Abstract
Objective
Male circumcision reduces female-to-male HIV-1 transmission risk by approximately 60%. Data assessing the effect of circumcision on male-to-female HIV-1 transmission are conflicting, with one observational study among HIV-1 serodiscordant couples showing reduced transmission but a randomized trial suggesting no short-term benefit of circumcision.
Design/Methods
Data collected as part of a prospective study among African HIV-1 serodiscordant couples were analyzed for the relationship between circumcision status of HIV-1 seropositive men and risk of HIV-1 acquisition among their female partners. Circumcision status was determined by physical examination. Cox proportional hazards analysis was used.
Results
1096 HIV-1 serodiscordant couples in which the male partner was HIV-1 infected were followed for a median of 18 months; 374 (34%) male partners were circumcised. Sixty-four female partners seroconverted to HIV-1 (incidence 3.8 per 100 person-years). Circumcision of the male partner was associated with a non-statistically significant ∼40% lower risk of HIV-1 acquisition by the female partner (hazard ratio [HR] 0.62, 95% confidence interval [CI] 0.35-1.10, p=0.10). The magnitude of this effect was similar when restricted to the subset of HIV-1 transmission events confirmed by viral sequencing to have occurred within the partnership (n=50, HR 0.57, p=0.11), after adjustment for male partner plasma HIV-1 concentrations (HR 0.60, p=0.13), and when excluding follow-up time for male partners who initiated antiretroviral therapy (HR 0.53, p=0.07).
Conclusions
Among HIV-1 serodiscordant couples in which the HIV-1 seropositive partner was male, we observed no increased risk and potentially decreased risk from circumcision on male-to-female transmission of HIV-1.
Keywords: male circumcision, HIV-1 transmission, HIV-1 discordant couples
Introduction
Randomized trials from Kenya, South Africa, and Uganda demonstrated that male circumcision reduces a man's risk of acquiring HIV-1 by approximately 60% [1-3]. In advance of the trial, 20 years of data, from more than three dozen observational studies, showed that circumcised men were at substantially decreased HIV-1 risk compared with uncircumcised men [4]. In response to this compelling body of evidence, WHO and UNAIDS recommended that male circumcision become part of a comprehensive HIV-1 prevention package in countries where HIV-1 is prevalent and circumcision is uncommon [5]. Programs to roll-out male circumcision have since been initiated in several African countries [6].
To avoid stigmatizing HIV-1 infected men, WHO/UNAIDS guidelines recommend that circumcision be provided to healthy men who request the procedure, regardless of HIV-1 serostatus, including for those declining HIV-1 testing [7]. Thus, HIV-1 infected men will undoubtedly undergo circumcision as roll-out programs are implemented. Moreover, some circumcised men will acquire HIV-1 in spite of the partial HIV-1 protection offered by circumcision. Few studies have examined the potential effect that the circumcision status of HIV-1 infected men might have on HIV-1 transmission risk to women. Two prospective observational studies found that female partners of circumcised HIV-1 infected men were less likely to acquire HIV-1 [8, 9]; however, another found no relationship between male circumcision status and women's HIV-1 risk [10]. A recent clinical trial found no statistically significant difference in HIV-1 incidence among female partners of HIV-1 infected men who were randomized to undergo circumcision compared with those whose partners remained uncircumcised, but with short-term increased HIV-1 risk for women whose partners underwent circumcision and resumed sex prior to wound healing [11].
Understanding the potential short- and long-term effects of circumcision in HIV-1 infected men on risk of HIV-1 transmission to their sexual partners is a public health priority. We analyzed data from a multinational prospective study among HIV-1 serodiscordant couples (i.e., in which one partner was infected with HIV-1 and the other was HIV-1 uninfected) to explore the relationship between male circumcision status and women's HIV-1 risk.
Methods
Population and procedures
Between November 2004 and April 2007, 3408 heterosexual HIV-1 serodiscordant couples in which the HIV-1 seropositive partner was also seropositive for herpes simplex virus type 2 (HSV-2) were enrolled in a randomized, double-blind, placebo-controlled, clinical trial of daily HSV-2 suppressive therapy (acyclovir 400 mg orally twice daily) as a potential intervention to decrease HIV-1 transmission to the HIV-1 seronegative partner [12]. The study was conducted at 7 sites in eastern Africa (Eldoret, Kisumu, Nairobi and Thika, Kenya; Kigali, Rwanda; Moshi, Tanzania; Kampala, Uganda) and 7 sites in southern Africa (Gaborone, Botswana; Cape Town, Orange Farm, and Soweto, South Africa; Kitwe, Lusaka, and Ndola, Zambia). Follow-up was completed in October 2008. As reported elsewhere, acyclovir HSV-2 suppressive therapy failed to reduce HIV-1 transmission within the couples, in spite of a 73% reduction in incident genital ulcer disease and a 0.25 log10 copies/mL reduction in HIV-1 plasma viral load among the HIV-1 infected partners [13].
