Abstract
Habituation is a simple form of memory, yet its neurobiological mechanisms are only beginning to be understood in mammals. In the olfactory system, the neural correlates of habituation at a fast experimental timescale involving very short intertrial intervals (tens of seconds) have been shown to depend on synaptic adaptation in olfactory cortex. In contrast, behavioral habituation to odorants on a longer timescale with intertrial intervals of several minutes depends on processes in the olfactory bulb, as demonstrated by pharmacological studies. We here show that behavioral habituation to odorants on this longer timescale has a neuronal activity correlate in the olfactory bulb. Spiking responses of mitral cells in the rat olfactory bulb adapt to, and recover from, repeated odorant stimulation with five-minute intertrial intervals with a timecourse similar to that of behavioral habituation. Moreover, both the behavioral and neuronal effects of odor habituation require functioning NMDA receptors in the olfactory bulb.
Keywords: Olfaction, habituation, adaptation, bulb, NMDA, rodent
Introduction
Animals are submitted to a flow of sensory information which the nervous system filters to identify information that may be of particular importance. Habituation is a simple form of nonassociative learning in which behavioral responses to repeated, non-reinforced sensory stimuli are progressively reduced, enabling an animal to perceptually deemphasize persistent or static stimuli in favor of novel or changing stimuli. Habituation has been described experimentally in multiple sensory modalities within various invertebrate and vertebrate species including sea slugs, fruit flies, nematodes, birds, and mammals (Christoffersen 1997; Rankin and Broster 1992). In the rodent olfactory system, both autonomic and behavioral habituating responses can be evoked by repeated odor stimulation (Wilson and Linster 2008).
Behaviorally, olfactory habituation can be induced by multiple paradigms that differ in timescale and are thought to be mediated by distinct mechanisms within different regions of the olfactory system (McNamara et al. 2008; Wilson and Linster 2008). For example, a form of short-timescale habituation, induced by repeated 20-second stimulations with 10-second intertrial intervals, persists for less than ten minutes and is mediated within piriform cortex, whereas a form of habituation induced by repeated 50-second stimulations with 5-minute intertrial intervals persists for at least 30 minutes and is mediated within the olfactory bulb. The neural correlates of this short-timescale behavioral habituation have been demonstrated in piriform cortical pyramidal cells in which adaptation is associated with mGluR II/III-mediated depression of the glutamatergic mitral-pyramidal cell synapse (Wilson 1998a; 2003; 1998b). In contrast, longer-timescale behavioral habituation requires functioning NMDA receptors within the olfactory bulb and is not affected by blockade of mGluR II/III receptors therein (McNamara et al. 2008).
Neuronal adaptation to persistent stimulation occurs at many levels in the olfactory system, including olfactory sensory neurons (Kurahashi and Menini 1997; Zufall and Leinders-Zufall 2000) as well as neurons within the olfactory bulb and piriform cortex. As in other sensory systems, central olfactory neurons show greater adaptation than do primary sensory neurons, and both olfactory bulb mitral cells and piriform cortical pyramidal cells have been shown to adapt to odorant stimulation under certain conditions (Chaput and Panhuber 1982; Gray and Skinner 1988; Shea et al. 2008; Wilson 2000).
In the present study, we investigated whether the adaptation of neuronal responses in olfactory bulb mitral cells could underlie a form of behavioral adaptation routinely used in olfactory behavioral studies (Bath et al. 2008; Cleland et al. 2002; Linster et al. 2001; Mandairon et al. 2006b; Wesson et al. 2008). We first show that odor responses in olfactory bulb mitral cells adapt to odorants in response to a similar stimulation paradigm than that used in behavioral habituation and that this neural adaptation depends on stimulus duration and intertrial interval paramters. We then show that both neuronal adaptation depends on functioning NMDA receptors in the olfactory bulb and follow up on this result by showing that behavioral habituation using the same experimental parameters also depends on functioning NMDA receptors in the olfactory bulb. We conclude that habituation to repeated odor stimulation in this paradigm is likely to be mediated by reductions in the odor responses of olfactory bulb mitral cells and that bulbar NMDA receptors are involved in this process.
Methods
Electrophysiology
Animals
Adult male Sprague-Dawley rats (200-250 g) were purchased from Charles River Laboratories (Wilmington, MA). Rats were singly-housed with water and food available ad libitum and maintained on a 12:12 hour light/dark cycle. All procedures were performed according to NIH guidelines under the supervision of the Institutional Animal Care and Use Committee of Cornell University.
Experimental preparation
Animals were anesthetized with urethane (1.5 g/kg intraperitoneal; Sigma-Aldrich, St. Louis, MO) and placed in a stereotaxic apparatus (Narishige Scientific Instruments, Tokyo, Japan). The skull was exposed by scalpel incision and a hole was drilled over each of the lateral olfactory tracts (AP +3.7 mm, ML ±3.4 mm) and over the olfactory bulbs (Paxinos and Watson, 1998). Respiratory activity was monitored throughout the experiments using a piezoelectric monitor strapped around the animal’s chest and sent to the computer, hence enabling synchronization of odor delivery with inhalation.
Drugs
The NMDA antagoni s t M K-801 ((+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine hydrogen maleate; 8 mM concentration) was dissolved in sterile 0.9% physiological saline and directly 6 uL were infused into the MOB at an infusion rate of 2 ul/min using a 50 ul Hamilton syringe attached to a Stoelting stereotaxic syringe pump. The dosage and volume were based on previously published studies (Mandairon et al. 2006b).
