Abstract
Wear particles generated from total joint arthroplasty (TJA) stimulate macrophages to release chemokines. The role of chemokines released from wear particle-stimulated macrophages on the migration of macrophages and osteoprogenitor cells in vitro has not been elucidated. In this study, we challenged murine macrophages (RAW 264.7) with clinically relevant polymethyl methacrylate (PMMA, 1-10 μm) and ultra high molecular weight polyethylene (UHMWPE, 2-3 μm) particles. The chemotactic effects of the conditioned media (CM) were tested in vitro using human macrophages (THP-1) and human mesenchymal stem cells (MSCs) as the migrating cells. CM collected from both particle types had a chemotactic effect on human macrophages, which could be eliminated by monocyte chemotactic protein-1 (MCP-1) neutralizing antibody. Blocking the CCR1 receptor eliminated the chemotactic effect, while CCR2 antibody only partially decreased THP-1 cell migration. CM from PMMA but not UHMWPE-exposed macrophages led to chemotaxis of MSCs; this effect could be eliminated by macrophage inflammatory protein-1 alpha (MIP-1α) neutralizing antibody. Neither CCR1 nor CCR2 blocking antibodies showed an effect on the migration of MSCs. Chemokines released by macrophages stimulated by wear particles can have an effect on the migration of macrophages and MSCs. This effect seems to be dependent on the particle type, and may be modulated by MCP-1 and MIP-1α, however more than one chemokine may be necessary for chemotaxis.
Keywords: Macrophage, Mesenchymal stem cell, Polymethylmethacrylate, Polyethylene, Chemotaxis, Chemokines
INTRODUCTION
Wear particles are inevitable byproducts of all joint replacements 1. The biological reaction associated with wear particles occurs in a unique microenvironment in which bone marrow cells / osteoprogenitors and macrophages are in direct contact with orthopaedic wear debris 2. Macrophages are considered one of the most essential cell types participating in the process of particle-associated osteolysis since they respond directly to particles by releasing inflammatory mediators such as interleukins (IL)-1, tumor necrosis factor (TNF)-α, MCP-1 and MIP-α 3.
Ultra high molecular weight polyethylene (UHMWPE) particles and polymethyl methacrylate (PMMA) particles are two common degradation products of cemented prostheses 4. With conventional bearing surfaces, polyethylene particles constitute the majority of the pool of wear debris 5,6. While larger particles and flakes are surrounded or ingested by foreign body multinucleated giant cells (MNGCs), the small particles (less than about 5-10 μm) are phagocytosed by macrophages which trigger a cascade of immunological events 4.
In aseptic loosening, it has been thought that the cytokines involved in local paracrine and autocrine events are derived from resident phagocytic macrophages, osteoblasts and other cells, resulting in a localized inflammatory and foreign body reaction 4. Although it is known that a class of chemoattractive cytokines, also known as chemokines, is produced by the cells present at the prosthesis-bone interface, the downstream effects of these chemokines on osteogenesis and osteoclastogenesis are largely unknown.
Chemokines are a large family of small molecules with similar structure that provide key signals for trafficking and homing of specific subpopulations of cells of the immune system in health (homeostatic) and disease (immunologic) 7. Most chemokines have four cysteine groups in conserved locations, and are classified into four groups, CC, CXC, C and CX3C chemokines, according to the location of the first two cysteines. The biological effects of chemokines are mediated by a family of closely related G protein–coupled receptors 7,8. Recent studies have shown that two of the CC chemokines, MCP-1 and MIP-1α, participate in the more widespread recruitment of cells to the area of particle generation 9-12,20 and play a critical role in osteolysis. The intracellular signal transduction of MCP-1 involves the C-C chemokine receptor 2 (CCR2). However, CCR2 is also activated by MCP-3, -4, and -5, suggesting redundancy in the chemokine system. Other chemokine receptors including CCR1 and CCR5 are postulated to mediate the effects of MIP-1α 8.
