Abstract
Cancer vaccines have undergone a renaissance due to recent clinical trials showing promising immunological data and some clinical benefit to patients. Current trials exploiting dendritic cells (DCs) as vaccines have shown durable tumor regressions in a fraction of patients. Clinical efficacy of current vaccines is hampered by myeloid-derived suppressor cells, inflammatory type 2 T cells and regulatory T cells (Tregs), all of which prevent the generation of effector cells. To improve the clinical efficacy of DC vaccines, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome Tregs and allow the breakdown of the immunosuppressive tumor microenvironment. This can be achieved by exploiting the fast increasing knowledge about the DC system, including the existence of distinct DC subsets. Critical to the design of better vaccines is the concept of distinct DC subsets and distinct DC activation pathways, all contributing to the generation of unique adaptive immune responses. Such novel DC vaccines will be used as monotherapy in patients with resected disease and in combination with antibodies and/or drugs targeting suppressor pathways and modulation of the tumor environment in patients with metastatic disease.
Keywords: dendritic cells, cancer, vaccines, priming
INTRODUCTION
Molecular identification of human cancer antigens has ushered in a new era of antigen specific cancer immunotherapy specifically targeting these antigens. One strategy is the transfusion of T cells, also called adoptive T cell therapy (reviewed in (1)). There, antigen specific T cells are expanded ex vivo and transfused to patients. Adoptive T cell therapy has been shown to be an effective treatment for viral infections (2) and has induced regression of cancer in early-stage clinical trials (3, 4). Another strategy is to expand T cells in vivo by means of vaccination. Initial attempts at vaccination (e.g. peptides, DNA vaccines, viral vectors and first generation DC-based vaccines) have thus far met with a limited success in the clinic. However, cancer vaccines are undergoing a renaissance due to increased knowledge on the regulation of immune responses as well as recent clinical trials showing promising immunological data and some clinical benefit to the patients. For example, an active immunotherapy product, sipuleucel-T (APC8015) based on antigen-loaded and GM-CSF activated PBMCs, appears to contribute to prolonged median survival in phase III trials in patients with prostate cancer (5). Sipuleucel-T has been recently approved by the FDA for treatment of metastatic prostate cancer thereby paving the regulatory path for the next generation of active immunotherapy products. A randomized phase II trial of a poxviral-based vaccine targeting PSA (PROSTVAC) in men with metastatic castration-resistant prostate cancer showed improved overall survival in patients who received PROSTVAC compared to patients receiving control vectors (6). While these first generation positive randomized phase II/III clinical trials need further analysis and mechanistic studies, they underline the therapeutic potential of the immune system that can be tapped into. Vaccines act through DCs which induce, regulate and maintain T cell immunity. Here we summarize our recent studies aimed at a better understanding of the DC system to unravel the pathophysiology of cancer and to design novel DC-based cancer vaccines with enhanced clinical relevance.
DENDRITIC CELLS
T cell priming is under the control of DCs. In the steady state, non-activated (immature) DCs present self-antigens to T cells, which leads to tolerance (7, 8). DCs induce immune tolerance in a number of ways including i) T cell deletion (9-11); ii) induction of T cell unresponsiveness (12); and iii) activation of regulatory T cells (Tregs) (13-16). Once activated (mature), antigen-loaded DCs are geared towards the launching of antigen-specific immunity (17, 18) leading to the T cell proliferation and differentiation into helper and effector cells (Figure 1). DCs are also important in launching humoral immunity partly due to their capacity to directly interact with B cells (19, 20) and to present unprocessed antigens (21-24).
Figure 1. Dendritic cells.