Electrophysiological recordings
Bipolar stimulating electrodes (100 um stainless steel, Formvar-insulated) were stereotaxically placed in the lateral olfactory tract (LOT; AP +3.7 mm, ML ±3.4 mm, DV 6.5 mm) in order to evoke antidromic action potentials in mitral cells. Stimulation currents (100 us duration, 200-900 uA) were delivered by a constant-current stimulus isolation unit (Grass model PSIU6) controlled by a Grass S88 stimulator (Grass Technologies, West Warwick, RI). Neuronal responses were recorded using tungsten stereotrodes (3-5 Mohm; World Precision Instruments, Sarasota, FL). Electrodes were lowered into the mitral cell layer using a stereotaxic micromanipulator (David Kopf Instruments, Tujunga, CA). Optimal placement of the recording electrode into the mitral cell layer of the MOB was achieved by monitoring the size and shape of field potentials (1000x amplification, 0.1Hz - 475Hz bandpass, 20 kHz sampling rate) following LOT stimulation. Single units (5000x amplification, 600 Hz – 6 kHz bandpass, 20 kHz sampling rate) were recorded in the ventrolateral and dorsomedial regions of the OB. Data were digitized and recorded to computer using a CED Power1401 digitizer and Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Odor Stimulation
After establishing stable single-unit recordings, but prior to beginning each experiment, each cell’s responsiveness to a variety of odors was measured. Odorants screened were the esters ethyl butyrate and ethyl pentanoate, the alcohols heptanol and hexanol, the aldehydes heptanal and hexanal, and the organic acids butyric acid and propanoic acid. The odorant that evoked the most robust excitatory response (greatest increase in overall spike rate) in the majority of presumed mitral cells within each test was subsequently used in that experiment. For odor delivery, odorant chambers in a custom-built olfactometer were loaded with stock solutions of odorants diluted in mineral oil so as to emit a consistent theoretical vapor-phase partial pressure of 100 Pa. Odors were then delivered by directing a 50 ml/min stream of humidified air through a selected odor chamber and subsequently into a carrier stream of charcoal-filtered, humidified air (1 L/min) resulting in an approximate 20x dilution to yield a final odorant vapor-phase partial pressure of ~5 Pa.
Odor response testing and adaptation proceeded as follows. First, the cell’s odor responses were probed with a response testing protocol: five two-second odor pulses separated by two-minute intertrial intervals (Pre; Figure 1A; Chaudhury et al., 2009; Wilson 2000). An adaptation protocol matching that of the corresponding behavioral experiments was then immediately administered: four 50-second odor pulses separated by five-minute intertrial intervals. Finally, the response testing protocol was repeated at both 5-minute and 60-minute timepoints following the end of the adaptation protocol (Post5 and Post60; Figure 1A). In experiments designed to further characterize the stimulation parameters necessary to induce adaptation, either the stimulus duration (d=2, 10, 20 or 30 seconds) or the interval between stimulations (ITI=1 or 2.5 minutes) was varied.
Figure 1.
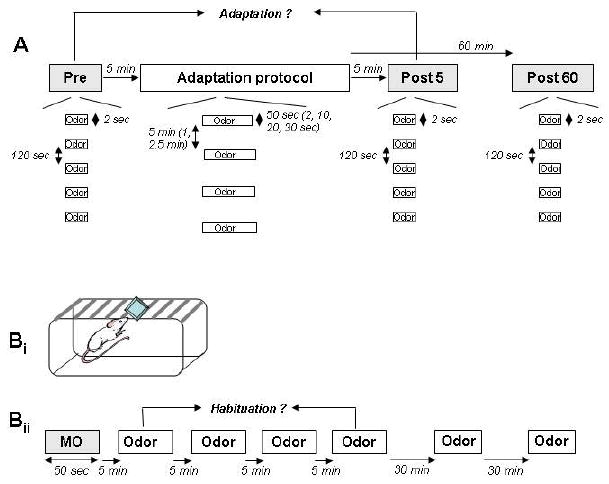
Schematic depiction of electrophysiological (A) and behavioral (B) experimental protocols. A. Electrophysiological protocol. During electrophysiological recordings, odor responses were first measured (Pre) by presenting a 2-second odor stimulus five times with 120 sec interstimulus intervals (response testing protocol). The adaptation protocol, consisting of four fifty-second presentations of the same odorant separated by five-minute intertrial intervals, was then administered. In separate experiments, stimulus duration and ITIs were varied independently of each other. Five and sixty minutes after the end of the last adaptation trial (Post), the response testing protocol was again delivered. Bi. Behavioral protocol. Rats were presented with a weighing dish containing either mineral oil (MO) or the habituation/test odorant. Bii. After a single presentation of mineral oil alone, the habituation/test odorant was presented for four consecutive trials, separated by five minute intertrial intervals, and then twice more at 30 and 60 minutes’ latency following the last habituation trial.
Spike Sorting
Single units were extracted offline using Spike2 software. Briefly, spike templates were derived and selected from the raw data and then used to extract units from the entire data set. Extracted spikes were further validated and separated using principal components analysis (PCA) whereby features from the data are extracted and clustered to groups of similar waveforms. Following spike sorting, the number of spikes for each single unit identified was automatically counted from 4 sec prior to 4 sec after each odor onset.
Histological verification of electrode placements
At the end of each experiment, positive current was passed through the recording electrodes (10 seconds duration, 1-15 mA) to produce a small lesion in the olfactory bulbs. Transcardial perfusion was then performed with saline and 10% neutral buffered formalin. Brains were removed, sectioned at 40 um, and subsequently stained with cresyl violet for electrode localization.
Data Analysis
We first determined whether a given cell exhibited a significant increase in firing rate in response to odor exposure by comparing the number of spikes evoked in the 4 second window immediately prior to odor delivery to the 4 second window beginning at odor onset (paired t-test, α=0.05; (Chaudhury et al. 2009; Wilson 2000). If so, the given cell/odor combination was included in the data analysis. The pre- and post-adaptation response magnitudes of each cell to the test odorant used were calculated as the difference between the number of action potentials evoked four seconds before and after each odor stimulation onset. Pre- and post-adaptation response magnitudes were then normalized with respect to the average pre-adaptation magnitude, hence, all graphs depict response magnitudes as percentages with respect to the mean pre-adaptation response. To determine if the adaptation protocol had a significant effect on mitral cell responses to odorants, an analysis of variance was performed on these normalized response magnitudes with test latency (i.e., pre-habituation, 5 min post-habituation, 60 min post-habituation) as main effect.
Behavioral experiments
Subjects
Eleven adult male Sprague-Dawley rats (250-300 grams), purchased from Charles River Laboratories (Wilmington, MA), were utilized for behavioral experiments. Animals were singly-housed and maintained at a constant temperature on a 12:12 light/dark cycle with water and food provided ad libitum. Prior to behavioral experiments, animals were provided with seven days’ acclimation time, during which investigators handled them for one hour daily. All procedures were performed according to NIH guidelines under the supervision of the Institutional Animal Care and Use Committee of Cornell University.
