Abstract
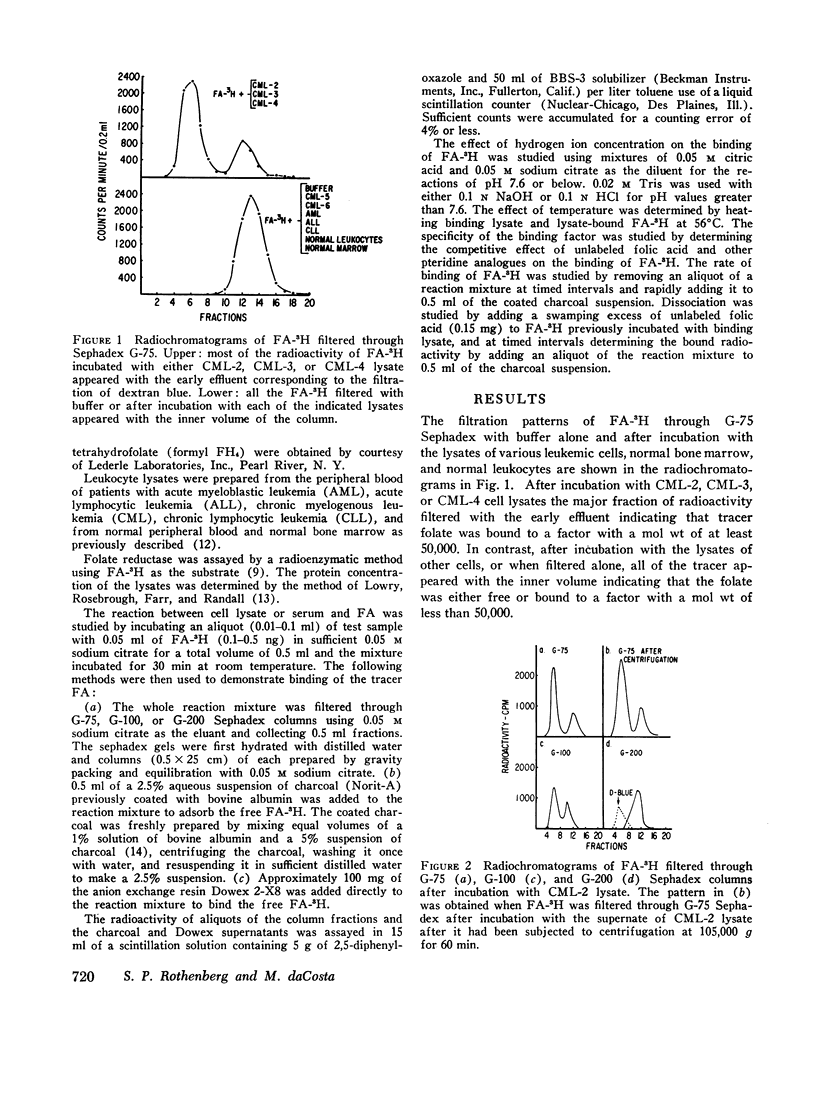
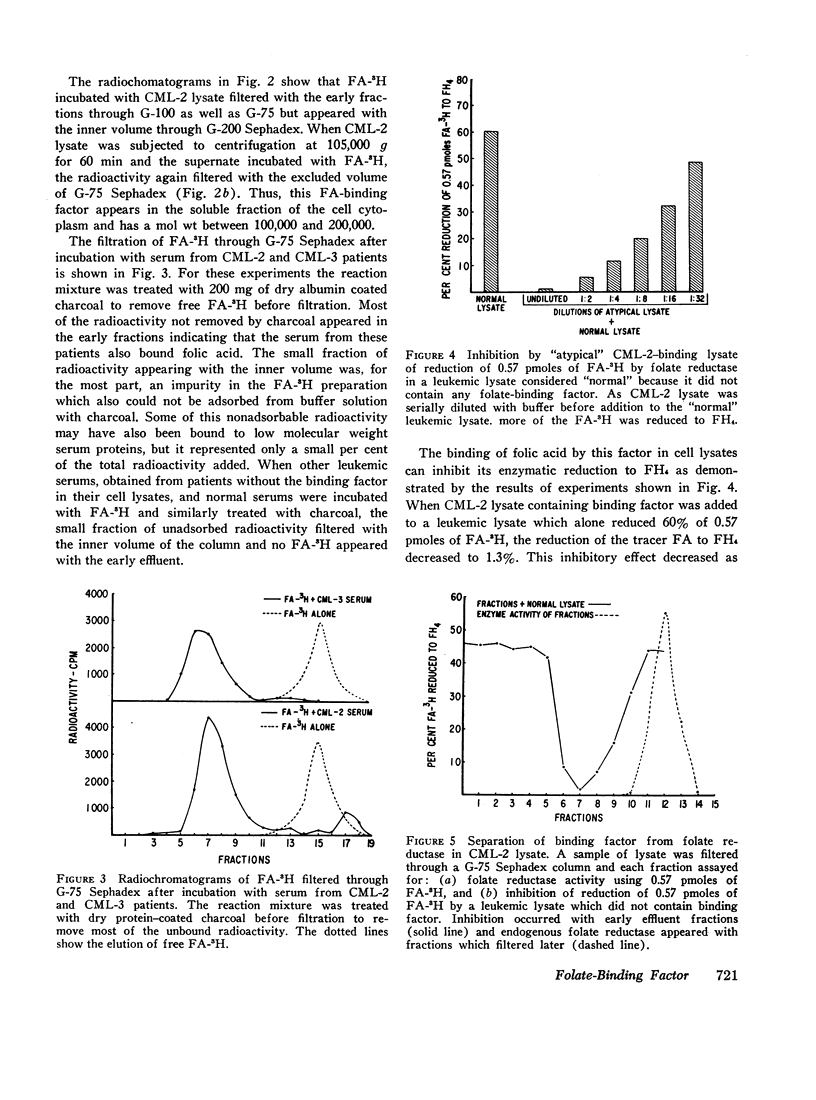
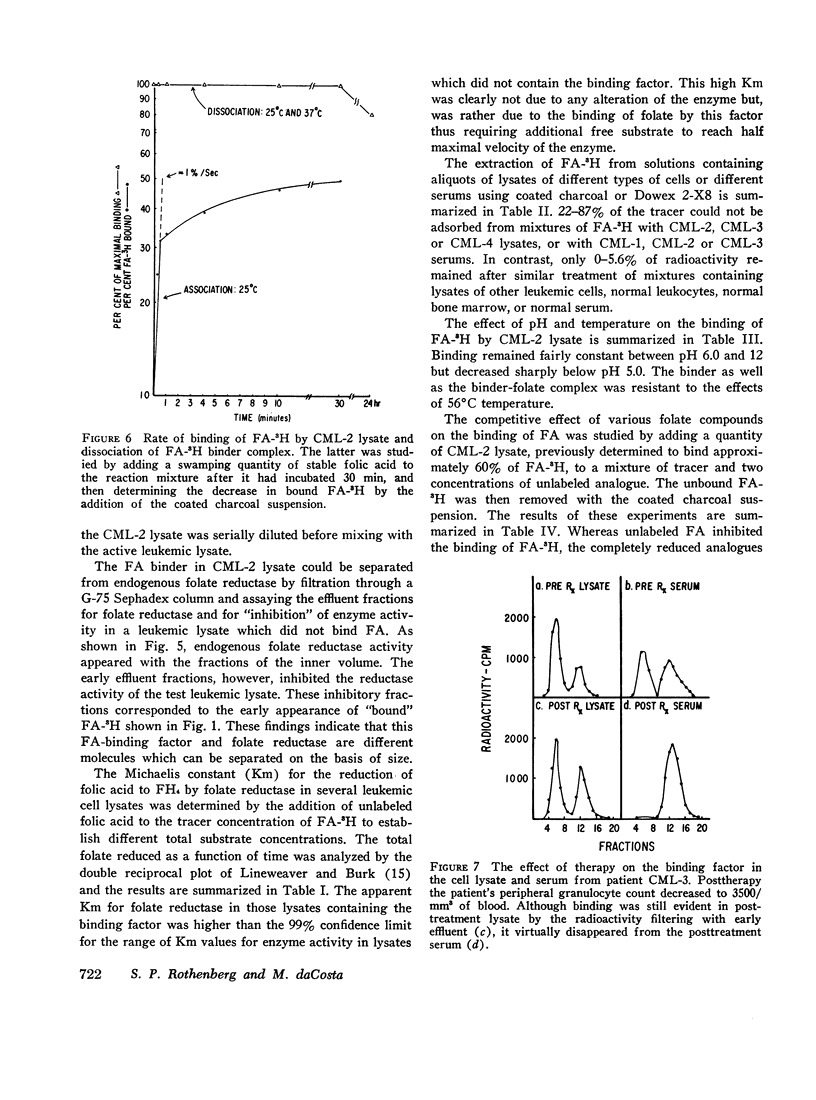
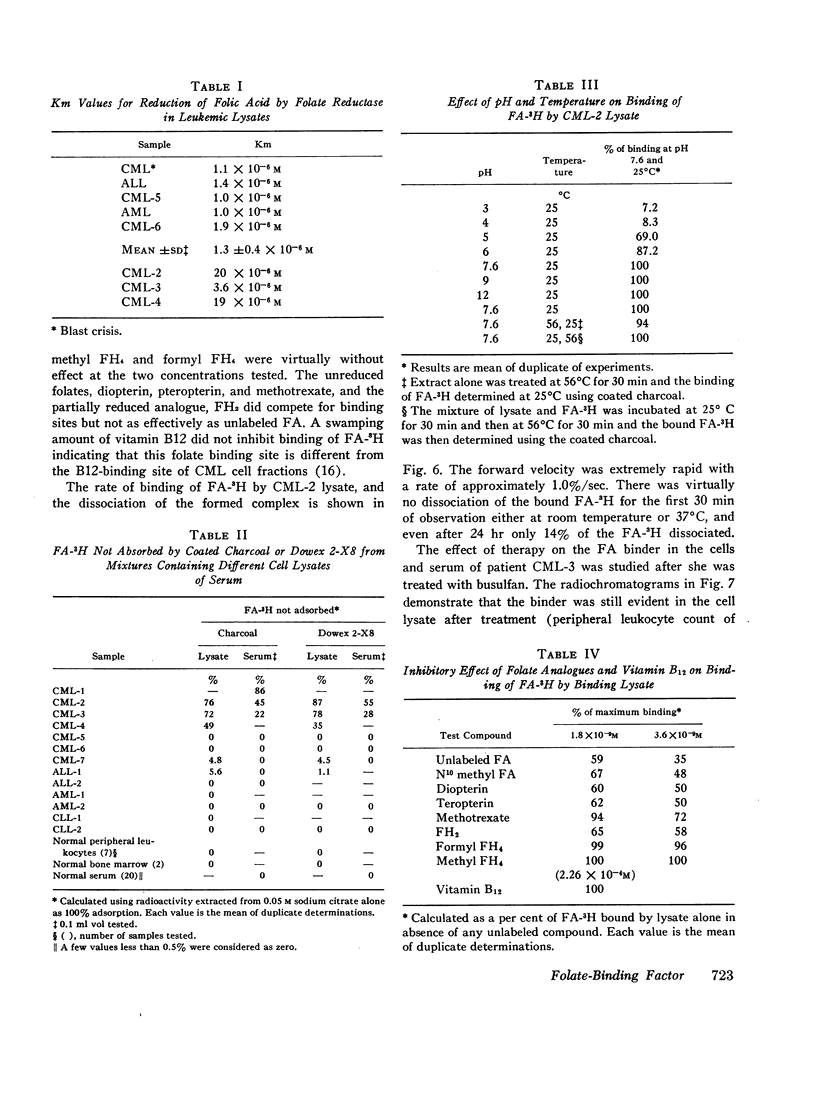
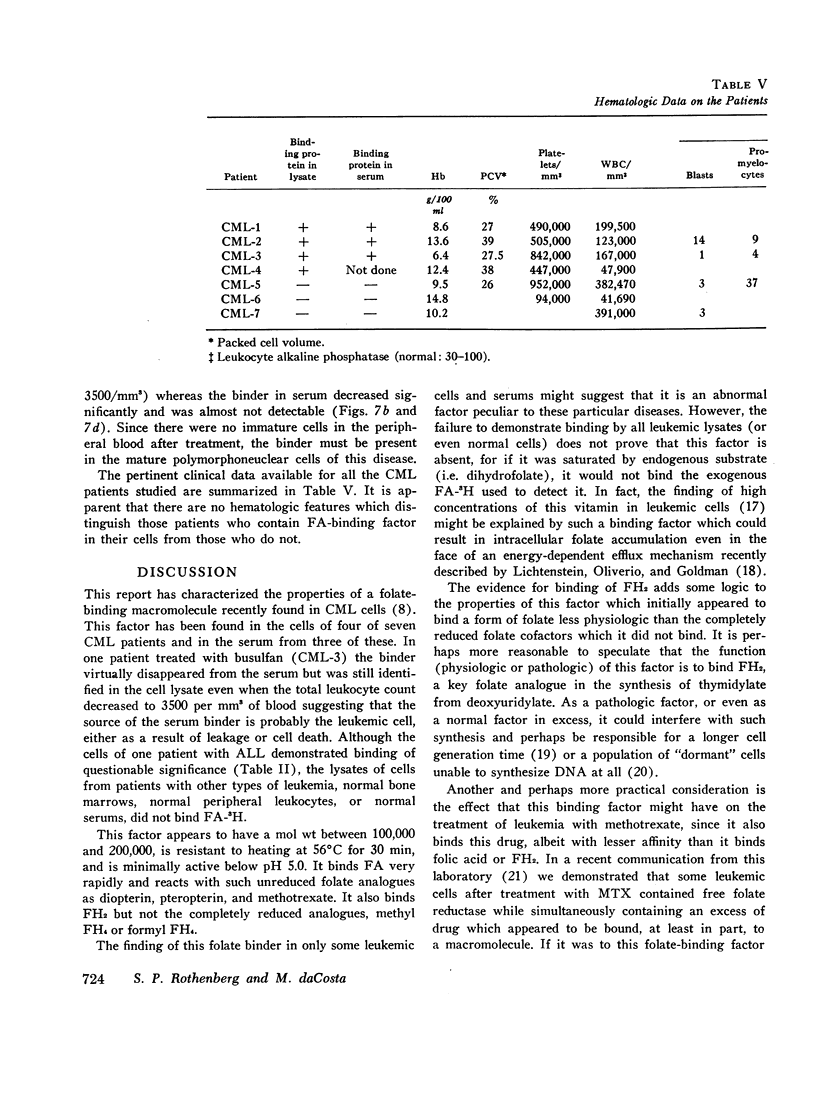
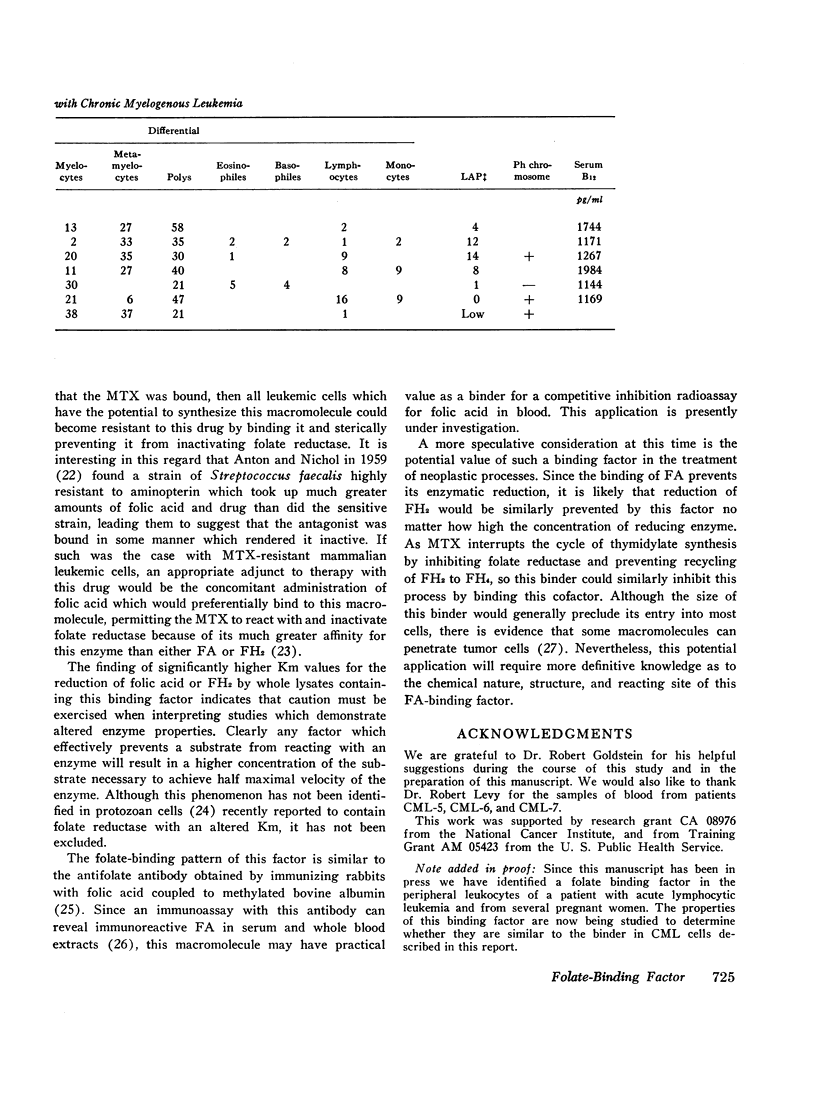
The lysates of peripheral cells as well as the serum from some patients with chronic myelogenous leukemia, contained a macromolecular factor which bound tritiated folic acid. Bound tracer folate filtered through Sephadex G-75 and G-100 columns with the early effluent and appeared with the inner volume through a Sephadex G-200 column. Bound tracer could not be extracted from solution by coated charcoal or the anion exchange resin Dowex 2-X8 and could not be reduced to tetrahydrofolate by folate reductase. The velocity of the binding reaction was very rapid and dissociation of bound tracer extremely slow. Binding decreased sharply below pH 5.0 and the binding factor as well as the folate-binder complex, resisted 56°C for 30 min. The binding factor in the leukemic lysate could be separated from endogenous folate reductase by filtration through a G-75 Sephadex column.
Competitive inhibition studies demonstrated little or no inhibition of binding of tritiated folic acid by formyltetrahydrofolate and methyltetrahydrofolate. Diopterin (pteroyldiglutamate), pteropterin (pteroyltriglutamate), methotrexate, and dihydrofolate inhibited binding of tracer folate but not as effectively as unlabeled folic acid.
