Abstract
Objective
Cutaneous disease associated with placental transport of maternal anti-SSA/Ro or anti-SSB/La antibodies is transient and children often appear otherwise healthy. However, the impact of this manifestation of neonatal lupus (NL) on the risk of cardiac disease in a future pregnancy is critical for family counseling and powering of preventive trials.
Methods
Fifty-eight families enrolled in the Research Registry for Neonatal Lupus (RRNL) met inclusion criteria: a) maternal anti-SSA/Ro or anti-SSB/La antibodies, b) a child with cutaneous-NL, c) a pregnancy subsequent to the child with cutaneous-NL.
Results
The majority (78%) of the 58 mothers were Caucasian. Of 77 pregnancies following a child with cutaneous-NL, the overall recurrence rate for any manifestation of NL was 49% (95%CI:37%-62%); 14 (18%) were complicated by cardiac-NL, 23 (30%) by cutaneous-NL, and 1 (1%) by hematologic/hepatic-NL. A subset analysis was restricted to the 39 children born prospectively after the initial cutaneous-NL child was enrolled in the RRNL. The overall recurrence rate for NL was 36% (95%CI:20%-52%); 5 (13%) had cardiac-NL and 9 (23%) had cutaneous-NL. There were no significant differences in the following maternal risk factors for having a subsequent child with cardiac or cutaneous-NL: age, race/ethnicity, anti-SSB/La status, diagnosis, use of non-fluorinated steroids, or breastfeeding. Fetal gender of the subsequent child did not influence the development of cardiac or cutaneous-NL.
Conclusion
Based on data from this large cohort, the identification of cutaneous-NL in an anti-SSA/Ro exposed infant is particularly important since it predicts a 6-10 fold risk for a subsequent child with cardiac-NL.
Keywords: cutaneous lupus, congenital heart block, anti-SSA/Ro antibodies, anti-SSB/La antibodies, neonatal lupus
Introduction
Neonatal lupus (NL) is a pathologic readout of maternal anti-SSA/Ro and/or anti-SSB/La autoantibodies with approximately 2% of exposed offspring being affected with cardiac disease [1-3]. The cardiac manifestations of NL (cardiac-NL) are well characterized including conduction abnormalities (first-, second-, or third-degree heart block) [1,4] and life-threatening cardiomyopathy which can be absent any conduction disease [5,6]. Cardiac-NL is associated with a significant mortality (20-30%, primarily fetal/neonatal) and morbidity (67% require permanent pacing before adulthood) [7,8].
The cutaneous manifestations of NL are also well characterized and in contrast to cardiac-NL, are generally transient [9]. The classical description comprises annular or elliptical lesions on the face, scalp, trunk, or extremities. The rash appears most often by 6 weeks postpartum and disappears generally without any sequelae by 6 months coincident with the clearance of maternal antibodies from the child’s circulation [9]. Biopsy reveals basal cell damage with a dermal mononuclear infiltrate [9].
The impact of cutaneous-NL on the risk of cardiac-NL in a future pregnancy is critical for family counseling and powering prospective trials. Two prospective studies of pregnancies involving mothers with maternal anti-SSA/Ro and or anti-SSB/La autoantibodies provide the rates of cutaneous-NL between 7-16%, both higher than the 2% associated with cardiac-NL [1,2]. Recurrence rates for cardiac-NL following a cardiac-NL child are tenfold higher [7,10-12]. One small study showed the rate of cardiac-NL following a cutaneous-NL child to be 30% but this rate may be overinflated due to inclusion of data obtained retrospectively and potential referral bias of cases with multiple affected children to the Research Registry for Neonatal Lupus (RRNL) [9]. While the cutaneous lesions of NL are themselves benign, cutaneous-NL may be an important risk factor for more serious disease in subsequent offspring, and predicting future maternal health. Accordingly, the present study was initiated to address the recurrence rates of NL, with a specific focus on cardiac-NL following a cutaneous-NL child in the RRNL.