Eligible couples reported ≥3 episodes of vaginal intercourse during the three months prior to screening and planned to remain together for the 24 months of study follow-up. HIV-1 infected partners were age ≥18 years, HIV-1 and HSV-2 seropositive, not on antiretroviral therapy, and had a CD4 count ≥250 cells/mm3 and no AIDS-defining conditions. HIV-1 uninfected partners were aged ≥18 years and HIV-1 seronegative; they could be HSV-2 seropositive or seronegative.
HIV-1 infected partners were seen monthly for provision of study drug and behavioral risk assessment, including risk-reduction counseling. Those who met national guidelines for initiation of antiretroviral therapy during follow-up were referred to local HIV-1 care clinics. Male circumcision status was determined by physical examination at the time of study enrollment. HIV-1 uninfected partners were seen quarterly. Visits included interviews about risk behavior and tests for HIV-1 antibodies.
All participants received an HIV-1 prevention package consisting of pre- and post-test counseling, risk reduction counseling (both individual and couple), free condoms, and management of sexually transmitted infections (STIs) according to WHO guidelines. Scheduled syndromic STI assessment was performed quarterly for all study participants and at non-quarterly visits when symptoms were reported. The study protocol was approved by the University of Washington Human Subjects Review Committee and ethical review committees at each collaborating institution. All participants provided written informed consent.
Laboratory analyses
HIV-1 serologic testing was by dual rapid HIV-1 antibody tests, confirmed by HIV-1 EIA and Western blot. For couples in which HIV-1 seroconversion occurred, virus sequence analysis was used to determine whether the HIV-1 transmission was “genetically linked” within the partnership or could not be linked, as detailed elsewhere [13]. HSV-2 serostatus was determined by HerpeSelect-2 EIA (Focus Technologies, Cypress CA) [14], confirmed by HSV Western blot [15]. Couples in which the HIV-1 infected partner did not have serologically-confirmed HIV-1 and HSV-2 infection at study entry were excluded from the dataset [13].
CD4 quantification was performed for HIV-1 infected participants at 6-month intervals using standard flow cytometry. Plasma HIV-1 concentrations from baseline and months 3, 6, 12, and 24 was quantified using the COBAS TaqMan HIV-1 RNA assay, version 1.0, (Roche Diagnostics, Indianapolis, IN); the limit of quantification was 240 copies/mL.
Batch testing at the end of the study was done from samples collected at enrollment by nucleic acid amplification for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis using endocervical swabs (women) and urine samples (men) (Gen-Probe, San Diego, CA) [12].
Data analysis
The present analysis was restricted to those couples in which the HIV-1 seropositive partner was male. Differences in baseline demographic and behavioral characteristics for couples with circumcised versus uncircumcised men were assessed using the Kruskal-Wallis test for continuous measures and the chi-squared test for proportions. For all sexual behavioral variables, data were analyzed based on the female partner's report.
Cox proportional hazards regression was used for assessing the effects of circumcision on risk of incident HIV-1 infection, defined as HIV-1 seroconversion among initially-HIV-1 seronegative female partners. For 9 HIV-1 seronegative partners, HIV-1 seroconversion occurred at the first follow-up visit and later testing of baseline plasma detected HIV-1 RNA at the time of study enrollment (i.e., thus, they were already infected with HIV-1 at enrollment although not yet seropositive); these HIV-1 seroconversion events were included in this analysis to maintain a consistent primary endpoint of HIV-1 seroconversion and because the male partner circumcision status was likely consistent over this time period.