Cannulation
Rats were anesthetized with an intramuscular injection of ketamine (50 mg/kg) and xylazine (7.5 mg/kg) and secured in a stereotaxic apparatus (Narishige Scientific Instruments, Tokyo, Japan). Guide cannulae (22-gauge; Plastics One, Roanoke, VA, USA) were inserted into both OBs according to standard procedures (Mandairon et al. 2006a). Cannulae were implanted at the following coordinates with respect to bregma: AP +8.0 mm, ML ±1.5 mm, DV 4.5 mm. The tips of the guide cannulae were positioned 1.0 mm dorsal to the target infusion site; consequently, infusion cannulae extended 1.0 mm from the end of the guide cannulae. Five screws were drilled into the skull, and dental cement was used to secure the guide cannulae to these screws and to cover the incision area. Dummy infusion cannulae were then placed into the guide cannulae to prevent blockage or infection. Following surgical implantation, rats were allowed to recover for 10 days.
Drug Administration
For drug/vehicle administration immediately prior to behavioral experiments, two infusion cannulae were fitted into the guide cannulae so that their tips protruded 1.0 mm beyond the ends of the guide cannulae into the center of each MOB. Two 10 μl Hamilton syringes containing either drug solution or vehicle were attached to the cannulae with a polyethylene tube and driven with paired infusion pumps (YA-12 Genie pumps, Kent Scientific). Drug dosage and infusion volume were matched with those of the electrophysiological experiments in this study and have been shown to be effective in previous studies (Mandairon et al. 2006b). Specifically, the NMDA antagonist MK-801 (8 mM and 4 mM, Sigma-Aldrich, Natick, MA) was dissolved at 37C in 0.9% saline; the drug (or saline vehicle) was then delivered bilaterally into awake rats at a rate of 2 ul/min for 3 minutes (6 μl total volume delivered per side). The infusion cannulae remained in place for 1 additional minute after the infusion ended in order to minimize backflow. Behavioral testing was performed 20 minutes after drug administration was completed. Infusion of 8mM MK-801 led to a general depression of the behavioral response which made results difficult to interpret; we therefore followed up with a lower dosage (4mM) and show results obtained with the lower dosage in the figures.
Odors
Two odorants, ethyl butyrate and ethyl pentanoate (Sigma-Aldrich, Natick, MA), were employed for habituation testing. Only one odorant was used during a single day of training/testing; the experiment then was repeated in each rat on a different day using the second odorant to enable counterbalancing of drug/vehicle administration groups. Prior to testing, odorants were diluted in mineral oil so as to each theoretically emit a vapor-phase partial pressure of 5 Pa (corresponding to vol/vol dilution of 0.09 for ethyl butyrate and 0.3% for ethyl pentanoate). Vapor pressures of pure odorants were estimated with the Hass-Newton equation as implemented in ACD/Boiling Point & Vapor Pressure Calculator (version 4.5; Advanced Chemistry Development, Toronto, Ontario, Canada).
Behavioral Experiments
An olfactory habituation task measures non-associative memory formation; cross-habituation testing with multiple odors can then be used to measure the specificity of this memory over time (Cleland et al. 2002). The behavioral experiments performed here replicate those previously performed in mice in our lab (McNamara et al., 2008); however, the experiments presented here were conducted with the goal of directly comparing electrophysiological and behavioral data by matching species, drug dosages, odors and odor concentration. All habituation experiments took place in the home cage under red light. Odors were presented by placing 60 ul of the diluted odor stimulus onto filter paper (Whatman #1) contained within a weighing dish that was placed on top of the cage lid (Figure 1Bi); this procedure enabled the observer to change the odor stimulus without disturbing the animal. Each test session was preceded by one 50-second presentation of plain mineral oil (MO). Test sessions comprised four 50-second presentations of diluted odorant separated by five-minute intertrial intervals, followed by one additional presentation of the same odorant at 30- and 60-minute time points after the last habituation trial (Figure 1Aii). Comparison of investigation times during the first and fourth odor habituation trials measures initial habituation (5-minute delay), whereas comparison between the first odor presentation and either of the two delayed (30- and 60-minute) trials measures the persistence of habituation memory. The 5-minute and 60-minute delayed trials (i.e., the fourth and sixth odor presentations) are emphasized in analyses for direct comparison with electrophysiological data. Active sniffing within one centimeter of the odor source was recorded with a stopwatch. The observer was blind as to the treatment group and in experiments involving more than one odor, odors were coded and randomized by a member of the lab. Figures show mean investigation times +/ standard error. All rats underwent both drug treatments using a separate odor for each and the order of drugs was counter balanced among the rats.
In a control experiment, rats were presented with the mineral oil carrier alone for four 50-second trials after which the odorant was presented in a single test trial, hence measuring rats’ ability to detect the odorants used. This control ensured that rats treated with the NMDA antagonist were not simply unable to detect the odors presented. As in the first experiment, all rats were tested with both odorants and drugs and the order of drug was counterbalanced.
Data analysis
An analysis of variance (ANOVA) was performed using SPSS statistical software (SPSS, Chicago, IL) with odor investigation time as the dependent variable, and drug group and odor presentation trial number as main effects. Fisher post hoc pairwise testing was then used to assess whether the time spent investigating during the fourth habituation trial or later test trial were significantly lower than the time measured during the first habituation trial (α = 0.05).