METHODS
Subjects
Mothers enrolled in the RRNL fulfill two requirements 1) presence of anti-SSA/Ro antibodies and/or anti-SSB/La antibodies and 2) a child with any manifestation of NL [7], verified by review of medical records. The RRNL and its informed consent are approved by the NYU School of Medicine IRB. The enrollment period for this study extended from September 1994 to July 2009. However, a mother could enter the RRNL in 1994 having had a child with NL many years prior. Inclusion criteria for the present study were 1) a child with cutaneous-NL described as annular or elliptical lesions on the face, scalp, trunk, or extremities and verified by medical records and/or photographs (JPB or LAL, dermatologic consultant for the RRNL), and 2) birth of a child following the birth of the cutaneous-NL child. The exclusion criteria were 1) birth of a cutaneous-NL child subsequent to a cardiac-NL child since the presence of cardiac-NL may have in itself increased the risk of subsequent cardiac-NL or 2) a cutaneous neonatal lupus child born to a mother with anti-RNP antibodies, in the absence of anti-SSA/Ro antibodies, as to date this antibody has not been associated with cardiac neonatal lupus.
Subsequent Pregnancies
An overview of the birth order data on families enrolled in the RRNL in which at least one child has cutaneous-NL and maternal anti-SSA/Ro and/or anti-SSB/La antibodies is presented in Figure 1. In families with more than one child following the birth of a baby with cutaneous-NL, the affected child could be any of the subsequent births. For the purposes of calculating the overall recurrence rate and potential risk factors for recurrence, all pregnancies from 58 families in which there were subsequent pregnancies following an initial child with cutaneous-NL were included (N=77). Twenty-seven families entered the RRNL with at least one subsequent child already being born, nine of those pregnancies resulted in a cardiac-NL child (thus supporting a potential bias to entering multiple affected families). Thirty-one families enrolled in the RRNL at the time of birth of the initial child with cutaneous-NL and all subsequent pregnancies were evaluated prospectively.
Figure 1. Birth order of cutaneous-NL families enrolled in the RRNL.
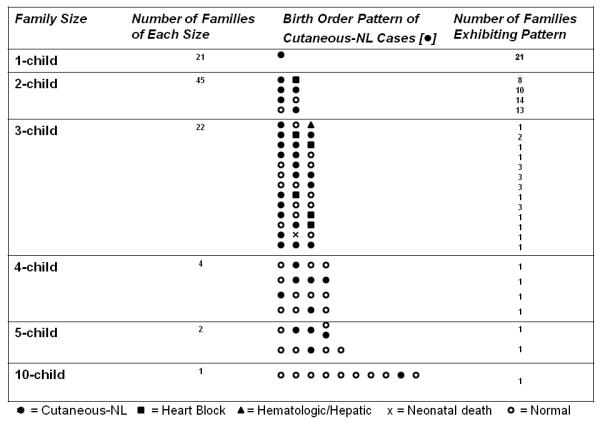
The figure summarizes the 95 cutaneous-NL families in the RRNL with regard to family size and birth order. There were 58 families with births subsequent to a child with cutaneous-NL. Children with cutaneous-NL are represented by the closed black circles, cardiac-NL by the closed black squares, hematologic/hepatic-NL by the closed triangles, neonatal death by an x and normal children by open circles.
Subsequent children’s outcomes were classified as a) cutaneous-NL, b) cardiac-NL defined herein as the presence of heart block (second-, or third-degree) documented by electrocardiogram, echocardiogram, history of pacemaker, or statement in the medical record; and/or presence of cardiac injury which specifically included autopsy evidence of a mononuclear infiltrate in the endocardium, myocardium and pericardium and/or endocardial fibroelastosis (EFE) on echocardiogram always associated with cardiac dysfunction, c) other manifestations of NL including abnormalities in hepatic and/or hematological laboratory values attributed to anti-SSA/SSB antibodies [13], or d) healthy with no manifestations of NL.
Additional information obtained from medical records and phone interviews and analyzed as risk factors for recurrence of cutaneous-NL or emergence of cardiac-NL included: maternal age, race/ethnicity, associated anti-SSB/La antibodies, and health status at the time of subsequent pregnancy, uses of systemic steroids (fluorinated and non-fluorinated) during pregnancy, breast feeding and fetal gender.
