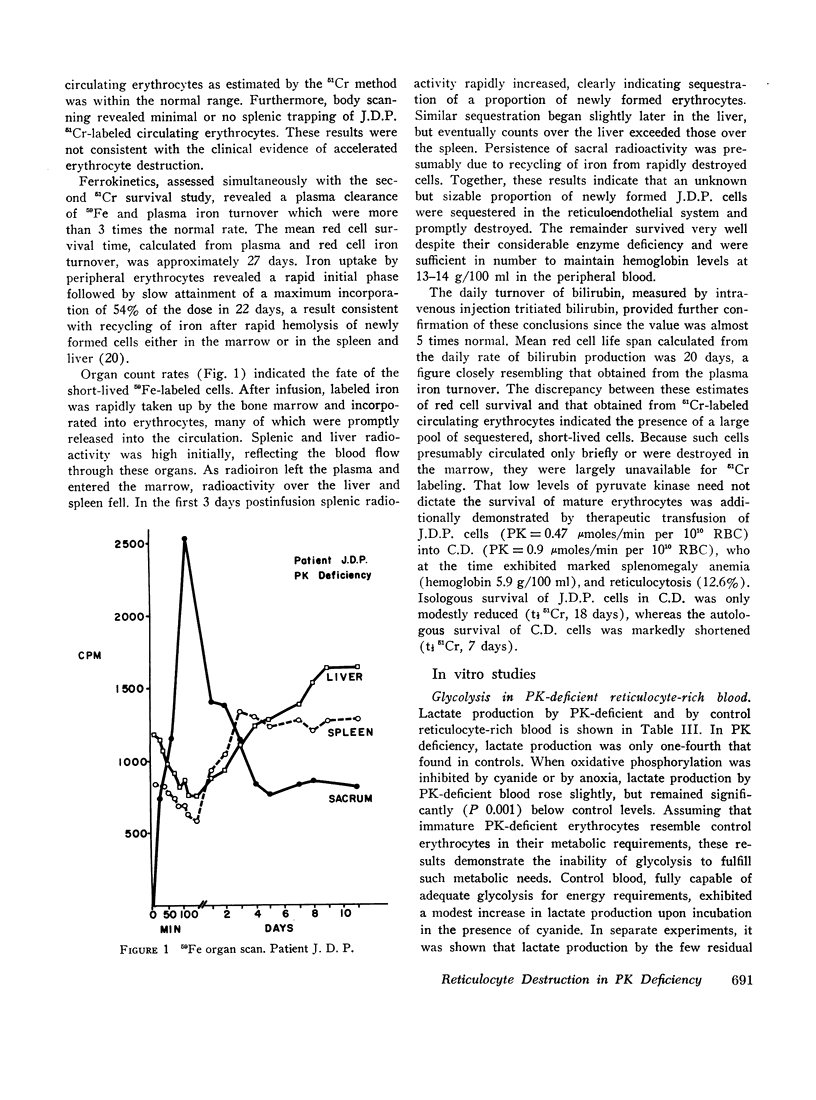
Abstract
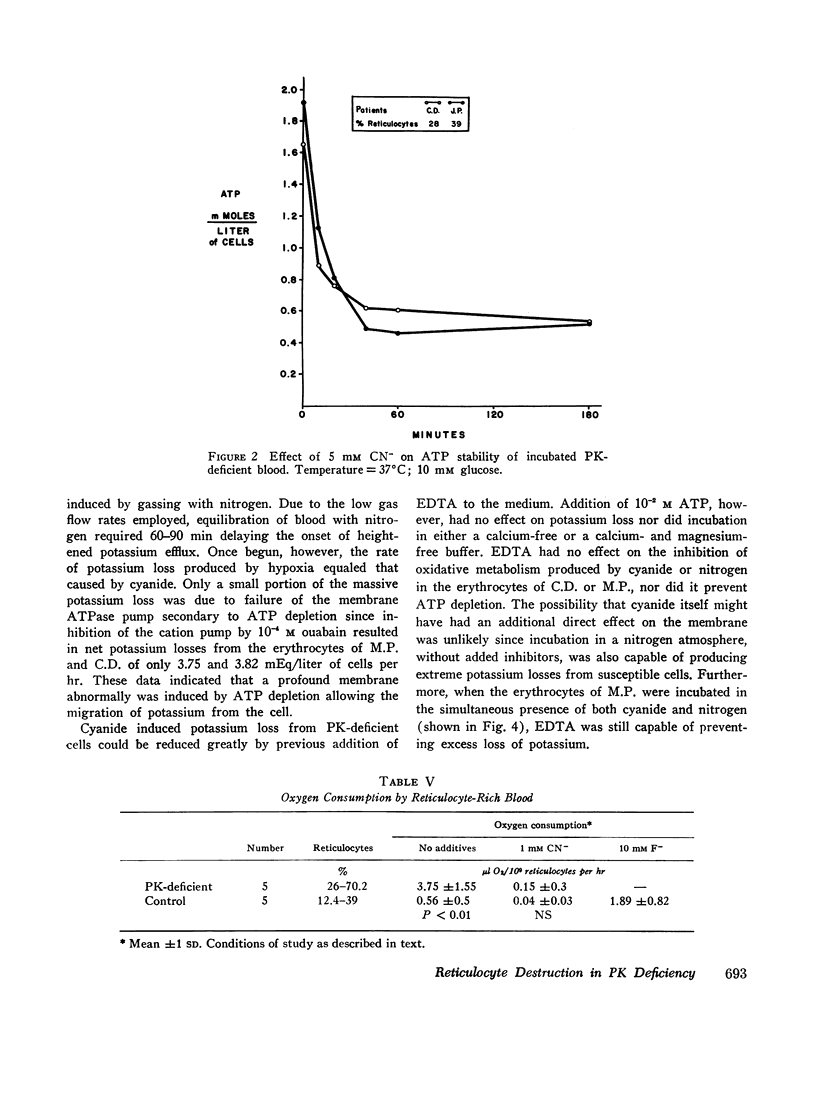
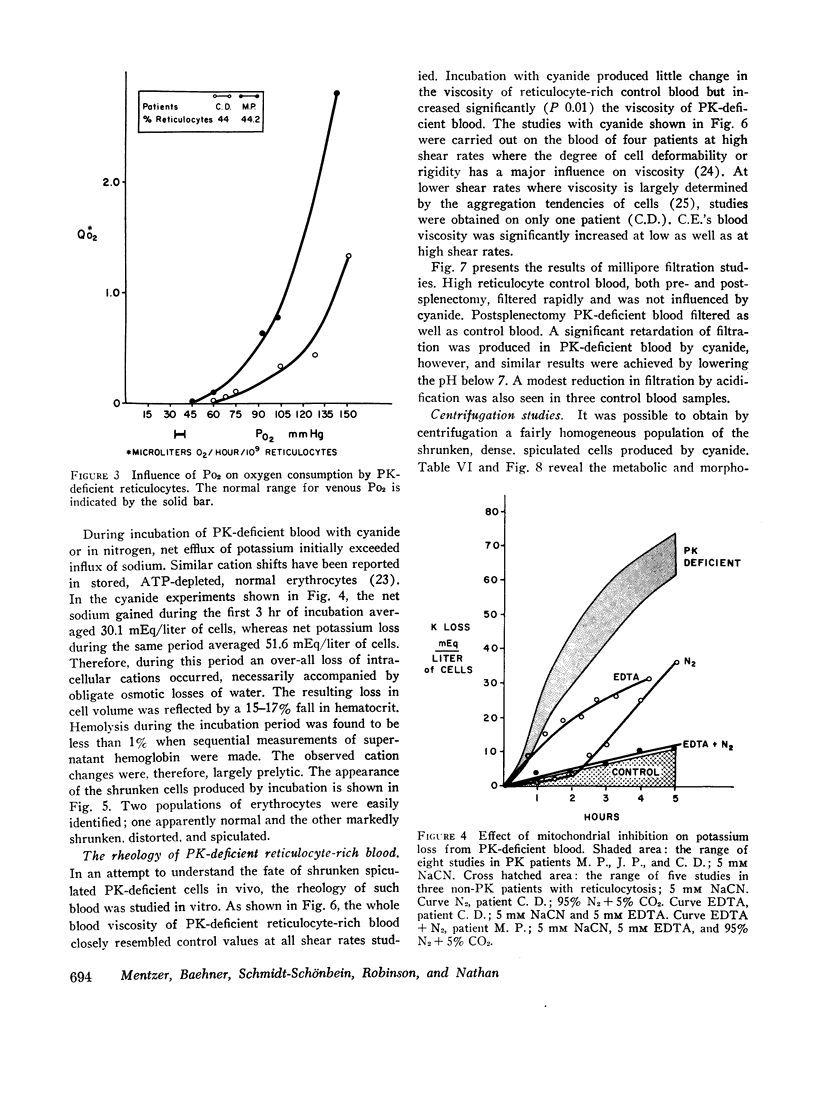
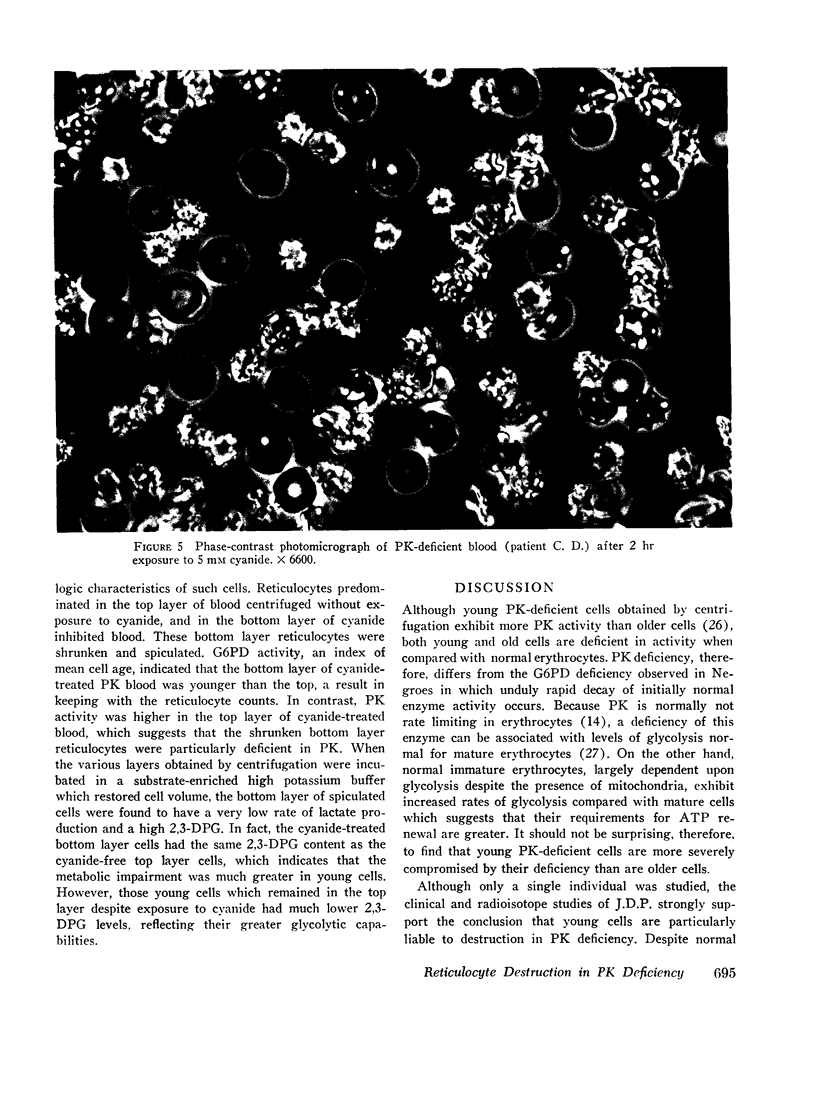
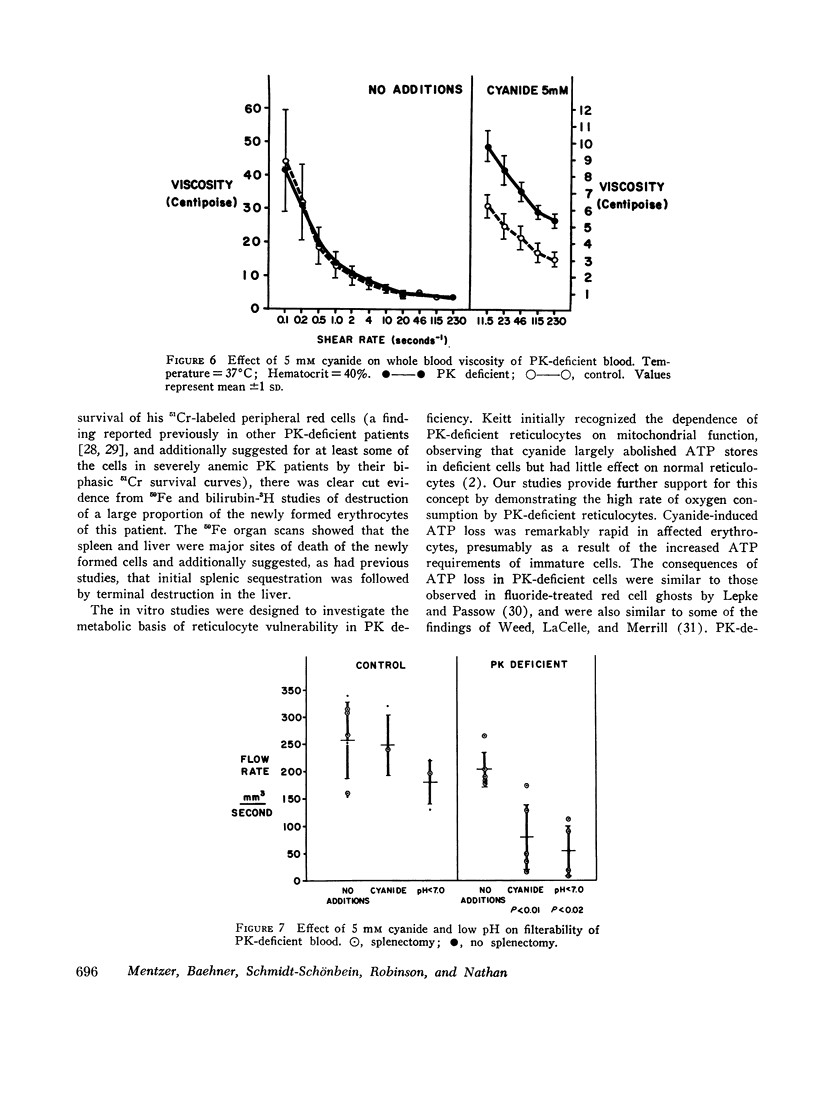
Radioisotope studies of bilirubin turnover, ferrokinetics, and red cell survival (51Cr) in a patient with erythrocyte PK deficiency have provided evidence for prompt reticulocyte sequestration and destruction by the reticuloendothelial system. More mature erythrocytes appeared to survive well despite their deficiency of PK. PK-deficient reticulocytes, dependent upon oxidative phosphorylation for ATP production, are exquisitely sensitive to cyanide- or nitrogen-induced mitochondrial inhibition. If oxidative phosphorylation is unavailable, ATP levels decline rapidly, producing alterations in the cell membrane which allow massive losses of potassium and water. The result is a shrunken, spiculated, viscous cell whose rheologic properties would favor its sequestration by the reticuloendothelial system. Those reticulocytes with particularly low levels of PK exhibit very low glycolytic rates and thus are uniquely reliant upon oxidative phosphorylation. Other reticulocytes, better endowed with PK activity, can meet the increased ATP requirements of young erythrocytes. Upon reaching maturity, such cells have diminished ATP needs and can, therefore, survive despite their enzyme deficiency.