Results
Neural adaptation to repeated odor stimulation
We recorded from a total of 33 presumed mitral cells, of which 20 (60%) exhibited a significant excitatory response to at least one of the odors used in the study and hence were included in analyses. Cells’ spontaneous activities ranged from 3 – 60 Hz with an average value of 29.23 Hz (+/- 5.17 Hz). Cells’ firing rates in response to odorants (difference between pre and post odor firing rate) ranged from 4 to 19 Hz with an average rate of 6.3 Hz (+/- 1.7 Hz). Figure 2 shows raw traces of one mitral cell over the course of the experimental paradigm. Overall, cells exhibited robust adaptation after exposure to the adaptation protocol of repeated odor stimulation, as evidenced by a significant reduction in mitral cell responses to the test odor during post-adaptation tests (Figure 3). There was no correlation between the initial response rate and the degree of adaptation (Pearson’s R = 0.38; p > 0.2). Specifically, analysis of variance revealed a significant difference during pre- and post-adaptation tests (Ftest(2,54) = 19.371; p < 0.001); subsequent post hoc comparisons showed that mitral cell odor responses at 5 minutes following the adaptation protocol were significantly attenuated relative to pre-adaptation responses (p < 0.001 in both cases; Fisher LSD), but not at 60 minutes (p > 0.05, Fisher LSD). Control experiments (47 cells, of which 7 (15%) exhibited significant odor responses and hence were included in analyses), in which only mineral oil odor was presented during the adaptation protocol, showed no significant effect on mitral cell odor responses among the three test latencies (Ftest(2, 18) = 0.532; p > 0.5), indicating that mitral cell adaptation is not due simply to fatigue and does not arise from stimulation with the carrier alone (Figure 3A). Moreover, mitral cells’ spontaneous activity (measured in the 4 second window before each odor stimulation) was not affected by repeated odor stimulation (ANOVA comparison of pre-adaptation, 5-minute post-adaptation and 60-minute post-adaptation latencies; Ftest(2, 27) = 0.18, p > 0.05).
Figure 2. Individual mitral cell responses to one odor over the course of the recording protocol.
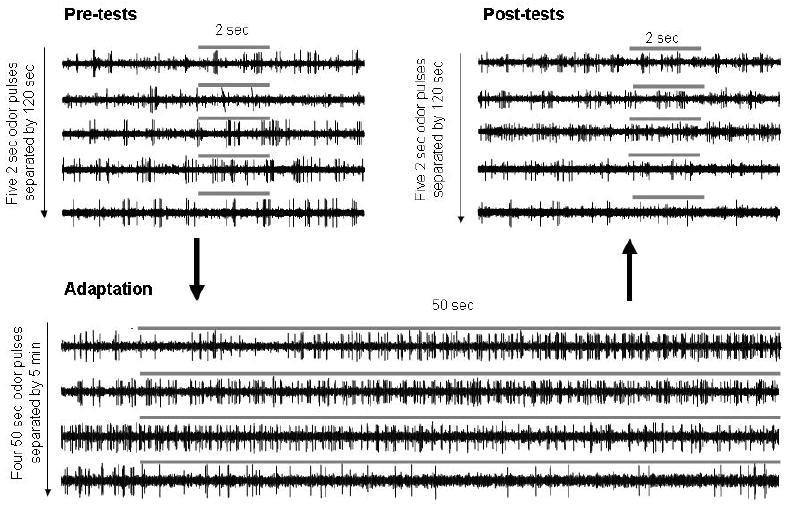
Raw recorded data from one mitral cell are shown during pre-tests (five 2-second odor presentations separated by 2-minute ITIs), followed by four 50-second adaptation stimuli with the same odorants separated by 5-minute ITIs (adaptation), followed 5 minutes later by a second series of five 2-second odor stimuli separated by 2-minute ITIs. Gray bars indicate odor stimulation times and durations.
Figure 3.
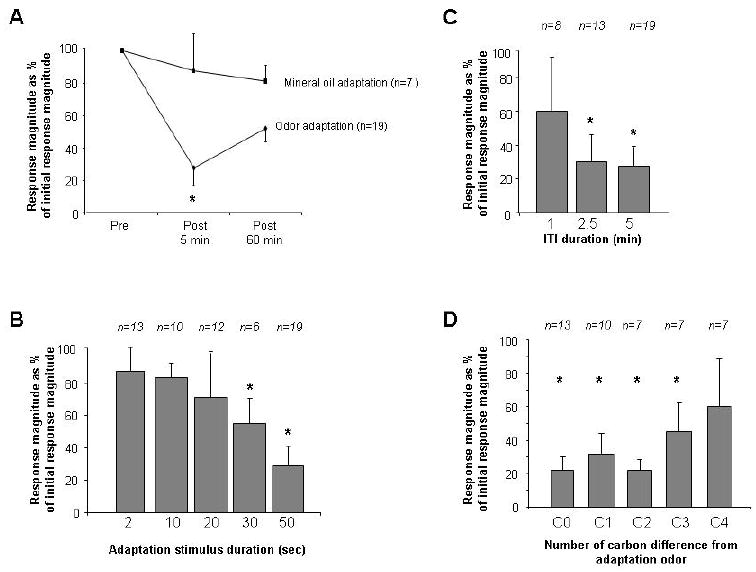
Neural adaptation to repeated odor presentations. A. Average number of spikes evoked by odor presentations during pre- and post- adaptation testing expressed as percentages of the response during pre- adaptation testing. Mitral cells stimulated with the adaptation odorant (Odor adaptation, solid line) over four trials during the adaptation protocol responded significantly less during both 5- but 60-minute post- test than during the pre- adaptation tests. Asterisks indicate a significant decrease as compared to pre-testing. In contrast, mitral cells stimulated with plain mineral oil (Mineral oil adaptation, dashed line) during the adaptation protocol did not change their responses significantly compared to pre- adaptation test responses. B. Post-test responses to odorants expressed as the percentage of the pre-test responses in cells stimulated with 5 repeated stimulations of 2, 10, 20, 30 and 50 second durations and 5 minute ITIs. C. Post-test responses to odorants expressed as the percentage of pre-test responses in cells in cells stimulated with 5 repeated stimulations of 50 seconds separated by 1, 2.5 and 5 minute ITIs. D. Post test responses to test odorants differing by 1, 2, 3 or 4 carbons from the odorant used as the adaptation odorant expressed as percentage of the pre-test response to this odorant. Asterisks indicate a significant reduction in response magnitude as compared to pre-adaption.