The function of this folate binder is unknown. However, that it reacts with dihydrofolate suggests some relationship (physiologic or pathologic) to DNA synthesis since this folate cofactor is essential for the de novo synthesis of thymidylate from deoxyuridylate. In addition, these findings also suggest that the binding of methotrexate may, like folate, inhibit its reaction with folate reductase, and thus be a mechanism by which leukemic cells become resistant to this drug.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., NICHOL C. A. Studies on the nature of resistance of Streptococcus faecalis to folic acid antagonists. Biochem Pharmacol. 1959 Dec;3:1–9. doi: 10.1016/0006-2952(59)90002-4. [DOI] [PubMed] [Google Scholar]
- CONDIT P. T., GROB D. Studies on the folic acid vitamins. I. Observations on the metabolism of folic acid in man and on the effect of aminopterin. Cancer. 1958 May-Jun;11(3):525–536. doi: 10.1002/1097-0142(195805/06)11:3<525::aid-cncr2820110311>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Clarkson B. D. Review of recent studies of cellular proliferation in acute leukemia. Natl Cancer Inst Monogr. 1969 May;30:81–120. [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- Ferone R., O'Shea M., Yoeli M. Altered dihydrofolate reductase associated with drug-resistance transfer between rodent plasmodia. Science. 1970 Feb 27;167(3922):1263–1264. doi: 10.1126/science.167.3922.1263. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A. V., Newcombe B. F. Leucocyte folate in vitamin B 12 and folate deficiency and in leukaemia. Br J Haematol. 1967 Nov;13(6):954–966. doi: 10.1111/j.1365-2141.1967.tb08866.x. [DOI] [PubMed] [Google Scholar]
- JOHNS D. G., PLENDERLEITH I. H. FOLIC ACID-DISPLACEMENT IN MAN. Biochem Pharmacol. 1963 Oct;12:1071–1074. doi: 10.1016/0006-2952(63)90081-9. [DOI] [PubMed] [Google Scholar]
- JOHNS D. G., SPERTI S., BURGEN A. S. The metabolism of tritiated folic acid in man. J Clin Invest. 1961 Sep;40:1684–1695. doi: 10.1172/JCI104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquez J. A. Permeability of Ehrlich ascites cells to folic acid, aminopterin, and amethopterin. Cancer Res. 1966 Aug;26(8):1616–1624. [PubMed] [Google Scholar]
- KERESZTESY J. C., DONALDSON K. P. Synthetic prefolic A. Biochem Biophys Res Commun. 1961 Jul 26;5:286–288. doi: 10.1016/0006-291x(61)90164-4. [DOI] [PubMed] [Google Scholar]
- LAU K. S., GOTTLIEB C., WASSERMAN L. R., HERBERT V. MEASUREMENT OF SERUM VITAMIN B12 LEVEL USING RADIOISOTOPE DILUTION AND COATED CHARCOAL. Blood. 1965 Aug;26:202–214. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtenstein N. S., Oliverio V. T., Goldman I. D. Characteristics of folic acid transport in the L1210 leukemia cell. Biochim Biophys Acta. 1969;193(2):456–467. doi: 10.1016/0005-2736(69)90204-1. [DOI] [PubMed] [Google Scholar]
- MEYER L. M., CRONKITE E. P., MILLER I. F., MULZAC C. W., JONES I. Co60 vitamin B12 binding capacity of human leukocytes. Blood. 1962 Feb;19:229–235. [PubMed] [Google Scholar]
- Mauer A. M., Saunders E. F., Lampkin C. Possible significance of nonproliferating leukemic cells. Natl Cancer Inst Monogr. 1969 May;30:63–79. [PubMed] [Google Scholar]
- Metz J., Zalusky R., Herbert V. Folic acid binding by serum and milk. Am J Clin Nutr. 1968 Apr;21(4):289–297. doi: 10.1093/ajcn/21.4.289. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Williams D. C. The fate of intravenously injected folate in rats. Biochem Pharmacol. 1965 Jun;14(6):903–914. doi: 10.1016/0006-2952(65)90241-8. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. P. A macromolecular factor in some leukemic cells which binds folic acid. Proc Soc Exp Biol Med. 1970 Feb;133(2):428–432. doi: 10.3181/00379727-133-34489. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. P. A rapid radioassay for folic acid reductase and amethopterin. Anal Biochem. 1966 Jul;16(1):176–178. doi: 10.1016/0003-2697(66)90095-9. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. P. Alteration of Methotrexate by leukemic cells: loss of affinity for an anion exchange resin. Cancer Res. 1969 Nov;29(11):2047–2055. [PubMed] [Google Scholar]
- Rothenberg S. P., Gizis F., Kamen B. Antibodies against folic acid. I. In vitro biophysical effect. J Lab Clin Med. 1969 Oct;74(4):662–671. [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]