We conducted analyses for all HIV-1 seroconversion events and for the subset of HIV-1 seroconversion events that were genetically linked by viral sequencing within the partnership. Female participants who had an unlinked HIV-1 seroconversion event contributed follow-up time until HIV-1 seroconversion and were censored thereafter. Analyses were stratified by region (eastern Africa sites versus southern Africa sites), because of differential median follow-up between the two regions. Adjusted analyses controlled for male partner HIV-1 plasma viral load, as a time-dependent variable, as plasma viral load has been shown to be the strongest predictor of HIV-1 transmission risk [16]. Additional analyses censored women's follow-up time when their male partners initiated antiretroviral therapy. Subgroup analyses – by female partner age, reported unprotected sexual activity during follow-up, male partner plasma viral load at enrollment (based on the results of a prior observational study that found circumcision reduced HIV-1 transmission only from men with viral loads <50,000 copies/mL [9]), region, genital ulcer disease in the HIV-1 infected male partner during follow-up, and trial randomization arm – were also performed.
Results
In total, 1109 heterosexual HIV-1 serodiscordant couples in which the HIV-1 infected partner was male were enrolled. One man's circumcision status was not assessed, and 12 women had no follow-up testing for HIV-1; the remaining 1096 were eligible for this analysis. The median age was 37 for men and 30 for their female partners (Table 1). Most couples were married and cohabitating, and the median duration of partnership was 4 years. The median frequency of sexual activity within the partnership during the month prior to enrollment was 4 (interquartile range [IQR] 2-8), and 27% of couples had sex unprotected by condoms during that month. Only 6 (1%) female partners reported sexual activity with another partner during the month prior to enrollment. Among the HIV-1 infected male partners, the median baseline CD4 count was 424 (IQR 334-571) cells/mm3, the median baseline plasma HIV-1 concentration was 4.3 (IQR 3.7-4.9) log10 copies/mL, and 51.4% were randomized to acyclovir. The majority (86%) of female partners were HSV-2 seropositive; all male partners were seropositive for HSV-2 (a study eligibility requirement).
Table 1. Enrollment characteristics for 1096 African HIV-1 serodiscordant couples with male HIV-1 seropositive partners.
| Median (IQR) or N (percent) | |||||||
|---|---|---|---|---|---|---|---|
| Total (N = 1096) | Couples with circumcised male partners (N = 374) | Couples with uncircumcised male partners (N = 722) | p-value | ||||
| Male (HIV-1 seropositive) partner characteristics | |||||||
| Age, years | 37 | (32-44) | 38 | (33-45) | 36 | (31-44) | 0.39 |
| CD4 count, cells/mm3 | 424 | (334-571) | 411 | (326-575) | 433 | (339-570) | 0.52 |
| Plasma HIV-1 RNA, log10 copies/mL | 4.3 | (3.7-4.9) | 4.3 | (3.6-4.9) | 4.4 | (3.7-4.9) | 0.50 |
| Any sexually transmitted infection1 | 71 | (6.5%) | 23 | (6.1%) | 48 | (6.6%) | 0.85 |
| Randomized to acyclovir (vs. placebo) | 564 | (51.4%) | 192 | (51.3%) | 372 | (51.5%) | 1.00 |
| Female (HIV-1 seronegative) partner characteristics | |||||||
| Age, years | 30 | (25-37) | 32 | (26-39) | 30 | (25-37) | 0.008 |
| Any sexually transmitted infection1 | 141 | (12.9%) | 47 | (12.6%) | 94 | (13.0%) | 0.91 |
| HSV-2 seropositive | 939 | (85.6%) | 325 | (86.9%) | 613 | (84.9%) | 0.42 |
| Couple characteristics | |||||||
| East Africa (vs. southern Africa) | 752 | (68.6%) | 293 | (78.3%) | 459 | (63.6%) | <0.001 |
| Married | 886 | (80.8%) | 309 | (82.6%) | 577 | (79.9%) | 0.32 |
| Living together | 1023 | (93.3%) | 348 | (93.0%) | 675 | (93.5%) | 0.88 |
| Duration of partnership, years | 4 | (2-8) | 4 | (2-7) | 4 | (2-8) | 0.48 |
| # years older (male partner) | 6 | (3-10) | 6 | (3-10) | 6 | (3-10) | 0.39 |
| # sexual contacts within couple, past month2 | 4 | (2-8) | 4 | (2-7) | 4 | (2-8) | 0.48 |
| Any unprotected sex, past month2 | 290 | (26.5%) | 99 | (26.5%) | 191 | (26.5%) | 0.95 |
Neisseria gonorrhoeae (0.8% men, 1.1% women), Chlamydia trachomatis (1.1% men, 1.6% women), or Trichomonas vaginalis (5% men, 11% women)
Sexual behavior data as reported by the female partner.