Additional experiments were conducted to further characterize the parameters of neural adaptation after repeated odor stimulation. First, experiments were performed to determine the duration of odor exposure required to induce response adaptation in mitral cells (Figure 3B). Rats were exposed to adaptation protocols of four odor stimulations with reduced durations (d = 2, 10, 20 or 30 seconds) separated by 5 minute ITIs. Recordings were made respectively in 24 (d = 2, 13 (54%) responsive), 19 (d = 10, 10 (58%) responsive), 27 (d = 20, 12 (44%) responsive) and 28 (d = 30, 6 (21%) responsive) cells. Repeated stimulation with either 2, 10 or 20 second odor durations did not evoke significant adaptation in mitral cells (d = 2: F(1, 36) = 0.892, p > 0.4; d = 10: F(1, 27) = 0.736, p > 0.4; d = 20: F(1, 33) = 1.504, p > 0.2); however, repeated stimulation with 30-second odor durations evoked significant adaptation (d = 30: F(1,15) = 4.331; p < 0.05). Second, we tested the length of the ITI required to induce significant adaptation (Figure 3C). Rats were exposed to four repeated odor stimulations of 50 seconds each, but with shorter ITI durations (ITI = 1.0 and 2.5 minutes; compare to the previous experiments using ITI = 5.0 minutes). Repeated stimulations with 50 second odor pulses and 1 minute ITIs did not induce significant adaptation of odor responses (F(1,21) = 2.4; p < 0.1), whereas repeated stimulations with 2.5 minute ITIs did induce significant adaptation (F(1,39) = 10.2; p < 0.005). The data from the above two experiments show that in order to induce the type of neural adaptation observed here, odor stimulations presented for longer than 20 seconds, with ITIs longer than 1 minute, are required. Third, we tested cross-adaptation between odorants (Figure 3D). Briefly, mitral cell responses to two odorants, differing by 1 (C1), 2 (C2) or 3 (C3) carbons in their carbon chain were tested (pre-test); the cell was then repeatedly stimulated with one of these two odorants using 50 second stimulus duration and 5 minute ITIs (adaptation). The cell’s responses to both test odorants after adaptation was then recorded five minutes after the adaptation protocol (post-test). Figure 3D shows that compared to the pre-test response, the responses to all tested odorants differing by 0 (CO = habituation odor), 1 (C1), 2 (c2) and 3 (C3) carbons from the habituated odor were significantly lower than during pre-tests (C0 or adaptation odor: F(1, 24) = 88.838, p < 0.001; C1: F(1, 17) = 127.849, p < 0.001; C2: F(1, 16) = 249.789, p < 0.001; C3: F(1, 15) = 15.081, p < 0.005). Responses to odors differing by 4 carbons from the habituated odor were not significantly decreased after the adaptation protocol (C4: F(1, 14) = 2.900, P > 0.1). This shows that as expected from previous behavioral and electrophysiological experiments, bulbar adaptation is relatively, but not completely, odor-nonspecific (Wilson, 2000; McNamara et al., 2008).
Neural adaptation is NMDA receptor-dependent
In electrophysiological experiments, pharmacological blockade of NMDA receptors in the olfactory bulb impaired the adaptation of mitral cell responses to odor stimulation. Recordings were made from 30 cells of which 13 (43%) exhibited significant excitatory responses to at least one odor. In contrast to the significant effect of adaptation on mitral cell odor responses in animals infused with saline (Figure 4A), mitral cells’ odor responses in the presence of the NMDA receptor antagonist MK-801 exhibited no significant effect of the adaptation protocol (Ftest(2,36) = 0.555; p > 0.5), demonstrating that mitral cell response adaptation to odors is NMDA receptor-dependent (Figure 4A). A second analysis was then performed on the same data under both drug conditions to compare the percentage of cells exhibiting significant odor responses 5 and 60 minutes after odor adaptation (among the cells tested, all of which had responded significantly to the test odor prior to adaptation). Analysis of variance demonstrated significant effects of both test number (pre-, 5-min post-, 60-min post-) and drug condition (saline or MK-801 infusion) as well as a significant interaction (Ftest(2,63) = 11.162, p < 0.01; Fdrug(1,63) = 7.513, p < 0.01; Fdrug*test(2,63) = 3.532, p < 0.05). Post hoc comparisons indicated that test latency was a significant main effect only in the vehicle-infused control group (Ftest(2, 27) = 10.8, p < 0.001), in which responses in all three test phases were significantly different from one another (p < 0.05 for all pairwise comparisons; Fisher LSD). In contrast, there was no significant effect of adaptation on mitral cell responses in the presence of MK-801 (Figure 4B), thereby confirming the role of NMDA receptors in mitral cell adaptation.
Figure 4.
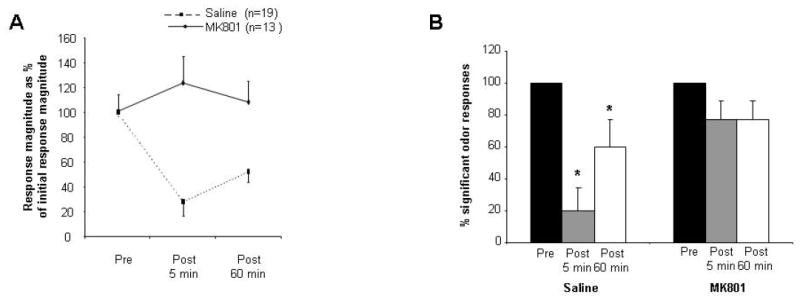
Effect of bulbar NMDA receptor blockade on neural adaptation. A. Average responses of mitral cells during pre-adaptation and post-adaptation odor tests in rats infused with MK-801, expressed as percentages of the average pre-adaptation test response. In these rats, responses to odorants post-habituation were not significantly different from those recorded pre-adaptation. B. Percentages of cells responding significantly to odor presentations in pre- adaptation and post- adaptation tests under control and MK-801 conditions. Asterisks indicate a significant decrease in response magnitude as compared to the first trial or pre- adaptation response (p < 0.05).