Statistical Analysis
The overall recurrence rate of NL and its manifestations were computed as the proportion of all NL among all pregnancies following an initial child with cutaneous-NL. Because data from multiple pregnancies from the same subject were included in the estimate, the 95% confidence interval for the recurrence rate was computed using a standard error based on the approach of [14] for clustered binary data. The effects of maternal age, race, health status, steroid use, presence of anti-SSB/La antibodies in addition to anti-SSA/Ro antibodies, breast feeding and gender of the child on risk of each manifestation of recurrent NL were evaluated with generalized estimating equations models. Two-sided p values less than 0.05 were considered statistically significant.
RESULTS
Outcome of overall pregnancies subsequent to a child with cutaneous-NL
Fifty-eight of the 95 families currently enrolled in the RRNL (verified to comprise a mother with anti-SSA/Ro antibodies and a child with cutaneous-NL not following a cardiac-NL child, no mother included had isolated anti-SSB/La antibodies) had a pregnancy subsequent to the cutaneous-NL child, Figure 1. Seventy-eight percent of these mothers were Caucasian, 3% were African-American, 10% were Hispanic, 7% were Asian, and one mother (2%) was mixed Hispanic/Asian.
The overall recurrence rate of any manifestation of NL, including all pregnancies following a cutaneous-NL child, was 49% (95%CI:37%-62%), Table 1A. Specifically, 14 (18%) were complicated by cardiac-NL. All cardiac-NL cases had 2nd/3rd degree congenital heart block (CHB); nine of which were associated with cutaneous-NL and two with hematological/hepatic abnormalities. Twenty-three (30%) of the subsequent children had cutaneous-NL (three accompanied by hematological/hepatic abnormalities). One child (1%) had isolated hematological/hepatic abnormalities and one child (1%) had a neonatal death from unknown reasons. Thirty-eight (49%) were unaffected.
Table 1A.
Outcome of 77 Children Subsequent to the Birth of a Child with Cutaneous-NL
| OUTCOME | N | % |
|---|---|---|
| Healthy | 38 | (49.4%) |
| Manifestations of NL | ||
| CHB (2nd or 3rd degree) | 14 | (18.2%) |
| CHB only | 3 | |
| CHB + Cutaneous-NL | 9 | |
| CHB + Hepatic/Hematologic | 2 | |
| Cutaneous-NL | 23 | (29.9%) |
| Cutaneous only | 20 | |
| Cutaneous + Hepatic/Hematologic | 3 | |
| Hepatic/Hematologic | 1 | (1.3%) |
| Neonatal Death | 1 | (1.3%) |
Outcome of prospective pregnancies subsequent to enrollment of a child with cutaneous-NL
In an attempt to limit potential referral bias of families with multiple affected children, a subset analysis was restricted to the 39 children born prospectively after the initial child was enrolled in the RRNL. In this analysis, the overall recurrence rate for NL was 36% (95%CI:20%-52%). Five of the children (13%) developed CHB (all 2nd/3rd degree, three accompanied by a rash and one associated with hematological/hepatic abnormalities). Nine children (23%) developed cutaneous-NL (three accompanied by hematological/hepatic abnormalities). The remaining 25 children (64%) were unaffected, Table 1B.
Table 1B.
Outcome of 39 Children Subsequent to the Birth of a Child with Cutaneous-NL Followed Prospectively
| OUTCOME | N | % |
|---|---|---|
| Healthy | 25 | (64.1%) |
| Manifestations of NL | ||
| CHB (2nd or 3rd degree) | 5 | (12.8%) |
| CHB only | 1 | |
| CHB + Cutaneous-NL | 3 | |
| CHB + Hepatic/Hematologic | 1 | |
| Cutaneous-NL | 9 | (23.1%) |
| Cutaneous only | 6 | |
| Cutaneous + Hepatic/Hematologic | 3 |
Maternal and fetal risk factors and association with recurrence of NL in children subsequent to cutaneous-NL
Maternal risk factors for emergence of cardiac manifestations were analyzed in the 14 subsequent CHB children and compared to the 62 children without cardiac-NL. There was no difference in maternal age (31.0 vs 32.5; p=0.14), maternal race/ethnicity (86% vs 71% Caucasian; p=0.25), maternal anti-SSB/La antibody status (92% vs 73%; p=0.14), and maternal diagnosis of SLE at the time of birth of the subsequent child (36% vs 40%; p=0.79) between the children who had CHB compared with those who did not. The use of maternal steroids was also evaluated and there was no difference in the use of non-fluorinated steroids (29% vs 19%; p=0.41) between the subsequent children who had CHB compared with those who did not. Maternal treatment with fluorinated steroids was significantly associated with CHB compared to children who did not have CHB (57% vs 3 % respectively, p=0.0003). However, this association reflected the prospect that fluorinated steroids might reverse conduction dysfunction or myocarditis [1], since treatment was given after diagnosis, not prior. The gender of the subsequent child did not influence the development of CHB (p=0.40).
