaravel TS, Evans MK, Han WF, Pizer ES. Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res. 2001;61:1493–1499. [PubMed] [Google Scholar]
- [85].Morikawa K, Ikeda C, Nonaka M, Suzuki I. Growth arrest and apoptosis induced by quercetin is not linked to adipogenic conversion of human preadipocytes. Metabolism. 2007;56:1656–1665. doi: 10.1016/j.metabol.2007.07.008. [DOI] [PubMed] [Google Scholar]
- [86].Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611–4615. [PubMed] [Google Scholar]
- [87].Knowles LM, Smith JW. Genome-wide changes accompanying knockdown of fatty acid synthase in breast cancer. BMC Genomics. 2007;8:168. doi: 10.1186/1471-2164-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T, Heemers H, Heyns W, Verhoeven G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- [89].Ross J, Najjar AM, Sankaranarayanapillai M, Tong WP, Kaluarachchi K, Ronen SM. Fatty acid synthase inhibition results in a magnetic resonance-detectable drop in phosphocholine. Mol Cancer Ther. 2008;7:2556–2565. doi: 10.1158/1535-7163.MCT-08-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jackowski S. Coordination of membrane phospholipid synthesis with the cell cycle. J Biol Chem. 1994;269:3858–3867. [PubMed] [Google Scholar]
- [91].Zeng L, Wu GZ, Goh KJ, Lee YM, Ng CC, You AB, Wang J, Jia D, Hao A, Yu Q, Li B. Saturated fatty acids modulate cell response to DNA damage: implication for their role in tumorigenesis. PLoS One. 2008;3:2329. doi: 10.1371/journal.pone.0002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kuhajda FP, Piantadosi S, Pasternack GR. Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med. 1989;321:636–641. doi: 10.1056/NEJM198909073211003. [DOI] [PubMed] [Google Scholar]
- [93].Alo PL, Visca P, Trombetta G, Mangoni A, Lenti L, Monaco S, Botti C, Serpieri DE, Di Tondo U. Fatty acid synthase (FAS) predictive strength in poorly differentiated early breast carcinomas. Tumori. 1999;85:35–40. doi: 10.1177/030089169908500108. [DOI] [PubMed] [Google Scholar]
- [94].Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917–921. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- [95].Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M. Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J Urol. 2008;180:729–736. doi: 10.1016/j.juro.2008.03.186. [DOI] [PubMed] [Google Scholar]
- [96].Sebastiani V, Visca P, Botti C, Santeusanio G, Galati GM, Piccini V, Capezzone de Joannon B, Di Tondo U, Alo PL. Fatty acid synthase is a marker of increased risk of recurrence in endometrial carcinoma. Gynecol Oncol. 2004;92:101–105. doi: 10.1016/j.ygyno.2003.10.027. [DOI] [PubMed] [Google Scholar]
- [97].Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Menendez JA, Lupu R, Colomer R. Inhibition of tumor-associated fatty acid synthase hyper-activity induces synergistic chemosensitization of HER -2/neu -overexpressing human breast cancer cells to docetaxel (taxotere) Breast Cancer Res Treat. 2004;84:183–195. doi: 10.1023/B:BREA.0000018409.59448.60. [DOI] [PubMed] [Google Scholar]
- [99].Menendez JA, Vellon L, Colomer R, Lupu R. Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity. Int J Cancer. 2005;115:19–35. doi: 10.1002/ijc.20754. [DOI] [PubMed] [Google Scholar]
- [100].Vazquez-Martin A, Ropero S, Brunet J, Colomer R, Menendez JA. Inhibition of Fatty Acid Synthase (FASN) synergistically enhances the efficacy of 5-fluorouracil in breast carcinoma cells. Oncol Rep. 2007;18:973–980. [PubMed] [Google Scholar]