Three hundred seventy-four (34%) male partners were circumcised. Demographic and behavioral characteristics were generally similar for couples with circumcised and uncircumcised men. Men from eastern Africa sites were more likely to be circumcised (39%) than men from southern African sites (24%, p<0.001), and female partners of circumcised men were older than partners of uncircumcised men (median age 32 vs. 30 years, p=0.008). There was no statistically significant difference in sexual behavior reporting for couples with circumcised versus uncircumcised men. Notably, there were no statistically significant differences between circumcised and uncircumcised men in terms of baseline plasma HIV-1 levels or CD4 count.
Follow-up of HIV-1 seronegative female partners and sexual behavior during follow-up
For the HIV-1 seronegative female partners, median follow-up was 18 months (IQR 15-24), and a total of 1685 person-years of follow-up were accrued. Follow-up time was shorter for partners of uncircumcised men than for partners of circumcised men (median 18 vs.21 months, p=0.03), in part reflecting earlier administrative site closure for some southern Africa sites compared with the eastern Africa sites. Within region, there were no statistically significant differences in median follow-up by circumcision status.
As described previously [13], the frequency of sexual activity decreased during follow-up, including sexual activity unprotected by condoms. The average number of sex acts per month across all follow-up visits was 4.4 (IQR 1-6), with no statistically significant difference between couples with circumcised versus uncircumcised men. Unprotected sex was reported at 7% of follow-up visits. During follow-up, 12% (79/733) of uncircumcised men and 13% of circumcised men (41/374) started antiretroviral therapy (p=0.9).
Male circumcision status and HIV-1 risk in female partners
Sixty-four women seroconverted to HIV-1 during follow-up (incidence 3.8 per 100 person-years). Of these, 50 (78%, incidence 3.0 per 100 person-years) were determined to be genetically linked within the partnership by viral sequence analysis. HIV-1 incidence was lower for partners of circumcised compared with uncircumcised men, both for total HIV-1 seroconversion events (hazard ratio [HR] 0.62, 95% confidence interval [CI] 0.35-1.10, p=0.10) and for genetically-linked events (HR 0.57, 95% CI 0.29-1.11, p=0.10), although these differences did not achieve statistically significance (Figure 1 and Table 2). In an analysis that adjusted for male partner plasma HIV-1 concentrations, female partners of circumcised men retained a non-statistically significant 40% reduced risk of HIV-1 acquisition, compared with partners of uncircumcised men (HR 0.60, 95% CI 0.31-1.16, p=0.13, for genetically-linked events). In an analysis excluding follow-up time occurring after male partners initiated antiretroviral therapy, the relationship between male circumcision status and risk of HIV-1 acquisition in female partners was strengthened and was of borderline statistical significance (HR 0.53, 95% CI 0.26-1.07, p=0.07, for genetically-linked events). Further adjustment for age of the female partner and for unprotected sex during follow-up (both of which were related to HIV-1 acquisition) did not substantially change this risk estimate (change <10%, data not shown).
Figure 1. Kaplan-Meier curve comparing cumulative HIV-1 incidence among female partners of circumcised versus uncircumcised HIV-1 seropositive men.
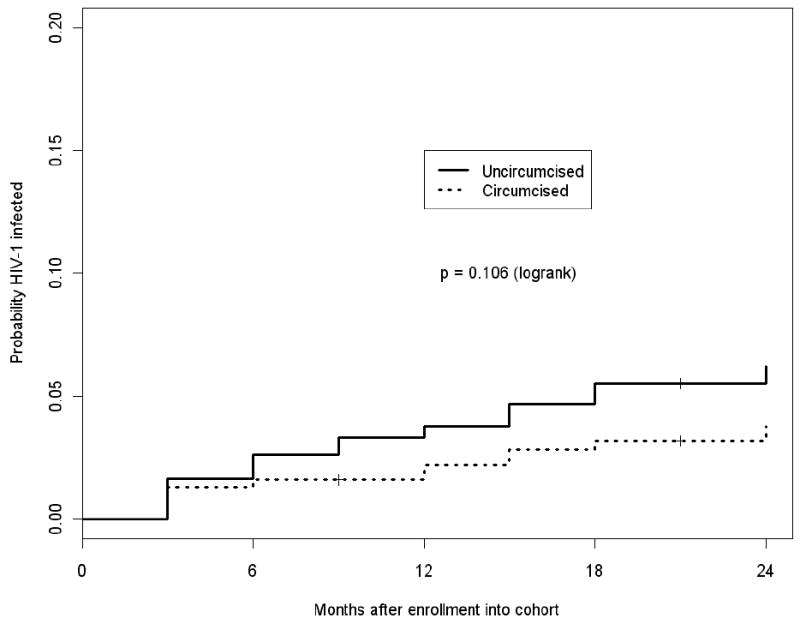
Cumulative HIV-1 incidence among female partners of circumcised (dashed) and uncircumcised (solid) HIV-1 infected men.
