Abstract
Mutations in presenilin (PS) and amyloid precursor protein (APP) genes are a major cause for early-onset familial Alzheimer disease (AD). We measured Aβ levels in the cortex of APPsw and PS1 (M146V) mutation carriers, sporadic AD (SAD) and non-demented individuals. Levels of insoluble and soluble Aβ40 and soluble Aβ42 in brain of APPsw mutation carriers did not differ much from those found in SAD, but lower levels of insoluble Aβ42 were detected in the frontal and temporal cortex of APPsw brain. Insoluble Aβ40 and Aβ42 were significant lower in all four cortical regions of PS1 brain compared with SAD, and Aβ40 was lower in frontal and occipital cortex compared with APPsw brain. The insoluble Aβ42/40 ratio was similar in SAD and APPsw but significantly higher in PS1 mutation carriers. Our results indicate that the pattern of Aβ deposition in PS1 mutation carriers differs from that in both APPsw and SAD, whereas the pattern in APPsw mutation carriers is more similar to that in SAD. The early onset and aggressive course of PS1 AD cannot solely be explained by elevated Aβ levels, at least in the PS1 M146V mutation carries investigated here.
Keywords: Alzheimer's disease, Amyloid β peptide, APP mutation, Cortex, Familial AD, Post mortem brain, Presenilin
Introduction
Extracellular senile plaques mainly comprised of amyloid-β (Aβ), and intracellular neurofibrillary tangles are the major pathological hallmarks of Alzheimer's disease (AD) (Selkoe 2001). Aβ is generated from the amyloid precursor protein (APP) by enzymatic cleavage involving β -secretase and the γ-secretase complex, which includes presenilin activities (Evin and Weidemann 2002; Selkoe and Schenk, 2003). Although the majority of AD cases (>90%) typically occur after the age of 60–65 years, a smaller proportion of cases correspond to the early-onset (<60 years) familial AD (FAD). Autosomal-dominant forms of FAD result from mutations in one of three genes: APP on chromosome 21, presenilin 1 (PS1) on chromosome 14 and presenilin 2 (PS2) on chromosome 1. Mutations in the PS1 gene are the most frequent cause of early onset FAD, and more than 160 mutations in 355 families have been reported, whereas AD families carrying APP mutations are less frequent (www.molgen.ua.ac.be/ADMutations). Mutations in these three genes share a common effect of abnormally processing APP and with an average age of onset at 50 years for APP mutations, 45 years for PS1 mutations and 52 years for PS2 mutations (Gomez-Isla et al. 1999). Except for the age of onset and the family history no pathological features that distinguish FAD from sporadic AD (SAD) have been reported (Menendez 2004; Ray et al. 1998). Both SAD and FAD share specific neuropathologic features, including neuritic plaques, neurofibrillary tangles and neuropil threads (Lippa et al. 1996). In some studies clinical differences between FAD and SAD have been found, such as unusual behavioural, psychiatric changes, seizures and myoclonus (Haltia et al. 1994; Lleo et al. 2004; Menendez 2004).
Neuropathological characterisation of the cortex of several cases with PS1 mutations revealed a predominant detection of Aβ 42 (Mann et al. 1996a, 2001). Similar increases in Aβ 42 levels have been observed in plasma and conditioned medium from skin fibroblasts from subjects carrying PS1 mutations (Scheuner et al. 1996), as well as in cells stably transfected with mutated PS1 and in the brain of transgenic mice expressing mutant PS1 (Borchelt et al. 1996, Citron et al. 1997; Duff et al 1996). It has been reported that PS1 mutations increase the Aβ 42/40 ratio (Duering et al. 2005; Murayama et al. 1999; Takeda et al. 2004). Yet, recent studies on mutant PS1 expressing cells suggest that the increased Aβ 42/40 ratio may be mainly due to reduction of Aβ40 rather than to increased production of Aβ42 (Bentahir et al. 2006; Shimojo et al. 2007). This increased proportion of Aβ42, which is more prone than Aβ40 to aggregate (Jarrett and Landsbury, 1993; McGowan et al. 2005), is thought to initiate the disease process. Mutations in the APP gene either cause increased production of both Aβ40 and Aβ42 or just Aβ42 alone (Citron et al. 1992; Mann et al. 1996b; Tamaoka et al. 1998).