Despite ongoing research into the role of chemokines in the cellular and molecular processes of particle-induced osteolysis, little is known about the effects of chemokines on chemotaxis of macrophages and osteoprogenitor cells. It has been postulated that both osteoclasts and osteoblasts respond to chemokines and that processes involving these cells might be tightly regulated via the control of precursor cell recruitment and proliferation 13-17. In this study, we challenged murine macrophages with clinically relevant PMMA and UHMWPE particles and examined the chemotactic ability of the conditioned medium. We hypothesized that murine macrophages challenged by clinically relevant particles release soluble substances that can trigger the chemotaxis of human macrophages and MSCs. Human (and not murine) macrophages and MSCs were used as the migrating cells because of their high level of responsiveness to chemotactic agents 18.
MATERIALS AND METHODS
Media and antibodies
All media and serum were purchased from Invitrogen (Carlsbad, CA). MCP-1, MIP-1α and their neutralizing antibodies were purchased from R&D Systems, Inc (Minneapolis, MN). Normal IgG and antibodies for CCR1 and CCR2 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). MCP-1 and MIP-1α were used as controls for the migration experiments. Normal IgG was used as the antibody control for the receptor blocking experiments.
Isolation of PMMA and UHMWPE
Conventional UHMWPE particles (GUR 1020), a generous gift from Dr. Tim Wright at the Hospital for Special Surgery in New York were obtained from knee joint simulator tests. The particles were isolated by density gradient centrifugation and sterilized by incubating with 95% ethanol overnight according to an established protocol 19. Briefly, frozen aliquots of the particle containing serum were lyophilized for 4-7 days. The dried material was digested in 5M sodium hydroxide at 70°C for 2 h. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40 K rpm at 10°C for 3 h. The collected particles at the surface of the sucrose solution were ultrasonicated and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40 K rpm at 10°C for 1 h. The purified particles at the interface between the two layers of isopropanol were harvested and the isopropanol was evaporated from the particle mixture until dry. Particles were then resuspended in 95% ethanol with shaking over night. The particle-ethanol suspension was then left in the tissue culture hood to allow the ethanol to evaporate completely. The diameters of the isolated particles were approximately 2-3 μm as revealed by scanning electron microscopy (SEM). The UHMWPE particle concentration of 108 particles/cm2 was achieved by suspending the calculated volume of particles in 200 μL sterile distilled water. The particle suspension was then coated directly onto 6-well culture plates. The coated plates were allowed to dry overnight to completely evaporate the water. Cells were then seeded onto the coated plate.
PMMA particles, ranging in diameter from 1-10 μm (mean=6.0 ± 1.8 μm), were purchased from Polysciences Inc (Warrington, PA). These particles have been used by our group and others in numerous in vitro and in vivo studies because they are commercially available and well documented for their ability to activate macrophages to release pro-inflammatory cytokines in vitro 20-24. Furthermore, the range of these particles is approximately the same order of magnitude as the retrieved polyethylene particles used in the current study. PMMA particles were sterilized by incubating them in 75% ethanol with shaking overnight and then washing with PBS. PMMA particle final concentration of 5.52×105 particles/cm2 was achieved by adding sterile PMMA particles directly into the 6-well culture plates. The absence of endotoxin for both particle types was confirmed by the Limulus Amoebocyte Lysate assay (Biowhittaker Inc, Walksville, MD).
Conditioned media
RAW 264.7 cells (Cat#: TIB-71, ATCC, Manassas, VA), a mouse macrophage cell line, were grown in 10% FBS in DMEM. 1×105/cm2 cells were seeded onto the culture wells of 6-well plates and either PMMA or UHMWPE particles were added, as outlined above. Cell-seeded culture plates without particles were used as a negative control. The conditioned media (CM) was collected after 48 hours and used for the chemotaxis assay. The concentration of the chemokines, MCP-1 and MIP-1α in the CM was assayed by ELISA (R&D Systems, Inc, Minneapolis, MN).
Chemotaxis assay
The experiments reported in this study were originally designed to investigate chemotaxis of primary murine macrophages and mesenchymal stem cells (MSCs) due to chemokines released by murine macrophages after polymer particle exposure. Preliminary experiments by our group showed very low levels of expression of the chemokine receptors CCR2 and CCR1, major receptors for the chemokines MCP-1 and MIP-1α, on primary murine macrophages and MSCs respectively. In our preliminary in vitro studies, murine macrophages and MSCs showed minimal directional migration towards either MCP-1 or MIP-1α positive controls, using final concentrations ranging from 100 pg/ml to 100 ng/ml, indicating low levels of expression of these chemokine receptors on murine primary cells. This fact was confirmed by the company (R&D Systems) who produced the antibodies to these receptors. In contrast, these chemokine receptors are highly expressed on human macrophages, which were verified in preliminary cell migration studies using MCP-1 or MIP-1α positive controls. Thus, we elected to use both human macrophages and MSCs, as the migrating reporter cells for the chemotaxis assay.