Table 2. Incident HIV-1 infections among female partners of circumcised versus uncircumcised HIV-1 infected men.
| Number of HIV-1 seroconversions (%) | HIV-1 incidence (per 100 person-years) | Unadjusted analysis1 | Adjusted for male partner HIV-1 plasma viral load1 | Adjusted for male partner HIV-1 plasma viral load and censored at male partner antiretroviral therapy initiation1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | (95% confidence interval) | p-value | Hazard ratio | (95% confidence interval) | p-value | Hazard ratio | (95% confidence interval) | p-value | |||
| All HIV-1 seroconversions | |||||||||||
| Circumcised | 16/374 (4.28%) | 2.72 | 0.62 | (0.35, 1.10) | 0.10 | 0.64 | (0.36, 1.14) | 0.13 | 0.56 | (0.30, 1.05) | 0.07 |
| Uncircumcised | 48/722 (6.65%) | 4.38 | |||||||||
| Genetically-linked HIV-1 seroconversions2 | |||||||||||
| Circumcised | 12/374 (3.21%) | 2.04 | 0.57 | (0.29, 1.11) | 0.10 | 0.60 | (0.31, 1.16) | 0.13 | 0.53 | (0.26, 1.07) | 0.07 |
| Uncircumcised | 38/722 (5.26%) | 3.47 | |||||||||
Cox proportional hazards analysis, stratified by region (eastern / southern Africa). Adjusted analyses control for male partner plasma HIV-1 viral load, as time-dependent variable. Censored analysis excludes person-time in the HIV-1 susceptible females after their HIV-1 infected male partners initiated antiretroviral therapy.
Genetically linked within the partnership, based on viral sequencing, as previously described [13]. For the analysis censored at the time of antiretroviral therapy initiation for the male partner, N=47 for genetically-linked HIV-1 seroconversion events.
Subgroup analyses were performed, restricted to linked transmission events and adjusted for plasma HIV-1 levels over time in the male partner (Table 3). In all subgroups, female partners of circumcised men were at decreased risk of HIV-1, although this effect wasnon-statistically significant. For all subgroup analyses, there was no suggestion of a statistically significant difference between the subgroup categories for the effect of male circumcision (i.e., no evidence for effect modification, p-values for interaction not shown). For participants from the southern Africa sites, male circumcision appeared not to protect against HIV-1 transmission (HR 1.06, p=0.93); however, this was based on a small number of HIV-1 seroconversion events (n=12) and did not differ statistically from the effect seen for eastern African sites (HR 0.51, p=0.08).
Table 3. Subgroup analyses: incident HIV-1 infections among female partners of circumcised versus uncircumcised HIV-1 infected men1.
| Number of HIV-1 seroconversions (incidence per 100 person-years) | Adjusted hazard ratio2 (95% confidence interval) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Female partners of circumcised men (N=374) | Female partners of uncircumcised men (N=722) | ||||||
| Female partner aged <30 years | 7/145 | (3.18) | 26/353 | (4.82) | 0.68 | (0.29, 1.58) | 0.37 |
| Female partner aged ≥30 years | 5/229 | (1.35) | 12/369 | (2.16) | 0.64 | (0.21, 1.89) | 0.42 |
| Unprotected sex3 | - | (3.33) | - | (12.83) | 0.23 | (0.03, 1.72) | 0.15 |
| No unprotected sex3 | - | (1.97) | - | (2.60) | 0.78 | (0.38, 1.60) | 0.49 |
| Male partner baseline HIV-1 plasma viral load < 50,000 copies/mL | 8/259 | (1.98) | 15/469 | (2.11) | 0.88 | (0.36, 2.14) | 0.79 |
| Male partner baseline HIV-1 plasma viral load VL ≥50,000 copies/mL | 4/111 | (2.25) | 23/246 | (6.17) | 0.37 | (0.13, 1.08) | 0.07 |
| Male partner with genital ulcer disease during follow-up | 1/51 | (1.19) | 8/102 | (5.08) | 0.24 | (0.03, 1.93) | 0.18 |
| Male partner without genital ulcer disease during follow-up | 11/323 | (2.18) | 30/620 | (3.20) | 0.69 | (0.34, 1.40) | 0.30 |
| Male partner randomized to acyclovir | 7/192 | (2.43) | 19/372 | (3.35) | 0.76 | (0.32, 1.82) | 0.54 |
| Male partner randomized to placebo | 5/182 | (1.66) | 19/350 | (3.60) | 0.44 | (0.16, 1.22) | 0.11 |
| Eastern African sites | 9/293 | (1.87) | 29/459 | (3.85) | 0.51 | (0.24, 1.08) | 0.08 |
| Southern African sites | 3/81 | (2.79) | 9/263 | (2.64) | 1.06 | (0.29, 3.89) | 0.93 |
Restricted to HIV-1 seroconversions determined by viral sequencing to be genetically linked within the partnership.