- [101].Menendez JA, Vellon L, Lupu R. Targeting fatty acid synthase-driven lipid rafts: a novel strategy to overcome trastuzumab resistance in breast cancer cells. Med Hypotheses. 2005;64:997–1001. doi: 10.1016/j.mehy.2004.09.027. [DOI] [PubMed] [Google Scholar]
- [102].Menendez JA, Vellon L, Lupu R. The an-tiobesity drug Orlistat induces cytotoxic effects, suppresses Her-2/neu (erbB-2) oncogene over-expression, and synergistically interacts with trastuzumab (Herceptin) in chemoresistant ovarian cancer cells. Int J Gynecol Cancer. 2006;16:219–221. doi: 10.1111/j.1525-1438.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- [103].Knowles LM, Yang C, Osterman A, Smith JW. Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up -regulating DDIT4. J Biol Chem. 2008;283:31378–31384. doi: 10.1074/jbc.M803384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, Nguyen PL, Migita T, Zam-poni R, Di Vizio D, Priolo C, Sharma C, Xie W, Hemler ME, Mucci L, Giovannucci E, Finn S, Loda M. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt 1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest. 2008;88:1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:rel4. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- [106].Bollinger CR, Teichgraber V, Gulbins E. Ce-ramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [107].Pike U. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- [108].Verkade P, Simons K. Robert Feulgen Lecture 1997. Lipid microdomains and membrane trafficking in mammalian cells. Histochem Cell Biol. 1997;108:211–220. doi: 10.1007/s004180050161. [DOI] [PubMed] [Google Scholar]
- [109].Rakheja D, Kapur P, Hoang MP, Roy LC, Bennett MJ. Increased ratio of saturated to unsaturated C18 fatty acids in colonic adenocarcinoma: implications for cryotherapy and lipid raft function. Med Hypotheses. 2005;65:1120–1123. doi: 10.1016/j.mehy.2005.05.045. [DOI] [PubMed] [Google Scholar]
- [110].Bandyopadhyay S, Zhan R, Wang Y, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, Furuta E, liizumi M, Mohinta S, Watabe M, Chalfant C, Watabe K. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 2006;66:5934–5940. doi: 10.1158/0008-5472.CAN-05-3197. [DOI] [PubMed] [Google Scholar]
- [111].Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3'-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- [112].Page C, Lin HJ, Jin Y, Castle VP, Nunez G, Huang M, Lin J. Overexpression of Akt/AKT can modulate chemotherapy-induced apoptosis. Anticancer Res. 2000;20:407–416. [PubMed] [Google Scholar]
- [113].Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu X, Shi Y, Giranda VL, Luo Y. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway sensitizes MDA-MB468 human breast cancer cells to cerulenin-induced apoptosis. Mol Cancer Ther. 2006;5:494–501. doi: 10.1158/1535-7163.MCT-05-0049. [DOI] [PubMed] [Google Scholar]
- [115].Vance D, Goldberg I, Mitsuhashi O, Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972;48:649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- [116].D'Agnolo G, Rosenfeld IS, Awaya J, Omura S, Vagelos PR. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein syn-thetase. Biochim Biophys Acta. 1973;326:155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- [117].Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 1988;256:357–361. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976;40:681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Omura S. Cerulenin. Methods Enzymol. 1981;72:520–532. [PubMed] [Google Scholar]