THP-1 cells (Cat#: TIB-202, ATCC, Manassas, VA), a human macrophage cell line, were grown in ATCC-formulated RPMI-1640 medium with 10% FBS and 50nM 2-mercaptoethanol. Human MSCs were purchased from Lonza (Basel, Switzerland) and grown in Lonza-formulated medium. Chemotx disposable chemotaxis systems (96-well format with 5um pore size, cat # 106-5) were purchased from Neuro Probe (Gaithersburg, MD). For the chemotaxis assay, 6×104 THP-1 cells or MSCs were loaded onto the migration membrane and 30μl CM was loaded in the migration chamber. The plates were read using a Spectra M2 microplate reader (Molecular devices, Sunnyvale, CA) set at 485/530nm after two hours incubation.
Neutralizing MCP-1 and MIP-1α antibodies, and blocking CCR1 and CCR2 using antibodies
Neutralizing antibodies (ligand /antibody = 1:100) were added into conditioned media and incubated for 30 minutes at 37 °C before the chemotaxis experiment. When blocking antibody was applied, cells were incubated with blocking antibodies (2.5 ng /mL) for 2 hours, followed by washing with DMEM media three times, and then the treated cells were ready to be used as chemotactic cells. Addition of MCP-1 and MIP-1α (10 ng/ml) were used as controls; IgG was used as the negative control for the blocking experiments.
Statistical analysis
A one way ANOVA (Post hoc multi-comparisons with the Tukey test) was conducted using SPSS 14.0 (SPSS Inc., Chicago, IL). Data were reported as mean ± standard error. A p value < 0.05 was chosen as the threshold of significance.
RESULTS
RAW 264.7 cells release MCP-1 and MIP-1α
RAW 264.7 cells constitutively released MCP-1 (2,667 pg/ml) in DMEM media without particles. After being exposed to PMMA particles for 48 hours, the level of MCP-1 released from RAW 264.7 cells increased by almost 4 fold to 8500 pg/ml (p<0.01). UHMWPE particles also increased the release of MCP-1 by 1.5 fold to 4,355pg/ml (Fig. 1), but the increase did not reach statistical significance (p=0.47). RAW 264.7 cells released a considerable amount of MIP-1α (15 ng/ml) in DMEM without particles, and particle challenge did not generate additional MIP-1α release.
Fig. 1.
MCP-1 was released from RAW264.7 cells after challenge with either PMMA or UHMWPE particles. a. p<0.05 vs. all other groups, One-Way ANOVA, n= 3.
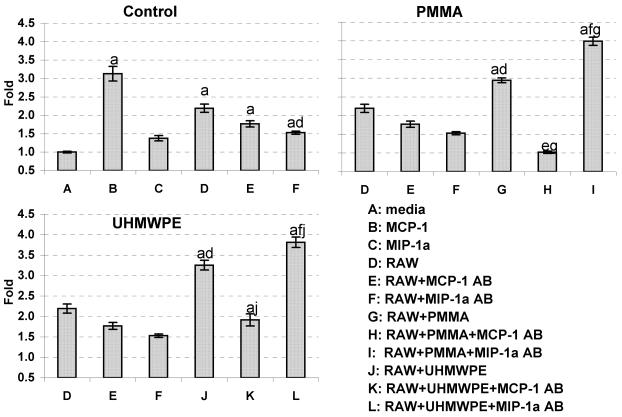
MCP-1 in CM led to the directional migration of human macrophages (THP-1 cells)
Exogenous MCP-1 and MIP-1α were used as migration controls. MCP-1 (Fig. 2, p<0.01 B vs. A), but not MIP-1α (Fig. 2, C), induced chemotactic migration of THP-1 cells. CM from RAW 264.7 cells without particles increased THP-1 migration 2.19 fold, (Fig.2, p<0.01, D vs. A), which was partially blocked by MIP-1α neutralizing antibody (Fig.2, p<0.01, F vs. D).
Fig. 2.
