Abstract
Visceral fat has been linked to insulin resistance and type 2 diabetes mellitus (T2DM); and emerging data links RBP4 gene expression in adipose tissue with insulin resistance. In this study, we examined RBP4 protein expression in omental adipose tissue obtained from 24 severely obese patients undergoing bariatric surgery, and 10 lean controls (4 males/6 females, BMI = 23.2 ± 1.5 kg/m2) undergoing elective abdominal surgeries. Twelve of the obese patients had T2DM (2 males/10 females, BMI: 44.7 ± 1.5 kg/m2) and 12 had normal glucose tolerance (NGT: 4 males/8 females, BMI: 47.6 ± 1.9 kg/m2). Adipose RBP4, glucose transport protein-4 (GLUT4), and p85 protein expression were determined by western blot. Blood samples from the bariatric patients were analyzed for serum RBP4, total cholesterol, triglycerides, and glucose. Adipose RBP4 protein expression (NGT: 11.0 ± 0.6; T2DM: 11.8 ± 0.7; lean: 8.7 ± 0.8 arbitrary units) was significantly increased in both NGT (P = 0.03) and T2DM (P = 0.005), compared to lean controls. GLUT4 protein was decreased in both NGT (P = 0.02) and T2DM (P = 0.03), and p85 expression was increased in T2DM subjects, compared to NGT (P = 0.03) and lean controls (P = 0.003). Regression analysis showed a strong correlation between adipose RBP4 protein and BMI for all subjects, as well as between adipose RBP4 and fasting glucose levels in T2DM subjects (r = 0.76, P = 0.004). Further, in T2DM, serum RBP4 was correlated with p85 expression (r = 0.68, P = 0.01), and adipose RBP4 protein trended toward an association with p85 protein (r = 0.55, P = 0.06). These data suggest that RBP4 may regulate adiposity, and p85 expression in obese-T2DM, thus providing a link to impaired insulin signaling and diabetes in severely obese patients.
INTRODUCTION
Obesity is strongly associated with insulin resistance and impaired glucose-mediated insulin secretion, and adds considerably to overall cardiovascular morbidity and mortality (1,2). Accumulation of abdominal fat may be even more problematic (3). It is now well established that adipose tissue is an active endocrine organ, and that adipocytes secrete a multitude of biologically active proteins and adipokines that mediate insulin resistance, including retinol-binding protein 4 (RBP4) (ref. 4). Yang et al. were the first to report that elevated serum RPB4 was associated with insulin resistance, and that ablation of RBP4 in mice improved insulin sensitivity (4). In humans, serum RBP4 levels are increased in obesity, impaired glucose tolerance, and type 2 diabetes mellitus (T2DM) (5). It is also known that RBP4 gene expression is increased in visceral as compared to subcutaneous adipose tissue in obese persons (6). However, there are no data showing an increase in RBP4 protein expression in visceral adipose tissue in humans who are obese or have T2DM.
Insulin resistance is partially characterized by impaired insulin signaling through the insulin receptor substrate-1/phosphoinositol 3-kinase signaling pathway (7). Phosphoinositol 3-kinase is comprised of a p110 catalytic subunit, and a p85 regulatory subunit (8), and is activated upon phosphorylation by insulin receptor substrate-1. Contrary to what one would expect, elevations of p85 protein have been reported to impair insulin signaling (8,9). Further, adipose tissue expression of glucose transport protein 4 (GLUT4) is downregulated in obesity and insulin resistance (10), resulting in impaired insulin-stimulated glucose transport into adipocytes. The relationship between RBP4 protein levels and insulin signaling and GLUT4 protein in visceral adipose tissue is not known.
Given that alterations in serum and RBP4 mRNA, as well as changes in GLUT4 and p85 protein expression, have all been reported in insulin resistance states, we sought to determine whether RBP4 protein expression was increased in visceral adipose tissue of obese humans with T2DM compared to obese-normal glucose tolerance (NGT), and if so whether there was also increased expression of p85 protein together with decreased expression of GLUT4 protein. We hypothesized that RBP4 would be more highly expressed in omental adipose tissue from obese-T2DM, as compared to obese-NGT and lean controls, and that RBP4 would be associated with p85 and GLUT4 expression.