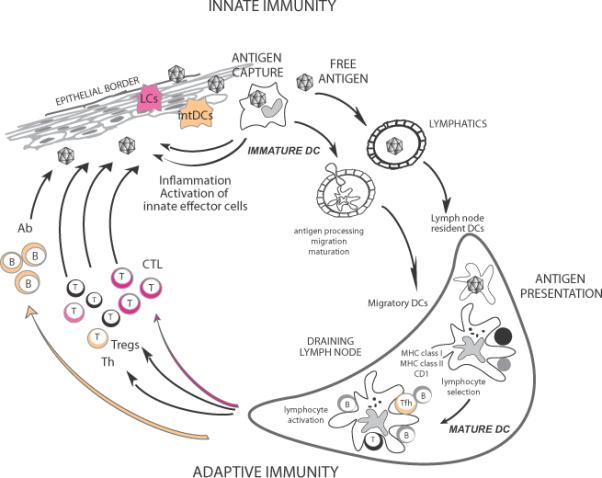
DCs reside in the tissue where they are poised to capture antigens (109). During inflammation, circulating precursor DC enter tissues as immature DC (109). DCs can encounter pathogens (e.g.: viruses) directly, which induce secretion of cytokines (e.g.: IFN-□); or indirectly through the pathogen effect on stromal cells. Cytokines secreted by DCs in turn activate effector cells of innate immunity such as eosinophils, macrophages and NK cells. Microbe activation triggers DCs migration towards secondary lymphoid organs and simultaneous activation (maturation). These activated migratory DCs that enter lymphoid organs display antigens in the context of classical MHC class I and class II or non-classical CD1 molecules, which allow selection of rare circulating antigen-specific T lymphocytes. Activated T cells help drive DCs toward their terminal maturation, which allows lymphocyte expansion and differentiation. Activated T lymphocytes traverse inflamed epithelia and reach the injured tissue, where they eliminate microbes and/or microbe-infected cells. B cells, activated by DCs and T cells, migrate into various areas where they mature into plasma cells that produce antibodies that neutralize the initial pathogen. Antigen can also reach draining lymph nodes without involvement of peripheral tissue DCs and be captured and presented by lymph node resident DCs (110). The quality of antibody responses is determined by intDCs while the quality of CTL responses is dictated by LCs.
To allow resistance to infection and tolerance to self, DCs are endowed with two critical features: subsets and functional plasticity (25). The two major subsets are the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs). The best studied human mDC subsets are those from skin, where three subsets can be identified. The epidermis hosts only Langerhans Cells (LCs) while the dermis displays two mDC subsets, CD1a+ DCs and CD14+ DCs, as well as macrophages (26-29). pDCs are considered as the front line in anti-viral immunity owning to their capacity to rapidly produce high amounts of type I interferon (30, 31). Similarly to mDCs (as discussed in detail below), pDCs display a remarkable functional plasticity. Thus, pDCs exposed to viruses, such as live influenza virus, are able to launch memory responses by inducing the expansion and differentiation of antigen-specific memory B and T lymphocytes into plasma cells (32), and CTLs (33, 34), respectively. On the contrary, pDCs activated with CpG or IL-3/CD40L induce in vitro IL-10-secreting regulatory CD4+ T cells (35) as well as suppressor CD8+ T cells through the expression of ICOS ligand (36). Their role in active immunotherapy of cancer is largely undefined. Hereunder, we will discuss in a greater detail the recent advances in our understanding of the biology of mDCs as it applies to vaccination.
Dermal DCs, antibody responses and IL-12
In the mid 90's, we observed that CD14+ DCs derived from CD34+ hematopoietic progenitor cells (HPCs) induce CD40-activated naïve B cells to differentiate into IgM-producing plasma cells through the secretion of IL-6 and IL-12 (37). A decade later, we found that CD14+ DCs, but not LCs, induce naïve CD4+ T cells to differentiate into cells with properties of T follicular helper cells (Tfh) (27), a CD4+ T cell subset specialized in B cell help (38, 39). There, CD4+ T cells primed by CD14+ DCs help naïve B cells to produce large amounts of IgM, and switch isotypes towards IgG and IgA. Our recent studies in human indicate that acquisition of Tfh phenotype and function depends on IL-12p70 (40).
Thus, IL-12 appears to contribute to humoral immunity in humans through a direct path in DC-B interaction, and an indirect path in DC-T cell interaction and induction of Tfh cells. These findings might explain the modest clinical efficacy of systemic IL-12 administration in cancer patients (41, 42). Furthermore, the injection of IL-12 into tumor sites of head and neck cancer patients resulted in the activation of B cells in the draining lymph nodes, which was associated with their infiltration into tumor sites and tumor regression (43).