- [120].Johansson P, Wiltschi B, Kumari P, Kessler B, Vonrhein C, Vonck J, Oesterhelt D, Grininger M. Inhibition of the fungal fatty acid synthase type I multienzyme complex. Proc Natl Acad Sci USA. 2008;105:12803–12808. doi: 10.1073/pnas.0805827105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Furuya Y, Akimoto S, Yasuda K, Ito H. Apop-tosis of androgen-independent prostate cell line induced by inhibition of fatty acid synthesis. Anticancer Res. 1997;17:4589–4593. [PubMed] [Google Scholar]
- [122].Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56:2745–2747. [PubMed] [Google Scholar]
- [123].Murata S, Yanagisawa K, Fukunaga K, Oda T, Kobayashi A, Sasaki R, Ohkohchi N. Fatty acid synthase inhibitor cerulenin suppresses liver metastasis of colon cancer in mice. Cancer Sci. 2010 doi: 10.1111/j.1349-7006.2010.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ikuta K, Coward J, Luftig RB. The effect of cerulenin on the synthesis of the precursor gag polyprotein in defective murine leukemia and sarcoma virus producing cell lines. Virology. 1986;154:207–213. doi: 10.1016/0042-6822(86)90442-3. [DOI] [PubMed] [Google Scholar]
- [125].Falo LD, Jr., Benacerraf B, Rothstein L, Rock KL. Cerulenin is a potent inhibitor of antigen processing by antigen-presenting cells. J Immunol. 1987;139:3918–3923. [PubMed] [Google Scholar]
- [126].Lawrence DS, Zilfou JT, Smith CD. Structure -activity studies of cerulenin analogues as protein palmitoylation inhibitors. J Med Chem. 1999;42:4932–4941. doi: 10.1021/jm980591s. [DOI] [PubMed] [Google Scholar]
- [127].Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Rendina AR, Cheng D. Characterization of the inactivation of rat fatty acid synthase by C75: inhibition of partial reactions and protection by substrates. Biochem J. 2005;388:895–903. doi: 10.1042/BJ20041963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Increased fatty acid synthase is a therapeutic target in mesothelioma. Clin Cancer Res. 2001;7:153–157. [PubMed] [Google Scholar]
- [130].Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kuhajda FP, Landree LE, Ronnett GV. The connections between C75 and obesity drug-target pathways. Trends Pharmacol Sci. 2005;26:541–544. doi: 10.1016/j.tips.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [132].Landree LE, Hanlon AL, Strong DW, Rumbaugh G, Miller IM, Thupari JN, Connolly EC, Huganir RL, Richardson C, Witters LA, Kuhajda FP, Ronnett GV. C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism. J Biol Chem. 2004;279:3817–3827. doi: 10.1074/jbc.M310991200. [DOI] [PubMed] [Google Scholar]
- [133].Nicot C, Napal L, Relat J, Gonzalez S, Llebaria A, Woldegiorgis G, Marrero PF, Haro D. C75 activates malonyl-CoA sensitive and insensitive components of the CPT system. Biochem Bio-phys Res Commun. 2004;325:660–664. doi: 10.1016/j.bbrc.2004.10.085. [DOI] [PubMed] [Google Scholar]
- [134].Puig T, Vazquez-Martin A, Relat J, Petriz J, Me-nendez JA, Porta R, Casals G, Marrero PF, Haro D, Brunet J, Colomer R. Fatty acid metabolism in breast cancer cells: differential inhibitory effects of epigallocatechin gallate (EGCG) and C75. Breast Cancer Res Treat. 2008;109:471–479. doi: 10.1007/s10549-007-9678-5. [DOI] [PubMed] [Google Scholar]
- [135].Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yang N, Kays JS, Skillman TR, Burris L, Seng TW, Hammond C. C75 4-methylene-2-octyl-5-oxo-tetrahydro-furan-3-carboxylic acid activates carnitine palmitoyltransferase-1 in isolated mitochondria and intact cells without displacement of bound malonyl CoA. J Pharmacol Exp Ther. 2005;312:127–133. doi: 10.1124/jpet.104.074104. [DOI] [PubMed] [Google Scholar]
- [137].Mobbs CV, Makimura H. Block the FAS, lose the fat. Nat Med. 2002;8:335–336. doi: 10.1038/nm0402-335. [DOI] [PubMed] [Google Scholar]