CM from RAW264.7 cells after challenge with either PMMA or UHMWPE particles induced direct migration of THP-1 cells. Neutralizing MCP-1 antibody blocked this induction while neutralizing MIP-1α antibody stimulated cell migration. a: p < 0.05 vs. group A; d: p < 0.05 vs. group D; e: p < 0.05 vs. group E; f: p < 0.05 vs. group F; g: p < 0.05 vs. group G; j: p < 0.05 vs. group J, One-Way ANOVA, n=5.
CM from RAW 264.7 cells challenged by PMMA particles significantly increased THP-1 cell migration by 34.3% (Fig.2, p<0.01, G vs. D), which was eliminated by MCP-1 neutralizing antibody (Fig.2, p<0.01, H vs. G). Surprisingly, MIP-1α neutralizing antibody further stimulated cell migration by 35% (Fig.2, p<0.01, I vs. G).
Similar results were found using UHMWPE particles. CM from RAW 264.7 cells challenged by UHMWPE particles significantly increased THP-1 cell migration by 48.4% (Fig. 2, p<0.01, J vs. D), which was eliminated by MCP-1 neutralizing antibody (Fig. 2, p<0.01, K vs. J). Neutralizing MIP-1α antibody also increased cell migration by an additional 17% (Fig. 2, p<0.05, L vs. J) (Table 1).
Table 1.
THP1 and MSC migrated towards CM. Numbers are fold compared with media only. CM: condition media, AB: antibody
| CM | THP1 | hMSC |
|---|---|---|
| media | 1.00 | 1.00 |
| media+MCP-1 | 3.13 | 0.98 |
| media+MIP-1α | 1.38 | 0.72 |
| RAW cells | 2.19 | 1.29 |
| RAW cells+MCP-1 AB | 1.77 | 1.40 |
| RAW cells+MIP-1α AB | 1.53 | 1.18 |
| RAW cells+PMMA | 2.94 | 2.55 |
| RAW cells+PMMA+MCP-1 AB | 1.02 | 2.22 |
| RAW cells+PMMA+MIP-1α AB | 3.99 | 0.73 |
| RAW cells+UHMWPE | 3.25 | 0.58 |
| RAW cells+UHMWPE+MCP-1 AB | 1.91 | 0.46 |
| RAW cells+UHMWPE+MIP-1α AB | 3.81 | 0.82 |
To find out the particular receptors responsible for the observed chemotactic effects, blocking antibodies to CCR1 receptor (bound by MIP-1α, RANTES, MCP-3 and other chemokines) and CCR2 receptor (bound by MCP-1, CCL8 and CCL16) were applied. The antibody control, IgG alone, did not change the migration profile of THP1 cells with particle-challenged CM (Fig. 3 IgG group). Blocking the CCR1 receptor eliminated the chemotactic effect of CM from both PMMA particles and UHMWPE particles (Fig. 3, p<0.01, CCR1 AB vs. control). CCR2 blocking antibody only partially decreased THP-1 migration to PMMA challenged CM by 24.5% (Fig. 3, p<0.05, CCR2 AB vs. control), and did not change THP-1 cell migration towards the UHMWPE particle-challenged CM.
Fig. 3.
Blocking the MIP-1α receptor CCR1, but not MCP-1 receptor CCR2, eliminated the migration of THP-1 towards CM from RAW 264.7 cells challenged by PMMA and UHMWPE particles. IgG was used as the protein control. a. p < 0.05 vs media, b. p <0.05 vs same CM from control and IgG groups, c,d. p <0.05 vs same CM from control groups, One-Way ANOVA, n=5.
MIP-1α is essential to human MSC chemotaxis
To test the effect of CM on the chemotaxis of MSCs, we repeated the experiment using human MSCs. Exogenous MCP-1 and MIP-1α did not induce chemotactic migration of human MSC cells (Fig. 4. B,C). The blank control, CM from RAW 264.7 cells without particles, did not significantly attract human MSC migration (Fig.4., D,E,F).
Fig. 4.
CM from RAW 264.7 cells challenged by PMMA particles induced direct migration of MSCs. MIP-1α, but not MCP-1, neutralization antibody eliminated this migration effect. a: p < 0.05 vs. group A; d: p < 0.05 vs. group D; e: p < 0.05 vs. group E; g: p < 0.05 vs. group G, One-Way ANOVA, n=5.