LCs and CD8+ T cell responses
LCs induce a robust proliferation of naïve allogeneic CD8+ T cells when compared to CD14+ DCs (27). Furthermore, when pulsed with MHC class I peptides derived from tumor or viral antigens, LCs are far more efficient than CD14+ DCs in the priming of antigen-specific CD8+ T cells. LCs are also efficient in cross-presenting peptides from protein antigens to CD8+ T cells. CD8+ T cells primed by LCs show high avidity in tetramer binding assays and express higher levels of cytotoxic molecules, such as granzymes and perforin. Accordingly, they are remarkably more efficient in killing target cells; in particular tumor cells that express low level of peptide/HLA complexes (27). IL-15 might explain the remarkable effects of LCs on the development of Cytotoxic T Lymphocyte (CTL) responses (44-46). Thus, the two different arms of adaptive immunity, i.e., humoral and cellular arms, might be differentially regulated by the two skin mDC subsets (Figure 1). Such framework might be of capital importance for the understanding of the immune alteration in malignancy and for development of novel and improved vaccination strategies against cancer, as well as chronic infections.
DENDRITIC CELLS IN VACCINATION AGAINST CANCER
Outcomes of current DC vaccination trials
Ex vivo-generated DCs have been used as therapeutic vaccines in patients with metastatic cancer for over a decade and early studies have been discussed in detail elsewhere (47). While a fraction of patients can experience durable tumor regressions (48), the most common outcome of the current DC vaccination protocols is a demonstration of expanded antigen-specific immunity, most often using IFN-γ ELISPOT, but no durable objective tumor regression.
Altogether, three outcomes emerge from our studies:
1) No immune response. Patients of this group usually progress quickly. These patients mount immune responses to control antigens such as KLH or viral peptides (Flu-M1 or CMV). In vitro experiments indicated that T cells of several patients can be primed to differentiate into CTLs with specificity for multiple melanoma antigens (49). Thus, tumor antigen-specific CD8+ T cells are kept anergic rather than deleted. This inability to mount immune responses to tumor antigens in vivo might be at least partly related to the presence of tumor antigen-specific Tregs (50, 51). Tregs limit the onset of protective immunity through several mechanisms, for example by eliminating DCs in lymph nodes (52). As discussed later, the control of Tregs becomes a key target to address for the coming vaccination trials. 2) Immune response without clinical response. The most common outcome of current DC vaccination protocols is the induction of immune responses in the absence of clinical responses. This might in part be explained by the quality of the elicited T cells including their capacity to migrate into tumors and penetrate tumor stroma (53). Improved immunomonitoring is expected to provide insights into the mechanisms of immune efficacy as discussed hereunder (54, 55). 3) Immune response and clinical response. Vaccination with DCs can elicit therapeutic immunity. These patients represent a formidable opportunity for the development of cancer immunotherapy. The challenge is two-fold. First, to establish the immunological mechanism that allowed tumor eradication. Second, we need to find ways to increase the fraction of patients experiencing durable tumor regression and/or prolonged survival.
The quality of elicited antigen-specific immune responses
Establishing causative links in clinical studies is a difficult task which often requires large patient cohorts. The current data suggest an association between the tumor-specific CD8+ T cell responses and clinical outcomes. In our view, four critical components will determine whether the induced immune response will be therapeutic: 1) the quality of elicited CTLs; 2) the quality of induced CD4+ helper T cells; 3) the elimination and/or non-activation of Tregs; and 4) the breakdown of immunosuppressive tumor microenvironment.
Indeed, the immune responses elicited by the first generation DC vaccines might not be of the quality required to allow the rejection of bulky tumors. For example, the induced T cells might not migrate into the tumor lesions (56, 57). Furthermore, low avidity T cells might be unable to recognize peptide-MHC class I complexes on tumor cells and/or to kill them (56). Finally, the tumor micro-environment might inhibit effector T cell functions, for example by action of myeloid derived suppressor cells and Tregs as summarized in recent reviews, respectively (58, 59).