Adjusted for log10 HIV-1 plasma viral load (time dependent) and stratified by region (eastern vs. southern Africa).
As reported by the HIV-1 seronegative (female) partner, analyzed as a time-dependent variable. Unprotected sex was assessed for each quarter of follow-up, and an individual woman may have contributed some follow-up time to both strata; thus, only incidence rates are reported.
Discussion
In this prospective observational study of 1096 African HIV-1 serodiscordant couples in which the HIV-1 seropositive partner was male, we found a non-statistically significant decreased risk of HIV-1 transmission from circumcised HIV-1 infected men to their female partners, compared to couples with uncircumcised HIV-1 infected men. This finding adds to a limited body of data relating circumcision status in HIV-1 infected men to the risk of male-to-female HIV-1 transmission, data which may be helpful for programs working to scale-up male circumcision for HIV-1 prevention.
Only one previous longitudinal observational study directly assessed the effect of male circumcision on male-to-female HIV-1 transmission risk by measuring incident HIV-1 infections within HIV-1 serodiscordant couples [9]. Among 223 couples in Rakai, Uganda, HIV-1 seroincidence was 5.2 versus 13.2 per 100 person-years among female partners of circumcised versus uncircumcised HIV-1 seropositive men, respectively (adjusted risk ratio 0.41, 95% confidence interval 0.10-1.14). No transmissions occurred within couples with circumcised men who had plasma viral loads <50,000 copies/mL, compared with an HIV-1 incidence of 9.6 per 100 person-years in couples with uncircumcised men (p=0.02). Like this study, our results suggest an overall decreased HIV-1 risk in partners of circumcised men, compared with partners of uncircumcised men, although our study did not find a statistically significant difference in circumcision effect for men with lower plasma HIV-1 concentrations compared to those with higher concentrations. Two additional prospective cohort studies in women assessed the relationship between male circumcision status and risk of HIV-1 acquisition: one found a statistically significant protective effect [8] and the other no effect [10]. An important limitation of these latter two studies was the potential for misclassification, as both relied on women's report of their partners' circumcision status.
Biologic mechanisms by which circumcision could reduce male-to-female HIV-1 risk include reduced risk of STIs, particularly genital ulcer disease, that may serve as co-factors for HIV-1 transmission [11, 17-20] or potentially through direct HIV-1 transmission via microtrauma or inflammation of the foreskin. However, we observed no evidence that genital ulcer disease was a substantial factor explaining the difference in HIV-1 transmission risk for circumcised versus uncircumcised men in our population. Social and behavioral factors could in part explain a relationship between circumcision status and HIV-1 transmission risk, although our analyses adjusting for region and for sexual behavior over time argue against a strong confounding effect be these factors.
A recently-completed clinical trial randomized uncircumcised, HIV-1 infected men to immediate versus delayed circumcision and assessed HIV-1 seroincidence for their partners [11]. The probability of HIV-1 acquisition was not statistically different for women whose partners became circumcised (21.7% at 24 months) compared with those whose partners remained uncircumcised (13.4%, HR 1.49, p=0.37). Notably, a post hoc analysis found the HIV-1 acquisition rate among partners of men who remained uncircumcised was 7.9% during the first 6 months after enrollment compared with 27.8% for partners of men who were circumcised and then resumed sexual activity prior to documented healing of the surgical wound (p=0.04), a substantially increased risk.