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWMAN H. S., PROCOPIO F. Hereditary non-spherocytic hemolytic anemia of the pyruvate-kinase deficient type. Ann Intern Med. 1963 Apr;58:567–591. doi: 10.7326/0003-4819-58-4-567. [DOI] [PubMed] [Google Scholar]
- Berk P. D., Howe R. B., Bloomer J. R., Berlin N. I. Studies of bilirubin kinetics in normal adults. J Clin Invest. 1969 Nov;48(11):2176–2190. doi: 10.1172/JCI106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S. F., Oski F. A. Red cell metabolism in the newborn infant. IV. Transmembrane potassium flux. Pediatrics. 1969 Mar;43(3):396–401. [PubMed] [Google Scholar]
- Cartier P., Najman A., Leroux J. P., Temkine H. Les anomalies de la glycolyse au cours de l'anémie hémolytique par déficit du globule rouge en pyruvate kinase. Clin Chim Acta. 1968 Oct;22(2):165–181. doi: 10.1016/0009-8981(68)90354-9. [DOI] [PubMed] [Google Scholar]
- ELMLINGER P. J., HUFF R. L., TOBIAS C. A., LAWRENCE J. H. Iron turnover abnormalities in patients having anemia; serial blood and in vivo tissue studies with Fe59. Acta Haematol. 1953 Feb;9(2):73–96. doi: 10.1159/000204273. [DOI] [PubMed] [Google Scholar]