The recent progresses in immunomonitoring of specific immune responses in the blood and at the tumor site should help us address these questions (48, 50, 54, 55, 60). Modern approaches including polychromatic flow cytometry rather than the analysis of a single cytokine (e.g., IFN-□ ELISPOT) and/or frequency of tetramer positive cells will contribute to a better assessment of the quality of the immune responses elicited in the patients (61, 62). Indeed, several studies, mostly performed in the context of HIV vaccines, have led to the conclusion that a mere measurement of the frequency of IFN-γ secreting CD8+ T cells is insufficient to evaluate the quality of vaccine-elicited immunity (56, 63, 64).
BUILDING ON DENDRITIC CELL SUBSETS TO IMPROVE CANCER VACCINES
Optimal DCs
The results summarized above prompted us to hypothesize that DCs with the properties of LCs might prove to be the best ones for the generation of strong cellular immunity (Figure 1). In line with this, the combination of cytokines used to differentiate monocytes into DCs play a critical role in determining the quality of the elicited T cell responses. For example, DCs generated with GM-CSF and IL-15 display the phenotype and characteristics of LCs. In particular, they are more efficient in priming melanoma-antigen specific CD8+ T cells in vitro than DCs derived with GM-CSF and IL-4 (44, 45). Thus, vaccination with IL15-DCs might elicit stronger CD8+ T cell responses that might lead to improved clinical responses. We are currently initiating such a clinical trial in patients with malignant melanoma. The selection of methods for activating DCs also represents a critical parameter in the design of DC vaccines. First, immature (non-activated) DCs induce antigen specific IL-10 producing T cells (65, 66). Second, IL-4 DCs activated with a cocktail of IFN-α, polyI:C, IL-1β, TNF, and IFN-γ induce up to 40 times more melanoma-specific CTLs in vitro than DCs matured with the “standard” cocktail of IL-1 □/TNF/IL-6/prostaglandin E2 (PGE2)(67-69).
Additional studies will be necessary to establish the therapeutic value of the newer generation DC vaccines in patients. Besides the quality of CD8+ T cells, the quality of CD4+ T cells will become one of the key parameters of immune efficacy. These studies are critical to the understanding of the human immune system because they permit us to assess in vivo the type of immune responses elicited by human DCs generated in different cytokine environments.
This in turn is essential for building a novel approach to vaccination that is based on the delivery of antigens directly to DCs in vivo using chimeric proteins that are made of an anti-DC receptor antibody molecularly fused to a selected antigen (DC targeting). Studies in mice demonstrate that the specific targeting of antigen to DCs in vivo results in considerable potentiation of antigen-specific CD4+ and CD8+ T cell immunity. The induction of immunity is observed only when the DC maturation signal is provided (7, 70, 71), and otherwise, tolerance ensues (7). Thus, selection of appropriate adjuvant is also a critical parameter for the induction of the immunity of the desired type. Although TLR-ligands are widely considered to promote protective immunity against infectious agents, selecting the appropriate ligand will be critical. For instance, TLR2 ligation, which promotes the induction of Tregs rather than Th1 or Th17 cells (72), does not appear to be a preferred option for cancer vaccines.
These pioneering studies have been already extended to demonstrate the targeting of tumor antigens to DCs (73) and Langerhans cells (LCs) in animal models (74, 75) and the generation of anti-tumor immunity (76). The therapeutic success of these vaccines will build on the recent knowledge and progress in our understanding of the biology of human DC subsets, cutaneous myeloid DCs (mDCs) in particular.
Optimal antigen loading
While much attention has been paid to CD8+ T cells, CD4+ T cells have long been known to be involved in anti-tumor immunity (77). A number of recent studies in murine models of cancer suggests the role of tumor antigen-specific CD4+ T cells in anti-tumor immunity, which appear to act through different mechanisms including i) help in the expansion of tumor antigen-specific CTLs (78), ii) activation of macrophages at tumor sites (79, 80), and iii) active killing of tumor cells (81, 82). Furthermore, it is now well established that antigen-specific CD4+ T cells are fundamental for the induction of long-term memory CD8+ T cells (83). Aiming at the induction of tumor antigen-specific CD4+ T cell responses, DC vaccines loaded with tumor antigen-derived HLA class II peptides (84), or transduced with a vector encoding a defined tumor antigen (85) have been tested in metastatic melanoma, and shown to induce tumor antigen-specific CD4+ T cell responses. Whether vaccination results in the development of durable memory CD4+ T cell response remains to be established.