One possible explanation for the difference between the results of the observational studies in HIV-1 serodiscordant couples and the recent clinical trial may be that men in the observational studies were likely circumcised in childhood or adolescence and thus had many years for full wound healing and keratinization of the glans. This raises the possibility that male circumcision may have long-term null or beneficial effects on male-to-female HIV-1 risk, but short-term risk for enhanced HIV-1 transmission during the post-operative period for men who are already HIV-1 infected when they become circumcised. Thus, circumcision earlier in life (e.g., in childhood, prior to initiation of sexual activity) might maximize the potential benefits of circumcision and minimize risks. Problematically, clinical trials to assess the effect of childhood circumcision on future HIV-1 transmission risk would likely be prohibitively long and expensive, as would trials of long-term follow-up (e.g., 5 years or more, to allow for keratinization of the glans) of partners of HIV-1 infected men who undergo circumcision.
Our study had several strengths, including its geographic diversity and enrollment of women at risk for HIV-1 and their partners, which allowed confirmation of circumcision status, adjustment for plasma HIV-1 levels in the male partner, and determination of viral transmission linkage within the partnership. Condom use was very high among this population of HIV-1 serodiscordant couples who received ongoing individual and couples risk-reduction counseling; unprotected sexual activity might be expected to be more common and HIV-1 incidence higher outside of a clinical trial. Sexual behavior risks did not differ between couples with circumcised and uncircumcised HIV-1 infected men in our population.
In spite of the large sample size (almost 1100 HIV-1 serodiscordant couples with HIV-1 seropositive males), our statistical power for adjusted and subgroup analyses was limited, and even larger observational studies might be necessary to detect a statistically significant effect of circumcision on reducing male-to-female HIV-1 transmission risk. Assuming the protective effect of circumcision on HIV-1 incidence we observed in this study held constant if we had enrolled a larger sample size, we would have achieved statistical significance with 76 linked HIV-1 transmission events, which we estimate we would have seen if we had followed 1302 couples. However, others have recently estimated that a randomized controlled trial of up to 10,000 HIV-1 serodiscordant couples might be necessary to definitively evaluate the effect of circumcising HIV-1 infected men on HIV-1 transmission risk to their female partners [21], for example, if the true magnitude of a protective effect of circumcision on male-to-female HIV-1 transmission risk is less than we observed in this study or if there is a period of increased HIV-1 transmission risk after circumcision.
Additional limitations include that HIV-1 subtype data are not available for this cohort (which might allow assessment of whether subtype modified the relationship between circumcision status and HIV-1 transmission risk) nor were data collected on the age of circumcision for circumcised men. Etiologic testing for STIs was performed only at enrolment and data on bacterial vaginosis are not available; however, STIs were rare in this population and syndromic assessment and treatment occurred quarterly.
Circumcision roll-out programs targeting HIV-1 uninfected men have recently been initiated in several countries. Mathematical modeling studies have demonstrated that male circumcision programs will lead to decreasing HIV-1 prevalence in women, over 10-20 years, by averting infections in men and onward transmission to their partners [22]. Women will also benefit from male circumcision programs through decreased risk of STIs, including T. vaginalis, bacterial vaginosis, herpes simplex virus type 2, and human papilloma virus [11, 17-20, 23]. Our results suggest that men who acquire HIV-1 in spite of being circumcised pose no greater HIV-1 risk to their female partners than if they were uncircumcised, and may actually pose a reduced risk. For men who are already HIV-1 infected and who desire circumcision, short-term interventions to protect against transmission risk during wound healing could be studied (e.g., a period of antiretroviral therapy or potentially antiretroviral pre-exposure prophylaxis for their female partners). Finally, programs should involve women for informed decision-making about circumcision [23], including couples HIV-1 counseling and testing and messaging on the risks and benefits of circumcision for HIV-1 seropositive men and their partners.
Acknowledgments
We thank the site staff, community partners, and institutions that contributed to the Partners in Prevention HSV/HIV Transmission Study. We gratefully acknowledge the invaluable contributions of the HIV-1 serodiscordant couples who participated in this study.
Funding: The Partners in Prevention HSV/HIV Transmission Study was funded by the Bill and Melinda Gates Foundation (grant ID #26469). Additional support provided by the US National Institutes of Health (National Institute of Allergy and Infectious Diseases grant R01 083034 and National Institute of Mental Health grant R01 66767, the latter for development of HIV testing and recruitment strategies for HIV serodiscordant conducted in part with the Emory-Rwanda-Zambia HIV Research Group). The trial was registered in clinicaltrials.gov (NCT00194519).
Role of the Funding Source: The authors designed and executed the study, had full access to the raw data, performed all analyses, wrote the manuscript, and had final responsibility for the decision to submit for publication.