CM from RAW 264.7 cells challenged by PMMA particles significantly increased human MSC migration by 98.1% (Fig.4, p<0.01, G vs. D), which was partially blocked by MCP-1 neutralizing antibody (Fig.4, p=0.332, H vs. G) and eliminated by MIP-1α neutralizing (Fig.4, p<0.01, I vs. G).
Comparing with media only, CM from RAW 264.7 cells challenged by UHMWPE particles did not attract human MSC cells (Table 1). On the contrary, migration in CM with UHMWPE treatment was even lower than CM without particles (Fig. 4, p<0.01, J vs. D).
Neither CCR1 nor CCR2 antibodies showed any effect on the migration of human MSCs to CM (data not shown).
DISCUSSION
Chemokines such as MCP-1 and MIP-1α have been shown to play a prominent role in initiating and perpetuating the chronic inflammatory response to wear particles 9,12. In this study, human macrophages (THP-1) directionally migrated towards the CM generated from cells exposed to clinically relevant PMMA and UHMWPE particles and the migration could be decreased significantly by MCP-1 neutralizing antibody. These observations agree with previous studies in which different types of particles and cell types were examined 12. The present data has suggested that both PMMA and UHMWPE particles are able to recruit neighboring macrophages to the bone-implant interface and that MCP-1 is a critical mediator of this process. In our study, MIP-1α by itself was not able to cause the migration of THP-1 cells in vitro. On the other hand, neutralizing MIP-1α in CM increased the chemotactic potential of the CM. These findings were surprising given the fact that MIP-1α normally functions as a chemoattractant for macrophages 12. Possible explanations for these observations include the specific in vitro conditions and cells used in the present experiments, and interactions of the MIP-1α antibody with other unknown chemoattractants.
Chemotaxis of macrophages and MSCs exposed to CM from PMMA challenged RAW264.7 cells was greater than that compared to CM from unchallenged macrophages. Although the level of MIP-1α remained unchanged (15 ng/mL) after exposing the RAW 264.7 cells to PMMA particles, migration of MSCs increased when exposed to CM from RAW 264.7 cells incubated with PMMA particles, and MIP-1α neutralizing antibody eliminated the increased migration of MSCs induced by the conditioned media. Unlike human monocytes that release extremely low levels of MIP-1 α (0.1 ng/mL) under normal conditions and are able to produce additional MIP-1 α upon exposure to PMMA particles 12, RAW264.7 cells did not produce more MIP-1 α after PMMA particle challenge. One reason might be that RAW 264.7 cells are a murine virus-transfected cell line that already produces high amounts of MIP-1α constitutively (15 ng/mL) in vitro. The effect of the MIP-1 α neutralizing antibody on MSC chemotaxis demonstrated the importance of MIP-α for MSC migration.
MSCs express a large number of chemokine receptors including receptors for MCP-1 and MIP-1α 25. The release of different signaling molecules and activation of specific receptors enables MSCs to respond to multiple homeostatic and pathological events involving tissue inflammation and repair. Inflammation-targeted homing of the MSCs has been reported in a tumor microenvironment 26. The systemic recruitment of MSCs to a fracture site has also been demonstrated 27. It appears that multiple molecular signaling substances have to be precisely orchestrated for the chemotaxis of MSCs to occur since MCP-1 and MIP-1α, when given separately at the doses applied, were not able to induce increased chemotaxis of MSCs. An alternative explanation for these findings might be that differences in the murine versus human chemokines, and the chemokine receptors on the human reporter MSCs lead to suboptimal ligand-receptor interaction. The current study has also shown that when PMMA wear particles are generated, MSCs can potentially be recruited by the chemokines produced by activated macrophages. These findings are concordant with the observation that periprosthetic osteolysis is associated with a heightened level of bone repair 28.