Comparison of mitral cell responses to odor before and after the administration of MK-801 (prior to any adaptation protocol) showed that the addition of MK-801 alone did not affect mitral cell responses to odors (p > 0.05; Fisher LSD). Furthermore, odor-independent spontaneous activity in mitral cells was not affected either by the application of MK-801 (in agreement with (Philpot et al. 1998)) or the administration of the odor adaptation protocol (comparison of pre-odor baseline activity levels from four experimental timepoints: before MK-801 administration, after MK-801 but prior to the adaptation protocol, and 5 and 60 minutes post-habituation; Ftest(3,12)=0.3; p = 0.8, data not shown).
Mitral cell odor responses decrease over the course of repetitive, but not single, 50 second odor stimulation
In the present experiments, mitral cell adaptation occurs when cells are stimulated multiple times with odor stimulations of at least 30 seconds, separated by more than 2.5 minute intertrial intervals. Previous experiments by Wilson (2000) showed that during a single 50-second odor stimulation mitral cells adapted to a much lesser degree than the adaptation shown here after four consecutive adaptation stimuli. To better compare these experiments, we analyzed the responses of mitral cells during the four 50-second adaptation trials in saline-infused and in MK-801-infused rats. No significant differences in spontaneous activity over the course of the four adaptation trials were observed in control (F(3, 54) = 0.094; p > 0.9) or MK-801 rats (F(3, 72) = 0.241; p > 0.5) (Figure 3Ai, Bi). When average numbers of evoked spikes during the four 50-second adaptation trials were compared, a significant effect of trial number was observed in saline control (F(3, 15) = 3.345; p < 0.05, Wilkens-Lambda, Figure 3Aii) but not MK-801 infused rats (F(3, 10) = 7.69; p > 0.05; Figure 3Bii). Further analysis showed that in saline infused control rats, average spike rates were significantly lower during the third adaptation trials as compared to the first (p < 0.05 with Fisher LSD). No significant changes in spike rate over the course of any adaptation trials were observed (p > 0.05 in all cases) when trials were analyzed in 10 second bins (Figure B), except for the third adaptation trial in saline infused rats (p < 0.05). These data suggest that the NMDA-dependent process leading to adaptation in this paradigm happens mostly in between odor stimulus presentations rather than during prolonged odor stimulation.
Behavioral habituation to repeated odor stimulation requires functioning NMDA receptors in the OB
Behavioral studies were performed to test the dependence of odor habituation on bulbar NMDA receptors in rats. One group of rats received infusions of the NMDA receptor antagonist MK-801 (4 mM) into the olfactory bulb while a control group was infused with saline vehicle (Figure ); rats were then presented with mineral oil, then four habituation trials separated by 5 minute ITIs, and finally two additional odor presentations at 30 and 60 minutes after the last habituation trial. Analysis of variance revealed significant main effects of both drug treatment (Fdrug(1, 114) = 11.462, p < 0.001) and trial number (Ftrial(5, 14) = 5.136, p < 0.001), as well as a significant interaction (Fdrug*trial(5, 114) = 2.394, p < 0.05). In vehicle-infused controls, there was a significant effect of trial (ANOVA; Ftrial(5, 60) = 7.555, p < 0.001) and a significant reduction of investigation time in the fourth trial as compared to the first (p < 0.001), as expected based on previous experiments. In contrast, no significant effect of trial was observed in MK-801-infused rats (Ftrial(5, 54) = 1.507; p > 0.2) and there was no significant difference between the response during the first and fourth trial (p > 0.7; Figure 6A).
Figure 6. Behavioral results.
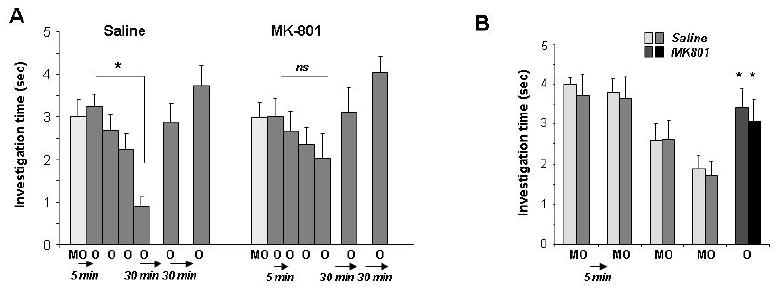
A. Average behavioral investigation times in saline and MK801 infused rats over the course of the behavioral experiment. Saline-injected control rats responded significantly less on the fourth habituation trial as compared to the first trial (* indicates a significant difference between responses during the first and fourth trial), whereas MK-801 infused rats did not significantly habituate, as indicated by a non-significant difference between investigation times during the fourth and first trials. B. Blockade of bulbar NMDA receptors does not impair odor detection. The graph shows the average investigation times of saline-infused and MK-801 infused rats presented with the mineral oil carrier during four trials followed by a single odor presentation. All rats responded significantly more to the odor than to the carrier alone, indicating that they detected the odorant.
To ensure that MK-801 treated animals were still able to detect odorants, we performed a behavioral control experiment in which vehicle-infused and MK-801-infused rats were presented with mineral oil over four successive trials, followed by a single presentation of a test odorant. We found no significant effect of drug group (Fdrug(1, 91) = 0.013, p > 0.9), indicating that both groups performed similarly in this control experiment. Specifically, in both groups rats investigated the odor significantly more than the mineral oil (p < 0.05 for both groups, Figure 6B).
Discussion
Repeated exposure to an odorant induces perceptual habituation evidenced by a reduction in active investigation behavior. The present results suggest that this behavioral phenomenon could be at least partially mediated by the adaptation of neural responses in mitral cells in the olfactory bulb. Single-unit recordings of presumptive mitral cells in freely breathing rats showed that repeated 50-second odor presentations delivered at five-minute intertrial intervals induced significant reductions in mitral cell responses to that odor when tested after completion of the adaptation protocol (Figures 2,3). Local blockade of NMDA receptors in the olfactory bulb abolished both the adaptation of mitral cell responses to odorants in these experiments (Figure 4), as well as the behavioral habituation to odorants (Figure 6) as previously shown in mice (McNamara et al., 2008), supporting the idea that the reduction of mitral cell responsiveness to odorants is the neural correlate of behavioral habituation to repeated odor stimuli at the timescales used here.