Intimately linked to the quality of CD4+ T cells responses is the nature of the antigen and the way to load tumor antigen on DC vaccines. As illustrated in Figure 2, in our own studies we have started with loading DC vaccines with short tumor antigen-derived peptides that can be presented by MHC class I. These were combined with KLH as a foreign helper antigen with the idea to provide CD4+ T cell help via generation of new CD4+ T cell responses. Both antigens elicited immune responses and we found long-lived melanoma specific CD8+ T cells in some patients (86-88). Loading DCs with exogenous peptides that bind MHC class I molecules can generate a limited set of high quality antigen-specific CD8+ T cells. Peptides are however limited to certain HLA types and to known tumor antigens. Furthermore, there is no evidence that the currently used melanoma-derived tumor antigens might be the best targets for vaccination. Foreign helper antigens (such as KLH) can be used to prime helper T cells and potentially avoid reactivation of tumor-antigen specific T regs. However, human tumor antigen-specific CD4+ T cells might prove more efficient in their helper function for tumor antigen-specific CD8+ T cells. An alternative strategy to generate broad immune responses is to load DCs with complex antigen preparations such as tumor-derived RNA (89) or killed tumor cells (90).
Figure 2. Loading antigen on ex vivo DC vaccines.
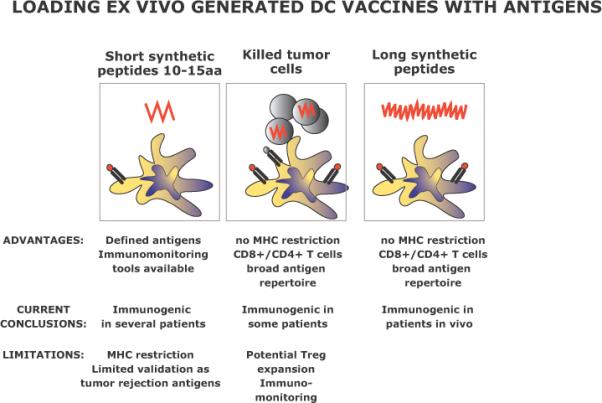
A number of ways by which the antigen can be delivered to DCs have been identified. The advantages and disadvantages are discussed. Currently, we are developing the strategy to load DCs with long peptides representing defined tumor antigens and providing both CD8+ and CD4+ T cell epitopes.
Loading DCs with killed tumor cells builds upon the unique capacity of DCs to present internalized antigens via MHC class I leading to cross-priming of naïve CD8+ T cells. A major advantage is that it is not limited to a selected haplotype such as HLA-A*0201. We have shown that DCs loaded with killed allogeneic tumor cells from melanoma (49, 91), prostate cancer (92) and breast cancer (93, 94) can cross-prime naive HLA-A*0201+ CD8+ T cells to differentiate into CTLs specific for defined tumor antigens. It also has the potential to allow presentation of tumor antigens on MHC class II molecules for tumor specific help. Type 1 CD8+ T cell immunity to MART-1 was found in three out of 13 patients with metastatic melanoma who received eight DC vaccines loaded with killed allogeneic melanoma cells (48). Two of these patients showed improved immunity in response to vaccination with DCs. Indeed, increased frequency of the specific CD8+ T cells and/or their proliferation in response to MART-1 derived peptides indicated in vivo cross-presentation of melanoma antigens by DC vaccine. In one patient, vaccination led to elicitation of IFN-γ producing CD8+ T cells specific to a MART-1 peptide to which no response could be detected at the onset. This suggests that in-vivo DC vaccines loaded with killed allogeneic melanoma cells are capable of cross-priming.
However, a potential limitation of such strategy is the expansion of tumor antigen-specific regulatory/suppressor cells. Furthermore, the assessment of immune responses in such trials represents a challenge as both antigens and their T cell epitopes as well as their HLA restriction elements are largely unknown. Recently we begun to assess the immune and clinical efficacy of DC vaccines loaded with long peptides derived from selected tumor antigens so as to allow the presentation of epitopes for both CD8+ and CD4+ T cells (Figure 2).