METHODS AND PROCEDURES
Participants
Omental fat samples were obtained from 24 patients with Grade 3 obesity, classified into obese-NGT or obese-T2DM (NGT: 4 males/8 females, BMI: 47.6 ± 1.9 kg/m2; T2DM: 2 males/10 females, BMI: 44.7 ± 1.5 kg/m2), undergoing Roux-en-Y gastric bypass surgery, and from 10 lean subjects (4 males/6 females, BMI= 23.2 ± 1.5 kg/m2) undergoing elective abdominal surgery. Samples were taken from the omental fat depot adjacent to the greater curvature of the stomach during laparoscopic surgery using endoscopic scissors. No cautery was used and samples were immediately placed in saline and were lysed in preparation for western blot analysis.
Analytical determinations
A fasting blood sample was drawn from bariatric surgery patients within 3 days before surgery and analyzed for plasma glucose, total cholesterol, and triglycerides by the clinical pathology lab at the Cleveland Clinic. A separate nonfasting blood draw was performed during the preoperative assessment visit, 1 week before surgery, and serum RBP4 was measured via sandwich enzyme-linked immunosorbent assay kit (ALPCO Diagnostics, Salem, NH) with interassay variations at two levels of 7.7% (1.2 ± 0.1) and 10.8% (2.4 ± 0.3).
Western blot analysis
Samples were lysed (20 mmol/l Tris; 1% Np-40; 137 mmol/l NaCl; 1 mmol/l CaCl2; 1 mmol/l MgCl2; 10% (v/v) glycerol; 1 mmol/l dithiothretol ; 1 mmol/l phenylmethanesulphonylfluoride; 2 mmol/l Na3VO4) and immediately stored at −80 °C. Total protein was determined via bicinchoninic acid method (Pierce, Rockford, IL). A 20-μg protein sample was loaded on 4–12% bis–tris gels and adjusted for volume with deionized water. Gels were transferred to a nitrocellulose membrane and blocked for 1 h at room temperature in 5% nonfat milk in 1% Tween–Tris-buffered saline. Membranes were washed and incubated in primary RBP4 antibody (1:1,000) (cat. no. HPA001641; Sigma, St Louis, MO) in Tween–Tris-buffered saline for 1.5 h at room temperature, anti-GLUT4 (1:1,000) (Cell Signaling Technologies, Beverly, MA), or p85 (1:1,000) (Cell Signaling Technologies) in 5% bovine serum albumin–Tween–Tris-buffered saline overnight at 4 °C. Membranes were washed and incubated with secondary antibody horseradish peroxidase goat anti-rabbit (1:2,000) (Pierce, Rockford, IL) and washed in Tween–Tris-buffered saline. The membranes were developed via enhanced chemiluminescent substrate (Super Signal West Pico; Pierce), followed by exposure to Kodak X-OMAT film (Rochester, NY). Membranes were stripped (0.5 mol/l NaOH), washed, and re-probed for β-actin (cat. no. A4700; Sigma). Films were scanned and quantitated using Image J (NIH, Bethesda, MD). A 20-μg sample of rat liver and adi-pose tissue were run as a reference control.
Data analysis
Statistical differences between groups were determined by ANOVA and paired two-tailed t-tests where appropriate. Data are presented as the mean ± s.e. The level of statistical significance was set a priori at P < 0.05.
RESULTS
Clinical characteristics and blood analysis
There was no difference between obese-NGT and obese-T2DM subjects for BMI, total cholesterol, low-density lipoprotein, or high-density lipoprotein. As expected, obese-T2DM subjects had significantly higher fasting blood glucose (NGT = 89.1 ± 3.3 mg/dl; T2DM: 147.9 ± 16.6 mg/dl, p = 0.0006). Further, there was no difference between groups for serum RBP4 (NGT = 48.5 ± 6.4 μg/ml; T2DM: 46.1 ± 4.2 μg/ml, P = 0.76).