Surprisingly, migration of human MSCs towards CM from UHMWPE exposed RAW 264.7 cells was decreased compared to CM without particles. This may be due to the properties of the material itself, or the dose of UHMWPE particles we applied to stimulate the RAW264.7 cells, which was optimized for TNF production and may not be optimal to maximize MCP-1 production. This is reflected by the levels of MCP-1 released by the macrophages exposed to UHMWPE particles, where the particles stimulated about half the MCP-1 release compared to the addition of PMMA particles. It is conceivable that other essential mediators responsible for MSC trafficking such as the chemokine CXCL16 and others 22 would also be considerably lower in UHMWPE conditioned media compared to media from PMMA particle exposure. Another possible reason is that UMHWPE CM could contain inhibitory substances to MSC migration that are absent in PMMA CM under these culture conditions. In addition, differences between murine- as apposed to human-derived chemokines from UHMWPE CM may have led to suboptimal ligand-receptor interaction on human MSCs. Nevertheless, with regards to both PMMA and UHMWPE particles, the presence of neutralizing MIP-1α antibody was associated with minimum levels of MSC migration.
We also conducted receptor-blocking studies to pinpoint the key receptors for chemotaxis. Blocking CCR2, one of the two receptors that MCP-1 binds, partially decreased the chemotactic effect of MCP-1 on THP-1 macrophages, which suggests that MCP-1 may bind to both CCR-2 and CCR4 receptors on THP-1 cells to trigger the chemotaxis. For human MSC migration, blocking CCR1, one of the cell surface receptors for MIP-1α, did not decrease the chemotactic effect of the PMMA CM. Taken together with the observation that neutralizing MIP-1α antibody was associated with minimum levels of MSC migration, our data suggested that MIP-1α may act through other receptors, such as CCR3 or CCR5.
In summary, under the conditions selected in this study, it has been demonstrated that clinically relevant PMMA and UHMWPE particles may trigger the chemotaxis of macrophages, and MCP-1 is the essential mediator of this homing process. PMMA particles may also induce the homing of MSCs via MIP-1α signaling. Future studies will focus on identifying the specific receptors responsible for these effects and the combined effects of multiple chemokines.
Acknowledgments
Funding is gratefully acknowledged from NIH grant R01AR55650.
Abbreviations
- UHMWPE
Ultra High Molecular Weight Polyethylene
- ELISA
enzyme-linked immunosorbent assay
- PMMA
Polymethyl methacrylate
- CM
conditioned Media
- MCP-1
monocyte chemotactic protein-1
- MIP-1α
macrophage inflammatory protein-1 alpha
- MSC
mesenchymal stem cell
References
- 1.Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 2008;5(28):1263–78. doi: 10.1098/rsif.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuan RS, Lee FY, TK Y, Wilkinson JM, Smith RL. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16(Suppl 1):S42–8. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostrom M, O'Keefe R. What experimental approaches (eg, in vivo, in vitro, tissue retrieval) are effective in investigating the biologic effects of particles? J Am Acad Orthop Surg. 2008;16(Suppl 1):S63–7. doi: 10.5435/00124635-200800001-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revell PA, al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng [H] 1997;211(2):187–97. doi: 10.1243/0954411971534304. [DOI] [PubMed] [Google Scholar]
- 5.Shanbhag AS, Jacobs JJ, Glant TT, Gilbert JL, Black J, Galante JO. Composition and morphology of wear debris in failed uncemented total hip replacement. J Bone Joint Surg Br. 1994;76(1):60–7. [PubMed] [Google Scholar]
- 6.Maloney WJ, Smith RL, Schmalzried TP, Chiba J, Huene D, Rubash H. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77(9):1301–10. doi: 10.2106/00004623-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–99. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 8.Rottman JB. Key role of chemokines and chemokine receptors in inflammation, immunity, neoplasia, and infectious disease. Vet Pathol. 1999;36(5):357–67. doi: 10.1354/vp.36-5-357. [DOI] [PubMed] [Google Scholar]
- 9.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Titanium particles induce the immediate early stress responsive chemokines IL-8 and MCP-1 in osteoblasts. J Orthop Res. 2002;20(3):490–8. doi: 10.1016/S0736-0266(01)00154-1. [DOI] [PubMed] [Google Scholar]