Combining DC vaccines with other therapies
In view of the remarkable diversity of regulatory/suppressive pathways present in patients with metastatic cancer, any durable clinical response elicited by vaccination with DCs is already a remarkable achievement. However, to improve the outcomes in metastatic disease, DC vaccines need to be combined with other therapies that offset the suppressive environment created by -tumors (95). Such combination regimens will involve several drugs that target different pathways (Figure 3).
Figure 3. DC vaccines in combination therapies.
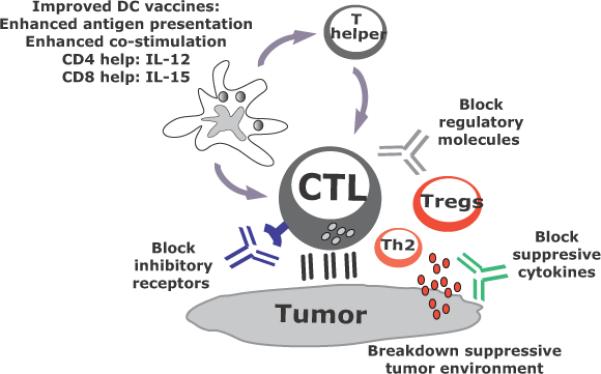
Current active immunotherapy trials have shown durable tumor regressions in a fraction of patients. However, clinical efficacy of current approaches is limited, possibly because tumors invade the immune system by means of myeloid-derived suppressor cells, inflammatory type 2 T cells and regulatory T cells (Tregs). To improve the clinical efficacy of immunotherapies, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome Tregs and allow the breakdown of an immunosuppressive tumor microenvironment. This can be achieved by developing combination therapies targeting these three major components.
In particular, blocking antibodies or soluble receptors can be exploited for the blockade of suppressive cytokines in the tumor microenvironment such as IL-10 (96), IL-13 (97), TGF-β (98, 99) and VEGF (100, 101). Such strategies can be used to block immune-inhibitory signals in lymphocytes as illustrated by anti-CTLA-4 (102, 103) and/or anti-PD1 (104-106), or to block their ligands expressed on tumors or DCs (for example anti-PD-L1).In contrast, agonistic antibodies (100, 101) might further promote co-stimulation of effector T cells as for example with anti-CD137 (107), a ligand for 4-1BB (108). Just as different tumors are treated with different combinations of cytostatic drugs and targeted therapies, we foresee development of clinical protocols combining DC vaccines with individualized adjunct therapies.
CONCLUDING REMARKS
The considerable progress made in the knowledge of DC biology as well as effector/regulatory T cell biology has clearly opened avenues for development of vastly improved clinical protocols. These, optimized DC vaccines eliciting strong and long-lived antigen-specific CD8+ and CD4+ T cell immunity will be offered to patients with early stage disease. For patients with late stage disease, strategies that combine novel highly immunogenic DC-based vaccines and immunomodulatory antibodies will have high impact on enhancing therapeutic immunity in cancer by simultaneously enhancing the potency of beneficial immune arms and offsetting immunoregulatory pathways (Figure 3). These optimized therapeutic strategies will be tailored to a single patient level, including strategies to break suppressive pathways (Figure 4).
Figure 4. Approaches to DC-based immune intervention in cancer.
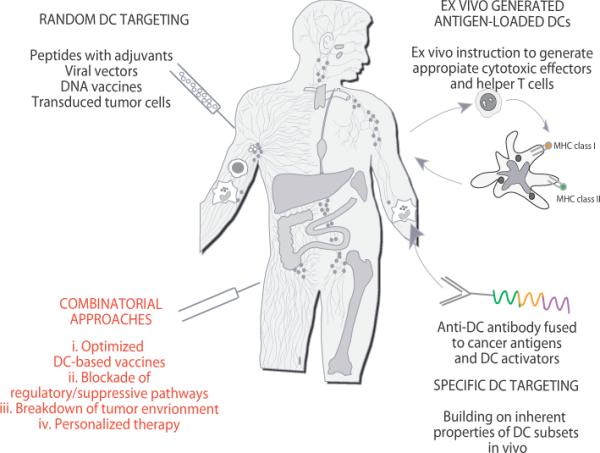
1) Vaccines based on antigen with or without adjuvant that target DCs randomly. That might result in vaccine antigens being taken up by a “wrong” type of DCs in the periphery which might lead to “unwanted” type of immune response. Vaccine antigens could also flow to draining lymph nodes where they can be captured by resident DCs; 2) Vaccines based on ex-vivo generated tumor antigen-loaded DCs that are injected back into patients; and 3) specific in vivo DC targeting with anti-DC antibodies fused with antigens and with DC activators. 4) Next generation clinical trials will test optimized DC vaccines combined with patient-adjusted approaches to block Tregs and to breakdown the tumor environment. These therapies will be tested in pre-selected patients thereby leading to personalized therapy.