- Errill E. W. Rheology of blood. Physiol Rev. 1969 Oct;49(4):863–888. doi: 10.1152/physrev.1969.49.4.863. [DOI] [PubMed] [Google Scholar]
- Jandl J. H., Aster R. H. Increased splenic pooling and the pathogenesis of hypersplenism. Am J Med Sci. 1967 Apr;253(4):383–398. doi: 10.1097/00000441-196704000-00001. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Pyruvate kinase deficiency and related disorders of red cell glycolysis. Am J Med. 1966 Nov;41(5):762–785. doi: 10.1016/0002-9343(66)90036-2. [DOI] [PubMed] [Google Scholar]
- MALLARME J., BOIVIN P., GERBEAUX J. L'AN'EMIE H'EMOLYTIQUE CONG'ENITALE NON SPH'EROCYTAIRE PAR D'EFICIT EN PYRUVATE-KINASE. Bull Mem Soc Med Hop Paris. 1964 Mar 6;115:483–491. [PubMed] [Google Scholar]
- MIWA S., TANAKA K. R., VALENTINE W. N. Enolase activity of erythrocytes in hereditary spherocytosis. Nature. 1962 Aug 11;195:613–614. doi: 10.1038/195613a0. [DOI] [PubMed] [Google Scholar]
- Murphy J. R. The influence of pH and temperature on some physical properties of normal erythrocytes and erythrocytes from patients with hereditary spherocytosis. J Lab Clin Med. 1967 May;69(5):758–775. [PubMed] [Google Scholar]