Maternal risk factors for the recurrence of cutaneous-NL manifestation were analyzed in the 23 subsequent cutaneous-NL children and compared to the 38 unaffected children. There was no difference in maternal age (32.4 vs 31.9; p=0.65), maternal race/ethnicity (70% vs 76% Caucasian; p=0.59), maternal anti-SSB/La antibody status (77% vs 76% p=0.88), and maternal diagnosis of SLE at the time of birth of the child (39% vs 39%; p=0.99) between the children who had cutaneous-NL compared with those who did not. The use of maternal steroids was also evaluated and there was no difference in the use of non-fluorinated steroids (10% vs 25%; p=0.16) between the subsequent children with cutaneous-NL and those who had cardiac-NL or were unaffected. The gender of the subsequent child did not influence the development of cutaneous-NL (p=0.34). In addition, breast feeding was not associated with the development of cutaneous-NL development after birth (p=0.91).
DISCUSSION
The challenge to studying NL is its rarity. The establishment of the RRNL has facilitated study of this disease by virtue of its large size and well characterized phenotyping and serial follow-up of enrolled families. Accordingly, the recurrence rate of NL subsequent to a cutaneous-NL child, in particular the cardiac manifestations of NL can be approximated. Based on data from the RRNL, the overall recurrence rate of NL was 49% with the rate of cardiac-NL being 18%, a nearly tenfold risk over the 2% rate reported without a previous affected child. Limiting this analysis to the 39 children born prospectively after enrollment of a cutaneous-NL child, demonstrated an overall recurrence rate for NL at 36% with 13% developing cardiac-NL (all CHB), a six-fold higher risk for CHB. There were no identifiable maternal or fetal risk factors associated with this increased recurrence risk.
Three prospective studies of women with anti-SSA/Ro antibodies have provided the risk of cardiac-NL at or near 2% if the mother has had no previously affected pregnancies [1-3]. Recurrence rates of cardiac-NL in a subsequent pregnancy are approximately tenfold this risk [7,10-12] and the estimated recurrence rate for a pregnancy subsequent to two consecutive cardiac NL-children is 50% [10]. Consistent with the study reported herein, to date there has been no identifiable maternal or fetal risk factor which associates with recurrence of cardiac-NL [10]. Since the recurrence rate in all these studies exceeds the overall rate of cardiac-NL in an anti-SSA/Ro exposed child, the contribution of a fetal genetic factor is likely.
The high morbidity, mortality, and irreversibility of cardiac-NL have promoted studies exploring preventative medication [15]. Given the low event rate of cardiac-NL in anti-SSA/Ro positive mothers overall, studies aimed at prevention of recurrence have taken precedence. This study now provides a robust rate to predict the expected frequency of cardiac-NL following a child with cutaneous-NL. The data are critical for powering future trials to evaluate preventative therapies in families with a prior affected child.
Several limitations of this study should be noted. As with our previously published report on the recurrence of cardiac-NL [10], the predominance of Caucasians restricts an extrapolation to other ethnicities. All the mothers included in this study are enrolled in the RRNL which specifically seeks families with NL. This may result in a bias attracting families with more than one affected child. However, if only prospectively evaluated pregnancies are included, the recurrence rate of NL remains substantial at 36% with 13% developing CHB. In addition, the RRNL contains a large number of asymptomatic women identified only as carrying anti-SSA/SSB antibodies when they have an affected child. Five cutaneous-NL cases in the RRNL were diagnosed retrospectively after a subsequent child was diagnosed with NL (four cutaneous, one cardiac) by evaluation of photographs of the first cutaneous-NL child which revealed the classic annular rash that had not been reported to a physician or had been misdiagnosed as seborrhea, dermatitis, or tinea, resulting in delay of autoantibody detection simply because the mother was asymptomatic and thus NL was not considered. It remains unclear how many more children have cutaneous-NL which remains undiagnosed since their mothers are unaware they carry these autoantibodies and how many of these mothers have subsequent unaffected pregnancies. Thus, the true denominator representing cutaneous-NL with subsequent pregnancies may be greater than what has been reported leading to an overestimation of the risk of recurrence.