ACKNOWLEDGMENTS
Dedicated to patients and volunteers who participated in our studies. We thank former and current members of the Institute for their contributions. Supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 3.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002 doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall Survival Analysis of a Phase II Randomized Controlled Trial of a Poxviral-Based PSA-Targeted Immunotherapy in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 10.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairchild PJ, Austyn JM. Thymic dendritic cells: phenotype and function. Int Rev Immunol. 1990;6:187–196. doi: 10.3109/08830189009056629. [DOI] [PubMed] [Google Scholar]
- 12.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 17.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 18.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–144. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 20.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 21.Zhong G, Reis e Sousa C, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility class II complexes after soluble protein exposure in vivo or in vitro. J. Exp. Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 23.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 26.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 29.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 32.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 33.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 34.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, Mellman I, Banchereau J, Connolly JE. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilliet M, Liu Y-J. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 38.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 39.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, Schwartz LH, Berg WJ, Bukowski RM. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–263. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- 42.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 43.van Herpen CM, van der Voort R, van der Laak JA, Klasen IS, de Graaf AO, van Kempen LC, de Vries IJ, Boer TD, Dolstra H, Torensma R, van Krieken JH, Adema GJ, De Mulder PH. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2008;123:2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 44.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 46.Klechevsky E, Liu M, Morita R, Banchereau R, Thompson-Snipes L, Palucka AK, Ueno H, Banchereau J. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70:281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 48.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 49.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-Priming of Naive CD8 T Cells against Melanoma Antigens Using Dendritic Cells Loaded with Killed Allogeneic Melanoma Cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews DM, Maraskovsky E, Smyth MJ. Cancer vaccines for established cancer: how to make them better? Immunol Rev. 2008;222:242–255. doi: 10.1111/j.1600-065X.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 52.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3(+) T Cells Induce Perforin-Dependent Dendritic Cell Death in Tumor-Draining Lymph Nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 54.Butterfield LH, Disis ML, Fox BA, Lee PP, Khleif SN, Thurin M, Trinchieri G, Wang E, Wigginton J, Chaussabel D, Coukos G, Dhodapkar M, Hakansson L, Janetzki S, Kleen TO, Kirkwood JM, Maccalli C, Maecker H, Maio M, Malyguine A, Masucci G, Palucka AK, Potter DM, Ribas A, Rivoltini L, Schendel D, Seliger B, Selvan S, Slingluff CL, Jr., Stroncek DF, Streicher H, Wu X, Zeskind B, Zhao Y, Zocca MB, Zwierzina H, Marincola FM. A systematic approach to biomarker discovery; preamble to “the iSBTc-FDA taskforce on immunotherapy biomarkers”. J Transl Med. 2008;6:81. doi: 10.1186/1479-5876-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tahara H, Sato M, Thurin M, Wang E, Butterfield LH, Disis ML, Fox BA, Lee PP, Khleif SN, Wigginton JM, Ambs S, Akutsu Y, Chaussabel D, Doki Y, Eremin O, Fridman WH, Hirohashi Y, Imai K, Jacobson J, Jinushi M, Kanamoto A, Kashani-Sabet M, Kato K, Kawakami Y, Kirkwood JM, Kleen TO, Lehmann PV, Liotta L, Lotze MT, Maio M, Malyguine A, Masucci G, Matsubara H, Mayrand-Chung S, Nakamura K, Nishikawa H, Palucka AK, Petricoin EF, Pos Z, Ribas A, Rivoltini L, Sato N, Shiku H, Slingluff CA, Streicher H, Stroncek DF, Takeuchi H, Toyota M, Wada H, Wu X, Wulfkuhle J, Yaguchi T, Zeskind B, Zhao Y, Zocca MB, Marincola FM. Emerging concepts in biomarker discovery; The US-Japan workshop on immunological molecular markers in oncology. J Transl Med. 2009;7:45. doi: 10.1186/1479-5876-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 57.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 60.Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, Old LJ, Romero P, Hoos A, Davis MM. “MIATA”-minimal information about T cell assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J. Immunol. 1999;163:6867–6875. [PubMed] [Google Scholar]