Previous behavioral studies have shown that habituation protocol variants can elicit odor habituation that differ in duration, odor specificity, neural location and pharmacology (McNamara et al. 2008) depending on the exact set of task parameter used. For example, a short-timescale habituation protocol utilizing brief, 4-second odor presentations separated by 10-second intertrial intervals resulted in relatively transient habituation (persisting for up to ten minutes) that was highly specific for the habituated odor and could be blocked by mGluRII/III antagonists administered in the anterior piriform cortex (Best et al. 2005; Yadon and Wilson 2005). A similar paradigm, using 20 second stimulations separated by 10 second ITIs, induced a highly odor-specific habituation that persisted for a few minutes (McNamara et al., 2008). The pharmacology, persistence and odor-specificity of behavioral habituation at this timescale mimics the characteristics of odor adaptation in piriform cortex pyramidal cells in urethane-anesthetized rats (Best et al. 2005; Best and Wilson 2004; McNamara et al. 2008; Wilson 2001; 1998b; Yadon and Wilson 2005). Our current data show that the pharmacology, persistence and specificity of behavioral habituation using a different paradigm (50-second odor presentations with five-minute ITIs) similarly mimics the properties of mitral cell odor responses in the olfactory bulbs of urethane-anesthetized rats.
Direct comparisons between behavioral phenomena in awake animals and recordings in anesthetized animals are not straightforward, as substantial differences between olfactory bulb odor responses in awake and anesthetized animals have been reported (Rinberg et al. 2006). However, behaviorally, olfactory habituation at the timescale used here does not depend on feedback connections to the olfactory bulb (Kiselycznyk et al. 2006), or on bulbar cholinergic or noradrenergic inputs (Chaudhury et al. 2009; Mandairon et al. 2006a; Mandairon et al. 2008), suggesting that, in this case, a predominantly feedforward model of processing may be utilized that could be minimally disrupted by anesthesia. Moreover, both behavioral and neuronal habituation are impaired by local blockade of NMDA receptors, an additional commonality between the two levels of analysis that is unlikely to depend on behavioral state. In the anesthetized preparation, significant adaptation could not be elicited with stimulus durations shorter than 30 seconds, which is significantly longer than the observed time range of active investigation during our behavioral tests. Behavioral trials lasted 50 seconds and exposed the animals to the odorant for the duration of the trial; differences between long duration passive exposure, as is the case in the electrophysiological experiments, and active short duration investigation, as is the case in the behavioral experiments, are difficult to assess. Given the observed similarities in specificity, dependence on ITI and pharmacology of mitral cell adaptation and behavioral habituation one may assume a causal, if not exclusive relationship between these two observations.
The observed NMDA receptor-dependent adaptation to repeated odor stimuli in mitral cells is most likely due to changes in inhibitory input to mitral cells by granule cells in the external plexiform layer. Excitation of mitral cells results in glutamate release from mitral cell secondary dendrites onto the dendritic spines of granule cells which, in turn, release the inhibitory neurotransmitter GABA back onto mitral cells (Balu et al. 2007; Chen et al. 2000; Halabisky et al. 2000; Urban and Sakmann 2002). An increase in granule cell excitation, whether mediated by enhanced inputs from mitral cells or from centrifugal glutamatergic inputs (Balu et al. 2007; Chen et al. 2000), would presumably lead to the suppression of mitral cell responses to odorants as demonstrated here. NMDA receptors in the olfactory bulb external plexiform layer mediate recurrent (self-) and lateral inhibition as well as auto-excitation of mitral cells and have been shown to underlie synaptic transmission and some forms of plasticity in the OB (Chen et al. 2000; Friedman and Strowbridge 2000; Halabisky et al. 2000; Satou et al. 2006). Furthermore, bulbar NMDA receptors have been shown to be involved in the coupling of mitral activity to respiratory patterns (Philpot et al. 1998). Recent experiments showing synaptic plasticity in response to theta-burst stimulation of centrifugal excitatory synapses onto granule cells suggest that this enhanced excitation of granule cells releases the Mg+ block of granule cell NMDA receptors that are activated by mitral cell inputs to granule cell spines, resulting in increased feedback inhibition onto mitral cells (Gao and Strowbridge 2009). This mechanism potentially could underlie the NMDA receptor-dependent adaptation to repeated odor stimulation observed in the present work. Behaviorally, NMDA receptors serve a functional role in modulating bulbar responses during sensory deprivation, olfactory enrichment, and associative and nonassociative learning on a long (weeks) timescale (Lincoln et al. 1988; Mandairon et al. 2006b; Wilson 1995). Our results, which demonstrate similarities between NMDA receptor-dependent behavioral habituation and neuronal adaptation responses, suggest a second, shorter (minutes to hours) role for NMDA receptor-mediated plasticity in the olfactory bulb.
The degree of adaption of mitral cell responses observed here differs substantially from that reported by other groups. Using a direct comparison of bulbar and cortical response adaptation during prolonged odor stimuli (20 – 50 seconds of continuous stimulation) Wilson (2000) reported that mitral cells adapted to about 50-75% of their initial response and that this adaptation was not specific to the odor used for prolonged stimulation. In our hands, repeated stimuli of that duration, separated by at least 2.5 minutes, resulted in greater adaptation during post-testing, suggesting a cumulative effect working on a minutes to hours time scale. Indeed, we observed no significant adaptation over the course of a single 50-second odor stimulation in our experiments, yet, a significant decrease in response was observed over the course of repeated 50-second stimuli. The induced adaptation was not specific to the odorant used during repeated stimulation, as reported in response to a single 50-second stimulation (Figure 3D; Wilson, 2000). In a previous report, Chaput and Panhuber (1982) saw a range of adaptation in mitral cells in response to continuous odor stimulation for up to 60 minutes. Recently, Shea et al. (2008) showed that using repeated 2-second odor stimulations with 30-second ITIs did not induce mitral cell adaptation; these results agree with our results showing that longer odor pulses separated by longer ITIs are needed to induce this form of adaptation (Figure 3 B,C). Interestingly, Shea et al. (2008) also showed that the stimulation of noradrenergic pathways to the olfactory bulb during these short pulsed odor stimuli with shorter ITIs resulted in significant adaptation of mitral cell odor responses.