In summary, based on data from this large cohort, the identification of cutaneous disease in an anti-SSA/Ro exposed infant is particularly important since it predicts increased risk for a subsequent child with cardiac-NL. Overall it is expected that these data will serve as an important reference point for family counseling. Given increased risk of cardiac-NL, these mothers should be followed closely during pregnancy.
Acknowledgments
We appreciate the help of Amy Lawless in the preparation of this manuscript. We would also like to acknowledge the families in the Research Registry for Neonatal Lupus for their generous contributions.
This work was funded by NIH, Contract NO1-AR-4-2220 (Research Registry for Neonatal Lupus) and NIAMS, grant RO1 AR42455-01 (Maternal Autoantibodies: Pathogenesis of Neonatal Lupus) to Dr. Buyon, and S.L.E. Foundation NY Inc. grants to Dr. Izmirly and Dr. Llanos.
References
- 1.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, et al. Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. The PR interval and Dexamethasone evaluation (PRIDE) Prospective Study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 2.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142:678–83. doi: 10.1067/mpd.2003.233. [DOI] [PubMed] [Google Scholar]
- 3.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of Congenital Complete Heart Block in Newborns of Mothers with Anti-Ro/SSA Antibodies Detected by Counterimmunoelectrophoresis: A prospective Study of 100 Women. Arthritis Rheum. 2001;44:1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Rein AJ, Mevorach D, Perles Z, Gavri S, Nadjari M, Nir A, Elchalal U. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circul. 2009;119(14):1867–72. doi: 10.1161/CIRCULATIONAHA.108.773143. [DOI] [PubMed] [Google Scholar]
- 5.Nield LE, Silverman ED, Smallhorn JF, Taylor GP, Mullen JB, Benson LN, et al. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. Circulation. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 6.Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–242. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 7.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: Mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 8.Waltuck J, Buyon J. Autoantibody-associated congenital heart block: Outcome in mothers and children. Annals Int Med. 1994;120:544–51. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Neinman AR, Lee LA, Weston WL, Buyon JB. Cutaneous Manifestations of Neonatal Lupus without heart block: Characteristics of Mothers and Children Enrolled in a National Registy. J Pediatr. 2000;142:674–80. doi: 10.1067/mpd.2000.109108. [DOI] [PubMed] [Google Scholar]
- 10.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence Rates of Cardiac Manifestations Associated with Neonatal Lupus and Maternal/Fetal Risk Factors. Arthritis Rheum. 2009 doi: 10.1002/art.24768. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julkunen H, Eronen M. The Rate of Recurrence of Isolated Congenital Heart Block: A Population Based Study. Arthritis Rheum. 2001;44:487–8. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Gladman G, Silverman ED, Yuk-Law, Luy L, Boutin C, Laskin C, et al. Fetal echocardiographic screening of pregnancies of mothers with anti-Ro and/or anti-La antibodies. Am J Perinatol. 2002;19:73–80. doi: 10.1055/s-2002-23555. [DOI] [PubMed] [Google Scholar]
- 13.Lee LA. Transient autoimmunity related to maternal autoantibodies: neonatal lupus. Autoimmun Rev. 2005;4(4):207–13. doi: 10.1016/j.autrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Donald A, Donner A. Adjustments to the Mantel-Haenszel chi-square statistic and odds ratio variance estimator when the data are clustered. Stat Med. 1987;6:491–9. doi: 10.1002/sim.4780060408. [DOI] [PubMed] [Google Scholar]
- 15.Friedman DM, Llanos C, Izmirly PM, et al. Evaluation of Fetuses in the Preventive IVIG Therapy for Congenital Heart Block (PITCH) study. Arthritis Rheum. 2009 doi: 10.1002/art.27308. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