- [138].Dridi S, Ververken C, Hillgartner FB, Arckens L, Van der Gucht E, Cnops L, Decuypere E, Buyse J. FAS inhibitor cerulenin reduces food intake and melanocortin receptor gene expression without modulating the other (an) orexigenic neuropeptides in chickens. Am J Physiol Regul Integr Comp Physiol. 2006;291:R138–147. doi: 10.1152/ajpregu.00899.2005. [DOI] [PubMed] [Google Scholar]
- [139].McFadden JM, Medghalchi SM, Thupari JN, Pinn ML, Vadlamudi A, Miller Kl, Kuhajda FP, Townsend CA. Application of a flexible synthesis of (5R)-thiolactomycin to develop new inhibitors of type I fatty acid synthase. J Med Chem. 2005;48:946–961. doi: 10.1021/jm049389h. [DOI] [PubMed] [Google Scholar]
- [140].Orita H, Coulter J, Lemmon C, Tully E, Vadlamudi A, Medghalchi SM, Kuhajda FP, Gabri-elson E. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13:7139–7145. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]
- [141].Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP. Fatty acid synthase inhibition activates AMP-activated protein kinase in SK0V3 human ovarian cancer cells. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- [142].McNeely W, Benfield P. Orlistat. Drugs. 1998;56:241–249. doi: 10.2165/00003495-199856020-00007. discussion 250. [DOI] [PubMed] [Google Scholar]
- [143].Borgstrom B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim Biophys Acta. 1988;962:308–316. doi: 10.1016/0005-2760(88)90260-3. [DOI] [PubMed] [Google Scholar]
- [144].Carvalho MA, Zecchin KG, Seguin F, Bastos DC, Agostini M, Rangel AL, Veiga SS, Raposo HF, Oliveira HC, Loda M, Coletta RD, Graner E. Fatty acid synthase inhibition with Orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. Int J Cancer. 2008;123:2557–2565. doi: 10.1002/ijc.23835. [DOI] [PubMed] [Google Scholar]
- [145].Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007;67:1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- [146].Menendez JA, Vellon L, Lupu R. Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann Oncol. 2005;16:1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- [147].Pemble CWt, Johnson LC, Kridel SJ, Lowther WT. Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by Orlistat. Nat Struct Mol Biol. 2007;14:704–709. doi: 10.1038/nsmb1265. [DOI] [PubMed] [Google Scholar]
- [148].Cheng F, Wang Q, Chen M, Quiocho FA, Ma J. Molecular docking study of the interactions between the thioesterase domain of human fatty acid synthase and its ligands. Proteins. 2008;70:1228–1234. doi: 10.1002/prot.21615. [DOI] [PubMed] [Google Scholar]
- [149].Wang X, Song KS, Guo QX, Tian WX. The galloyl moiety of green tea catechins is the critical structural feature to inhibit fatty-acid synthase. Biochem Pharmacol. 2003;66:2039–2047. doi: 10.1016/s0006-2952(03)00585-9. [DOI] [PubMed] [Google Scholar]
- [150].Wang X, Tian W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem Biophys Res Commun. 2001;288:1200–1206. doi: 10.1006/bbrc.2001.5923. [DOI] [PubMed] [Google Scholar]
- [151].Brusselmans K, De Schrijver E, Heyns W, Verho-even G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- [152].Yeh CW, Chen WJ, Chiang CT, Lin-Shiau SY, Lin JK. Suppression of fatty acid synthase in MCF-7 breast cancer cells by tea and tea poly-phenols: a possible mechanism for their hypol-ipidemic effects. Pharmacogenomics J. 2003;3:267–276. doi: 10.1038/sj.tpj.6500192. [DOI] [PubMed] [Google Scholar]
- [153].Vergote D, Cren-Olive C, Chopin V, Toillon RA, Rolando C, Hondermarck H, Le Bourhis X. (-)-Epigallocatechin (EGC) of green tea induces apoptosis of human breast cancer cells but not of their normal counterparts. Breast Cancer Res Treat. 2002;76:195–201. doi: 10.1023/a:1020833410523. [DOI] [PubMed] [Google Scholar]