In summary, our results, taken together with experiments from other groups, further support the idea that olfactory behavioral habituation can be mediated by a variety of neural phenomena operating at different timescales and located in different neural structures, including the olfactory bulb.
Figure 5. Average responses of mitral cells during the adaptation protocol in control and MK-801 rats.
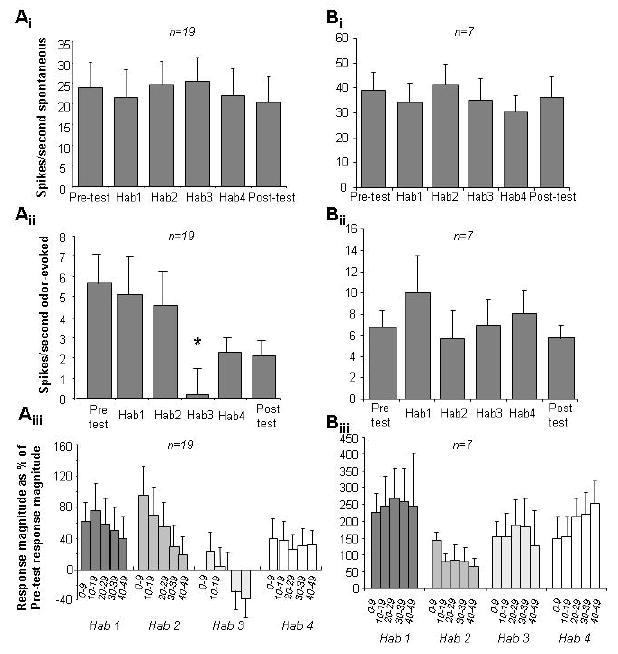
Ai and Bi. Spontaneous activity, recorded for 4 seconds before each odor presentation, did not change significantly over the course of a recording protocol (Ai: control rats, Bi: MK-801 infused rats). Aii and Bii. Average number of evoked spikes per second during pre-test, adaptation trials and posttests. In control rats, the average number of odor evoked spikes per second significantly decreased after the first two adaptation trials (Aii), whereas in MK-801 infused rats no significant decrease was observed (Bii). Asterisk indicates a significant difference to the first adaptation trial. Aiii and Biii. Time course of mitral cell responses during adaptation trials. The graphs show the average number of odor-evoked spikes normalized by the number evoked during pre-tests recorded in 10-second intervals. No significant change over the course of a 50-second odor presentation was observed in either group of animals, except for the first adaptation trial in control rats.
Acknowledgments
This work has been supported by National Science Foundation Grant CNS 0338981 (CL), NIH training grant T32 GM007469 (LM) and a SUNY predoctoral fellowship (AA). The authors thank Sasha Devore and Nathalie Mandairon for comments on earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci. 2007;27:5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Thompson JV, Fletcher ML, Wilson DA. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. J Neurosci. 2005;25:2513–2517. doi: 10.1523/JNEUROSCI.5298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci. 2004;24:652–660. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput MA, Panhuber H. Effects of long duration odor exposure on the unit activity of olfactory bulb cells in awake rabbits. Brain Res. 1982;250:41–52. doi: 10.1016/0006-8993(82)90951-9. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci. 2009;29:52–60. doi: 10.1523/JNEUROSCI.4036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Christoffersen GR. Habituation: events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Prog Neurobiol. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Friedman D, Strowbridge BW. Functional role of NMDA autoreceptors in olfactory mitral cells. J Neurophysiol. 2000;84:39–50. doi: 10.1152/jn.2000.84.1.39. [DOI] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci. 2009;12:731–733. doi: 10.1038/nn.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Skinner JE. Field potential response changes in the rabbit olfactory bulb accompany behavioral habituation during the repeated presentation of unreinforced odors. Exp Brain Res. 1988;73:189–197. doi: 10.1007/BF00279672. [DOI] [PubMed] [Google Scholar]
- Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk CL, Zhang S, Linster C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn Mem. 2006;13:575–579. doi: 10.1101/lm.285706. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Coopersmith R, Harris EW, Cotman CW, Leon M. NMDA receptor activation and early olfactory learning. Brain Res. 1988;467:309–312. doi: 10.1016/0165-3806(88)90036-3. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006a;24:3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci. 2008;27:1210–1219. doi: 10.1111/j.1460-9568.2008.06101.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci U S A. 2006b;103:13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15:117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Lyders EM, Brunjes PC. The NMDA receptor participates in respiration-related mitral cell synchrony. Exp Brain Res. 1998;118:205–209. doi: 10.1007/s002210050273. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Broster BS. Factors affecting habituation and recovery from habituation in the nematode Caenorhabditis elegans. Behav Neurosci. 1992;106:239–249. doi: 10.1037//0735-7044.106.2.239. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou M, Hoshikawa R, Sato Y, Okawa K. An in vitro study of long-term potentiation in the carp (Cyprinus carpio L.) olfactory bulb. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:135–150. doi: 10.1007/s00359-005-0056-7. [DOI] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci. 2008;28:10711–10719. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol. 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biol. 2008;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol. 2000;84:3036–3042. doi: 10.1152/jn.2000.84.6.3036. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998a;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson DA. NMDA receptors mediate expression of one form of functional plasticity induced by olfactory deprivation. Brain Res. 1995;677:238–242. doi: 10.1016/0006-8993(95)00151-f. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Receptive fields in the rat piriform cortex. Chem Senses. 2001;26:577–584. doi: 10.1093/chemse/26.5.577. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Synaptic correlates of odor habituation in the rat anterior piriform cortex. J Neurophysiol. 1998b;80:998–1001. doi: 10.1152/jn.1998.80.2.998. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Linster C. Neurobiology of a simple memory. J Neurophysiol. 2008;100:2–7. doi: 10.1152/jn.90479.2008. [DOI] [PubMed] [Google Scholar]
- Yadon CA, Wilson DA. The role of metabotropic glutamate receptors and cortical adaptation in habituation of odor-guided behavior. Learn Mem. 2005;12:601–605. doi: 10.1101/lm.41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses. 2000;25:473–481. doi: 10.1093/chemse/25.4.473. [DOI] [PubMed] [Google Scholar]