- NATHAN D. G., OSKI F. A., SIDEL V. W., DIAMOND L. K. EXTREME HEMOLYSIS AND RED-CELL DISTORTION IN ERYTHROCYTE PYRUVATE KINASE DEFICIENCY. II. MEASUREMENTS OF ERYTHROCYTE GLUCOSE CONSUMPTION, POTASSIUM FLUX AND ADENOSINE TRIPHOSPHATE STABILITY. N Engl J Med. 1965 Jan 21;272:118–123. doi: 10.1056/NEJM196501212720302. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Oski F. A., Miller D. R., Gardner F. H. Life-span and organ sequestration of the red cells in pyruvate kinase deficiency. N Engl J Med. 1968 Jan 11;278(2):73–81. doi: 10.1056/NEJM196801112780203. [DOI] [PubMed] [Google Scholar]
- OSKI F. A., NATHAN D. G., SIDEL V. W., DIAMOND L. K. EXTREME HEMOLYSIS AND RED-CELL DISTORTION IN ERYTHROCYTE PYRUVATE KINASE DEFICIENCY. I. MORPHOLOGY, ERYTHROKINETICS AND FAMILY ENZYME STUDIES. N Engl J Med. 1964 May 14;270:1023–1030. doi: 10.1056/NEJM196405142702001. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Bowman H. A low Km phosphoenolpyruvate mutant in the Amish with red cell pyruvate kinase deficiency. Br J Haematol. 1969 Sep;17(3):289–297. doi: 10.1111/j.1365-2141.1969.tb01375.x. [DOI] [PubMed] [Google Scholar]
- READ R. C., WILSON G. W., GARDNER F. H. The use of radioactive sodium chromate to evaluate the life span of the red blood cell in health and certain hematologic disorders. Am J Med Sci. 1954 Jul;228(1):40–52. doi: 10.1097/00000441-195407000-00005. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Schmid-Schönbein H., Wells R., Goldstone J. Influence of deformability of human red cells upon blood viscosity. Circ Res. 1969 Aug;25(2):131–143. doi: 10.1161/01.res.25.2.131. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein H., Wells R. Rheological consequences of osmotic red cell crenation. Pflugers Arch. 1969;307(1):59–69. doi: 10.1007/BF00589459. [DOI] [PubMed] [Google Scholar]
- TANAKA K. R., VALENTINE W. N., MIWA S. Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood. 1962 Mar;19:267–295. [PubMed] [Google Scholar]
- Valentine W. N. Hereditary hemolytic anemias associated with specific erythrocyte enzymopathies. Calif Med. 1968 Apr;108(4):280–294. [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINKHAM W. H., LENHARD R. E., Jr, CHILDS B. A deficiency of glucose-6-phosphate dehydrogenase activity in erythrocytes from patients with favism. Bull Johns Hopkins Hosp. 1958 Apr;102(4):169–175. [PubMed] [Google Scholar]
- Zuelzer W. W., Robinson A. R., Hsu T. H. Erythrocyte pyruvate kinase deficiency in non-spherocytic hemolytic anemia: a system of multiple genetic markers? Blood. 1968 Jul;32(1):33–48. [PubMed] [Google Scholar]