Footnotes
Conference presentation: Presented in part at the 5th International AIDS Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, South Africa, 19-22 July 2009 (abstract LBPEC06).
Conflict of Interest: No authors report conflicts of interest regarding content for this manuscript.
Authorship: Data were collected as part of the Partners in Prevention HSV/HIV Transmission Study, designed by CC. All authors contributed to the design of the present analysis and to the final draft of the manuscript. JB completed the first draft of the manuscript; DD performed the statistical analyses.
Partners in Prevention HSV/HIV Transmission Study Team:
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared Baeten, Mary S. Campbell, Robert W. Coombs, Lawrence Corey, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, James I. Mullins, William L. H. Whittington
Study sites and site principal investigators:
Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex, Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Kayitesi Kayitenkore, Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, William Kanweka; Lusaka, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga, Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Mubiana Inambao; Orange Farm, South Africa (Reproductive Health Research Unit, University of the Witwatersrand): Sinead Delany-Moretlwe, Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, James McIntyre; Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 3.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RC, Plummer FA, Moses S. Male circumcision and HIV prevention: current knowledge and future research directions. Lancet Infect Dis. 2001;1:223–231. doi: 10.1016/S1473-3099(01)00117-7. [DOI] [PubMed] [Google Scholar]
- 5.Biesalski HK. Comparative assessment of the toxicology of vitamin A and retinoids in man. Toxicology. 1989;57:117–161. doi: 10.1016/0300-483x(89)90161-3. [DOI] [PubMed] [Google Scholar]
- 6.Weiss HA, Halperin D, Bailey RC, Hayes RJ, Schmid G, Hankins CA. Male circumcision for HIV prevention: from evidence to action? AIDS. 2008;22:567–574. doi: 10.1097/QAD.0b013e3282f3f406. [DOI] [PubMed] [Google Scholar]
- 7.WHO/UNAIDS. New data on male circumcision and HIV prevention: policy and programme implications. Geneva: WHO; 2007. [Google Scholar]
- 8.Kapiga SH, Lyamuya EF, Kwihula GK, Hunter DJ. The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS. 1998;12:75–84. doi: 10.1097/00002030-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, Mangen FW, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team AIDS. 2000;14:2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 10.Turner AN, Morrison CS, Padian NS, Kaufman JS, Salata RA, Chipato T, et al. Men's circumcision status and women's risk of HIV acquisition in Zimbabwe and Uganda. AIDS. 2007;21:1779–1789. doi: 10.1097/QAD.0b013e32827b144c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wawer MJ, Makumbi F, Kigozi G, Serwadda D, Watya S, Nalugoda F, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374:229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingappa JR, Kahle E, Mugo N, Mujugira A, Magaret A, Baeten J, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the Partners Study. PLoS ONE. 2009;4:e5272. doi: 10.1371/journal.pone.0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celum C, Wald A, Lingappa JR, Magaret A, Wang RS, Mugo N, et al. Twice-daily acyclovir to reduce HIV-1 transmission from HIV-1/HSV-2 dually-infected persons within HIV-1 serodiscordant couples: a randomized, double-blind, placebo-controlled trial. Submitted. [Google Scholar]
- 14.Laeyendecker O, Henson C, Gray RH, Nguyen RH, Horne BJ, Wawer MJ, et al. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J Clin Microbiol. 2004;42:1794–1796. doi: 10.1128/JCM.42.4.1794-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 17.Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobngwi-Tambekou J, Taljaard D, Lissouba P, Zarca K, Puren A, Lagarde E, Auvert B. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009;199:958–964. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, Lissouba P, Puren A, Auvert B. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sex Transm Infect. 2009;85:116–120. doi: 10.1136/sti.2008.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200:42 e41–47. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss HA, Hankins CA, Dickson K. Male circumcision and risk of HIV infection in women: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:669–677. doi: 10.1016/S1473-3099(09)70235-X. [DOI] [PubMed] [Google Scholar]
- 22.Hallett TB, Singh K, Smith JA, White RG, Abu-Raddad LJ, Garnett GP. Understanding the impact of male circumcision interventions on the spread of HIV in southern Africa. PLoS ONE. 2008;3:e2212. doi: 10.1371/journal.pone.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeten JM, Celum C, Coates TJ. Male circumcision and HIV risks and benefits for women. Lancet. 2009;374:182–184. doi: 10.1016/S0140-6736(09)61311-8. [DOI] [PubMed] [Google Scholar]