- 62.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 63.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 65.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 67.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 68.Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, McDonald HA, Gibson GA, Watkins SC, Muthuswamy R, Kalinski P, Okada H. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells--significant roles of CXCL10. Cancer Res. 2009;69:1587–1595. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 74.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, Saeland S. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei H, Wang S, Zhang D, Hou S, Qian W, Li B, Guo H, Kou G, He J, Wang H, Guo Y. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. doi: 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- 77.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 78.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen Is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-g. Proc. Natl. Acad. Sci. USA. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butterfield LH, Comin-Anduix B, Vujanovic L, Lee Y, Dissette VB, Yang JQ, Vu HT, Seja E, Oseguera DK, Potter DM, Glaspy JA, Economou JS, Ribas A. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008;31:294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palucka AK, Dhodapkar MV, Paczesny S, Burkeholder S, Wittkowski KM, Steinman RM, Fay J, Banchereau J. Single injection of CD34+ progenitor-derived dendritic cell vaccine can lead to induction of T-cell immunity in patients with stage IV melanoma. J Immunother. 2003;26:432–439. doi: 10.1097/00002371-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Palucka AK, Connolly J, Ueno H, Kohl J, Paczesny S, Dhodapkar M, Fay J, Banchereau J. Spontaneous proliferation and type 2 cytokine secretion by CD4+T cells in patients with metastatic melanoma vaccinated with antigen-pulsed dendritic cells. J Clin Immunol. 2005;25:288–295. doi: 10.1007/s10875-005-4089-z. [DOI] [PubMed] [Google Scholar]
- 88.Palucka AK, Dhodapkar MV, Paczesny S, Ueno H, Fay J, Banchereau J. Boosting Vaccinations with Peptide-Pulsed CD34+ Progenitor-Derived Dendritic Cells Can Expand Long-Lived Melanoma Peptide-Specific CD8+ T Cells in Patients with Metastatic Melanoma. J Immunother. 2005;28:158–168. doi: 10.1097/01.cji.0000154249.74383.17. [DOI] [PubMed] [Google Scholar]
- 89.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 91.Cao T, Ueno H, Rida W, Glaser C, Fay JW, Palucka AK, B. J. Both Langerhans cells and Interstitial DCs derived from CD34+ progenitor cells efficiently cross-present tumor-associated antigens to CD8+ T cells from metastatic melanoma patients. Eur J Immunol. 2007 doi: 10.1002/eji.200636499. in press. [DOI] [PubMed] [Google Scholar]
- 92.Nouri-Shirazi M, Banchereau J, Bell D, Burkeholder S, Kraus ET, Davoust J, Palucka KA. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165:3797–3803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 93.Neidhardt-Berard EM, Berard F, Banchereau J, Palucka AK. Dendritic cells loaded with killed breast cancer cells induce differentiation of tumor-specific cytotoxic T lymphocytes. Breast Cancer Res. 2004;6:R322–328. doi: 10.1186/bcr794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saito H, Dubsky P, Dantin C, Finn OJ, Banchereau J, Palucka AK. Cross-priming of cyclin B1, MUC-1 and survivin-specific CD8+ T cells by dendritic cells loaded with killed allogeneic breast cancer cells. Breast Cancer Res. 2006;8:R65. doi: 10.1186/bcr1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 96.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 97.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 98.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming Growth Factor-beta Regulation of Immune Responses. Annu Rev Immunol. 2005 doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 99.Terabe M, Ambrosino E, Takaku S, O'Konek JJ, Venzon D, Lonning S, McPherson JM, Berzofsky JA. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 101.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 105.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of Programmed Death Ligand 1 Enhances the Therapeutic Efficacy of Combination Immunotherapy against Melanoma. J Immunol. 2010 doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 108.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 109.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]