Protein expression in omental fat
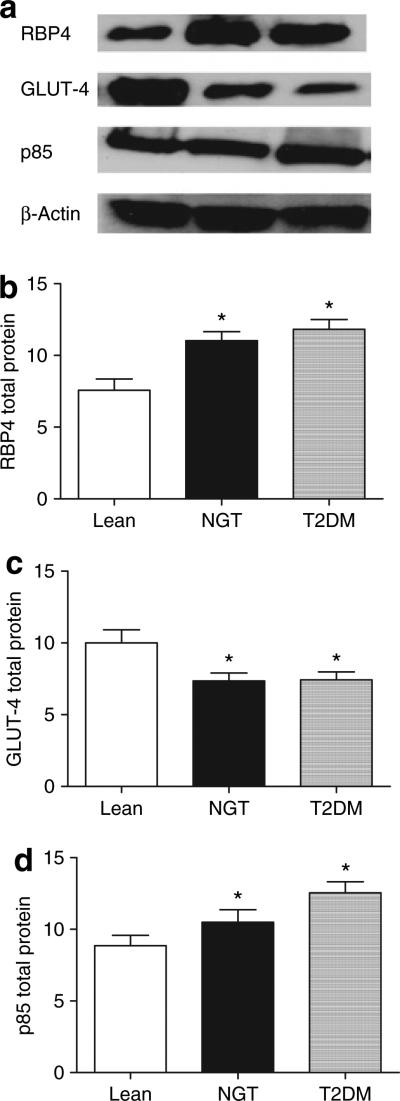
Adipose RBP4 protein expression was significantly elevated in both the obese-NGT (P = 0.03) and obese-T2DM (P = 0.005) groups compared to lean controls (Figure 1a,b). There was no difference between obese-NGT and obese-T2DM (P = 0.42). Total GLUT4 protein was significantly reduced in the obese patients (NGT: 7.4 ± 0.6 arbitrary units, P = 0.02; T2DM: 7.5 ± 0.6 arbitrary units, P = 0.03) compared to lean controls (9.7 ± 0.9 arbitrary units) (Figure 1c). There was no difference in GLUT4 expression between the obese subgroups (NGT vs. T2DM, P = 0.94). Total p85 protein was significantly elevated in obese-T2DM as compared to lean controls (P = 0.003) and obese-NGT (P = 0.03) (Figure 1d).
Figure 1.
Omental fat samples were obtained from lean, obese-normal glucose tolerant (NGT), and obese-type 2 diabetic patients (T2DM) and 20 μg of protein was run on 4–12% bis–tris gels. Blots were probed for (b) RBP4, (c) GLUT-4, and (d) p85 total protein and were normalized to β-actin . Representative blots are shown in a. *P < 0.05, significantly different from lean control.
Correlation analyses
The combined data from all subjects revealed that omental RBP4 protein was significantly correlated with BMI (r = 0.37, P = 0.04). Strong association trends were noted between total GLUT4 protein and BMI (r = −0.35, P = 0.08), and p85 protein and BMI (r = 0.31, P = 0.08). Fasting blood glucose levels were significantly correlated with both adipose RBP4 (r = 0.76, P = 0.004) and p85 total protein (r = 0.63, P = 0.03) for the T2DM subjects. Further, in the obese-T2DM group, there was a strong association between serum RBP4 levels and p85 protein (r = 0.68, P = 0.01), while total adipose RBP4 protein and p85 protein trended toward a significant correlation (r = 0.55, P = 0.06).
DISCUSSION
The strong relationship between adipose RBP4 protein and adiposity as reflected by BMI suggests that adipose tissue RBP4 expression may play a role in adipogenesis. Retinaldehyde, which is an oxidized derivative of retinol is expressed in adipose tissue and has been shown to inhibit adipogenesis, possibly through suppression of nuclear receptor responses (11). Although speculative on our part, there is the possibility that increased RBP4 in adipose tissue binds and holds retinol, thus reducing retinaldehyde formation and the inhibition of adipogenesis in the cell. We also found a strong association between adipose RBP4 expression and fasting glucose for the T2DM subjects, suggesting that upregulation of tissue RBP4 may be secondary to hyperglycemia.
RBP4 has been linked to insulin resistance (4,5). Insulin resistance is characterized by decreased insulin-stimulated glucose uptake and defective signaling in the phosphoinositol 3-kinase pathway in metabolically active tissues including adipose tissue (1,12). Herein, we report an increase in p85 protein expression in severely obese-T2DM subjects. The strong correlation between p85 and serum RBP4 protein, and the trend toward an association between p85 and adipose RBP4 protein, suggests that elevations in RBP4, both systemically and locally, may upregulate expression of p85 and this may contribute to impaired insulin signaling and decreased glucose uptake resulting in hyperglycemia (8,13). This possibility is supported by the observation that p85 protein expression in adipose tissue is associated with fasting glucose levels in the obese-T2DM subjects.