- [154].Tian WX. Inhibition of fatty acid synthase by polyphenols. Curr Med Chem. 2006;13:967–977. doi: 10.2174/092986706776361012. [DOI] [PubMed] [Google Scholar]
- [155].Zhang SY, Ma XF, Zheng CG, Wang Y, Cao XL, Tian WX. Novel and potent inhibitors of fatty acid synthase derived from catechins and their inhibition on MCF-7 cells. J Enzyme Inhib Med Chem. 2009;24:623–631. doi: 10.1080/14756360802319678. [DOI] [PubMed] [Google Scholar]
- [156].Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- [157].Liu B, Wang Y, Fillgrove KL, Anderson VE. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemo-ther Pharmacol. 2002;49:187–193. doi: 10.1007/s00280-001-0399-x. [DOI] [PubMed] [Google Scholar]
- [158].Purohit VC, Richardson RD, Smith JW, Romo D. Practical, catalytic, asymmetric synthesis of beta-lactones via a sequential ketene dimerization/hydrogenation process: inhibitors of the thioesterase domain of fatty acid synthase. J OrgChem. 2006;71:4549–4558. doi: 10.1021/jo060392d. [DOI] [PubMed] [Google Scholar]
- [159].Richardson RD, Ma G, Oyola Y, Zancanella M, Knowles LM, Cieplak P, Romo D, Smith JW. Synthesis of novel beta-lactone inhibitors of fatty acid synthase. J Med Chem. 2008;51:5285–5296. doi: 10.1021/jm800321h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zhang W, Richardson RD, Chamni S, Smith JW, Romo D. Beta-lactam congeners of orlistat as inhibitors of fatty acid synthase. Bioorg Med Chem Lett. 2008;18:2491–2494. doi: 10.1016/j.bmcl.2008.02.043. [DOI] [PubMed] [Google Scholar]
- [161].Richardson RD, Smith JW. Novel antagonists of the thioesterase domain of human fatty acid synthase. Mol Cancer Ther. 2007;6:2120–2126. doi: 10.1158/1535-7163.MCT-07-0187. [DOI] [PubMed] [Google Scholar]
- [162].Hiltunen JK, Chen Z, Haapalainen AM, Wier-enga RK, Kastaniotis AJ. Mitochondrial fatty acid synthesis-an adopted set of enzymes making a pathway of major importance for the cellular metabolism. Prog Lipid Res. 49:27–45. doi: 10.1016/j.plipres.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [163].Hiltunen JK, Okubo F, Kursu VA, Autio KJ, Kastaniotis AJ. Mitochondrial fatty acid synthesis and maintenance of respiratory competent mitochondria in yeast. Biochem Soc Trans. 2005;33:1162–1165. doi: 10.1042/BST20051162. [DOI] [PubMed] [Google Scholar]
- [164].Olsen JG, Rasmussen AV, von Wettstein-Knowles P, Henriksen A. Structure of the mitochondrial beta-ketoacyl-acyl carrier protein synthase from Arabidopsis and its role in fatty acid synthesis. FEBS Lett. 2004;577:170–174. doi: 10.1016/j.febslet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- [165].Yoshida I, Sweetman L, Kulovich S, Nyhan WL, Robinson BH. Effect of lipoic acid in a patient with defective activity of pyruvate dehydro-genase, 2-oxoglutarate dehydrogenase, and branched-chain keto acid dehydrogenase. Pedi-atr Res. 1990;27:75–79. doi: 10.1203/00006450-199001000-00020. [DOI] [PubMed] [Google Scholar]
- [166].Feng D, Witkowski A, Smith S. Down-regulation of mitochondrial acyl carrier protein in mammalian cells compromises protein lipoy-lation and respiratory complex I and results in cell death. J Biol Chem. 2009;284:11436–11445. doi: 10.1074/jbc.M806991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wang YY, Kuhajda FP, Li J, Finch TT, Cheng P, Koh C, Li T, Sokoll U, Chan DW. Fatty acid synthase as a tumor marker: its extracellular expression in human breast cancer. J Exp Ther Oncol. 2004;4:101–110. [PubMed] [Google Scholar]