- 10.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Chemokine gene activation in human bone marrow-derived osteoblasts following exposure to particulate wear debris. J Biomed Mater Res A. 2006;77(1):192–201. doi: 10.1002/jbm.a.30609. [DOI] [PubMed] [Google Scholar]
- 11.Fritz EA, Jacobs JJ, Glant TT, Roebuck KA. Chemokine IL-8 induction by particulate wear debris in osteoblasts is mediated by NF-kappaB. J Orthop Res. 2005;23(6):1249–57. doi: 10.1016/j.orthres.2005.03.013.1100230603. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima Y, Sun DH, Trindade MC, Chun LE, Song Y, Goodman SB, Schurman DJ, Maloney WJ, Smith RL. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg Br. 1999;81(1):155–62. doi: 10.1302/0301-620x.81b1.8884. [DOI] [PubMed] [Google Scholar]
- 13.Fuller K, Owens JM, Chambers TJ. Macrophage inflammatory protein-1 alpha and IL-8 stimulate the motility but suppress the resorption of isolated rat osteoclasts. J Immunol. 1995;154(11):6065–72. [PubMed] [Google Scholar]
- 14.Scheven BA, Milne JS, Hunter I, Robins SP. Macrophage-inflammatory protein-1alpha regulates preosteoclast differentiation in vitro. Biochem Biophys Res Commun. 1999;254(3):773–8. doi: 10.1006/bbrc.1998.9909. [DOI] [PubMed] [Google Scholar]
- 15.Votta BJ, Levy MA, Badger A, Bradbeer J, Dodds RA, James IE, Thompson S, Bossard MJ, Carr T, Connor JR. Peptide aldehyde inhibitors of cathepsin K inhibit bone resorption both in vitro and in vivo. J Bone Miner Res. 1997;12(9):1396–406. doi: 10.1359/jbmr.1997.12.9.1396. and others. [DOI] [PubMed] [Google Scholar]
- 16.Kukita T, Nomiyama H, Ohmoto Y, Kukita A, Shuto T, Hotokebuchi T, Sugioka Y, Miura R, Iijima T. Macrophage inflammatory protein-1 alpha (LD78) expressed in human bone marrow: its role in regulation of hematopoiesis and osteoclast recruitment. Lab Invest. 1997;76(3):399–406. [PubMed] [Google Scholar]
- 17.Graves DT, Jiang Y, Valente AJ. The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Front Biosci. 1999;4:D571–80. doi: 10.2741/graves. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169(4):1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell P, Ma S, Yeom B, McKellop H, Schmalzried TP, Amstutz HC. Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res. 1995;29(1):127–31. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 20.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26(1):106–16. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 21.Trindade MC, Lind M, Nakashima Y, Sun D, Goodman SB, Schurman DJ, Smith RL. Interleukin-10 inhibits polymethylmethacrylate particle induced interleukin-6 and tumor necrosis factor-alpha release by human monocyte/macrophages in vitro. Biomaterials. 2001;22(15):2067–73. doi: 10.1016/s0142-9612(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 22.Trindade MC, Lind M, Sun D, Schurman DJ, Goodman SB, Smith RL. In vitro reaction to orthopaedic biomaterials by macrophages and lymphocytes isolated from patients undergoing revision surgery. Biomaterials. 2001;22(3):253–9. doi: 10.1016/s0142-9612(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 23.Trindade MC, Schurman DJ, Maloney WJ, Goodman SB, Smith RL. G-protein activity requirement for polymethylmethacrylate and titanium particle-induced fibroblast interleukin-6 and monocyte chemoattractant protein-1 release in vitro. J Biomed Mater Res. 2000;51(3):360–8. doi: 10.1002/1097-4636(20000905)51:3<360::aid-jbm9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Yamanaka Y, Abu-Amer W, Foglia D, Otero J, Clohisy JC, Abu-Amer Y. NFAT2 is an essential mediator of orthopedic particle-induced osteoclastogenesis. J Orthop Res. 2008;26(12):1577–84. doi: 10.1002/jor.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS ONE. 2008;3(8):e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malek S, Kaplan E, Wang JF, Ke Q, Rana JS, Chen Y, Rahim BG, Li M, Huang Q, Xiao YF. Successful implantation of intravenously administered stem cells correlates with severity of inflammation in murine myocarditis. Pflugers Arch. 2006;452(3):268–75. doi: 10.1007/s00424-005-0035-4. and others. [DOI] [PubMed] [Google Scholar]
- 27.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60(3):813–23. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 28.Kadoya Y, Revell PA, Kobayashi A, al-Saffar N, Scott G, Freeman MA. Wear particulate species and bone loss in failed total joint arthroplasties. Clin Orthop Relat Res. 1997;(340):118–29. doi: 10.1097/00003086-199707000-00016. [DOI] [PubMed] [Google Scholar]