Insulin-facilitated glucose transport via GLUT4 is a critical component of insulin action in adipose tissue. Similar to previously published data (10,14), we find that GLUT4 protein is decreased in adipose tissue from obese patients. It has been suggested that RBP4 is produced and secreted in response to downregulation of GLUT4 in adipose tissue and this in turn acts to mediate insulin resistance in muscle and glucose production in the liver (10). However, we found no correlation between adipose RBP4 protein expression and GLUT4 protein in adipose tissue in this study. Associations between serum RBP4, as well as RBP4 mRNA, and adipose tissue GLUT4 expression have been inconsistent. Some studies report an inverse correlation (5,6), others a positive correlation (14–16). Our lack of association suggests that suppression of GLUT4 might be independent of adipose RBP4 expression and might be due to other factors such as macrophage migration into adipose tissue (17), or overproduction of macrophage-derived cytokines, which result in reduced GLUT4 protein (18). Alternatively, resistin is an adipocyte-secreted hormone that is elevated in obesity (19) and similar to RBP4 is abundant in the liver (20) and involved in adipogenesis. Thus, it may be that RBP4 expression is regulated via resistin or another adipokine in a paracrine manner. However, further studies are needed to evaluate these possibilities.
In summary, our data show for the first time that total RBP4 protein is elevated in omental adipose tissue in severely obese patients. We found a strong correlation between BMI and adipose RBP4, suggesting that RBP4 may be linked in some way to adipogenesis. Further, our data agree with previously published reports that adipose GLUT4 protein is downregulated in obese subjects, and that p85 is upregulated. We also observed correlations between serum RBP4 and p85 protein, and fasting blood glucose and adipose RBP4 expression, and fasting blood glucose and p85 expression in obese-T2DM subjects. Collectively, these data suggest that RBP4 may be involved in adipogenesis in severely obese patients, and in the downregulation of insulin signaling and subsequent hyperglycemia observed in severely obese-T2DM patients.
ACKNOWLEDGMENTS
We thank the surgical nursing staff of the Bariatric and Metabolic institute, Dr Silverstein's laboratory for preparation of human adipose tissue samples, and Dr Nagy for rat liver and adipose tissue samples. This research was supported by National institutes of Health grants aG12834 (J.p.K.), RR018390, and T32DK007319, National Center for Research Resources UL1RR024989, and Multidisciplinary Clinical Research Career Development programs Grant K12RR023264 (S.R.K.).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 2.Bourey RE, Kohrt WM, Kirwan JP, et al. Relationship between glucose tolerance and glucose-stimulated insulin response in 65-year-olds. J Gerontol. 1993;48:M122–M127. doi: 10.1093/geronj/48.4.m122. [DOI] [PubMed] [Google Scholar]
- 3.Kohrt WM, Kirwan JP, Staten MA, et al. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- 4.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 5.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 6.Klöting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54:2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- 9.Kirwan JP, Varastehpour A, Jing M, et al. Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol Metab. 2004;89:4678–4684. doi: 10.1210/jc.2004-0749. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd PR, Kahn BB. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 11.Ziouzenkova O, Orasanu G, Sharlach M, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-α. J Clin Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F, Ueki K, Fruman DA, et al. Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J Clin Invest. 2002;109:141–149. doi: 10.1172/JCI13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janke J, Engeli S, Boschmann M, et al. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 15.Yao-Borengasser A, Varma V, Bodles AM, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribel-Madsen R, Friedrichsen M, Vaag A, Poulsen P. Retinol-binding protein 4 in twins: regulatory mechanisms and impact of circulating and tissue expression levels on insulin secretion and action. Diabetes. 2009;58:54–60. doi: 10.2337/db08-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikκβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 19.Vázquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–728. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Szalowska E, Elferink MG, Hoek A, Groothuis GM, Vonk RJ. Resistin is more abundant in liver than adipose tissue and is not up-regulated by lipopolysaccharide. J Clin Endocrinol Metab. 2009;94:3051–3057. doi: 10.1210/jc.2008-2787. [DOI] [PubMed] [Google Scholar]