Detailed comparisons of Aβ levels in rare dominant mutation carriers with common late-onset AD have not been performed previously even though several neuropathological descriptions of FAD cases have been published to date. Differences in the levels of Aβ peptides and their regional distribution in FAD and SAD cases may provide an understanding of the molecular mechanisms by which Aβ is deposited in these forms of the disease and may also be an important consideration in future Aβ vaccination therapies. This study is the first to report the pattern and levels of soluble and insoluble Aβ species in cortical regions of FAD and SAD brain. Our studies were conducted using postmortem brain tissue obtained from five relatives who carried the Swedish APP double mutation (KM670/671NL) (Axelman et al. 1994; Lannfelt et al. 1994) and three PS1 (M146V) mutation carriers from a Finnish/Swedish family (Clark et al. 1995; Haltia et al.1994). In the Swedish APP 670/671 and PS1 M146V mutation families AD has been traced through eight and four generations, respectively.
Materials and Methods
Post mortem human brain tissues
Frontal, temporal, parietal and occipital cortices from 5 FAD cases with the Swedish APP670/671 double mutation and 3 cases with the PS1 M146V mutation were obtained from the Huddinge Brain Bank, Huddinge University Hospital, Sweden. Neuropathological examination of the brains of APP sw and PS1 mutation carriers confirmed the clinical diagnosis of AD in these families, and a large number of neuritic plaques were detected throughout the cortex (Bogdanovic et al. 2002; Lannfelt et al. 1994; Mann et al. 1996a; Marutle et al. 1999). Brain tissue from 9 sporadic AD cases and 18 control individuals were obtained from the Netherlands Brain Bank. Autopsies were performed on donors from whom written informed consent was obtained either from the donor or from next of kin. The clinical diagnosis of dementia was performed according to the NINCDS-ADRDA criteria (Mc Khann et al. 1984) and all subjects were confirmed with AD and met the established CERAD criteria. The control individuals had no known history or symptoms of neurological or psychiatric disorders. The individual case histories of the FAD subjects are listed in Table 1. Samples from each of the four cortical regions were not obtained from all cases.
Table 1.
Demografics of AD patients and non-demented controls
| Group | Gender | Apo E | Age of | Age at | Disease |
|---|---|---|---|---|---|
| Female/Male | genotyp | onset (ys) | death (ys) | duration (ys) | |
| PS1 | |||||
| 1 | F | 3/4 | 37 | 47 | 10 |
| 2 | F | 3/4 | 39 | 51 | 11 |
| 3 | F | 3/3 | 37 | 43 | 5 |
| mean±SE | 38±1* | 47±2* | 9±2 | ||
| APPsw | |||||
| 1 | M | 4/4 | 45 | 56 | 11 |
| 2 | F | 3/3 | 50 | 62 | 12 |
| 3 | M | 2/3 | 61 | 66 | 5 |
| 4 | M | 2/3 | 56 | 68 | 12 |
| 5 | M | 3/3 | 53 | 62 | 9 |
| mean±SE | 53±3 | 63±2 | 10±1 | ||
| Sporadic AD | 8F/1M | 3/3 (3) 3/2 (1) | 75.3a | 84±3 | 8.3b |
| 4/3 (2) 4/4 (3) | |||||
| Controls | 3F/3M | 3/3 (5) 4/3 (1) | 46±1 | ||
| Controls | 5F/2M | 3/3 (4) 4/3 (3) | 68±12 | ||
| Controls | 4F/1M | 3/4 (4) 3/2 (1) | 88±2 |
significant different from APPsw mutation carriers (P<0.01)
from Engelborghs et al. (2003), 504 patients
from Gomez-Isla et al. (1999), 51 patients
Measurements of A β levels
Brain tissues were homogenized in 7 volumes of 20 mM Tris-HCl, pH 8.5 including protease inhibitors (Complete, Roche Diagnostics) and then centrifuged at 100 000 × g at 4 °C for 1 h. The soluble fraction of Aβ40 and Aβ42 was measured in the supernatant. For quantification of Aβ in the insoluble fraction the pellet was extracted with 10 volumes of 5.0 M guanidine-HCl in 20 mM Tris-HCl, pH 8.0 and then diluted 1:10 with phosphobuffered saline containing 0.5% BSA, 0.05% Tween 20 and protease inhibitor (standard buffer) and centrifuged at 13,100 × g for 25 min at 4 °C. This guanidine-HCl-extractable fraction is hereafter referred as insoluble Aβ. The supernatants were further diluted with standard buffer plus 0.1 M guanidine-HCl before assays in order to analyse all samples in the linear range of the ELISA. The levels of Aβ40 and Aβ42 were analysed by colorimetric sandwich ELISA kits according to the manufacturer’s instructions (Signal Select™ Human β Amyloid 1–40 and 1–42 kit, respectively, BioSource International). The C-terminal specific ELISA uses a monoclonal capture antibody directed against the first 16 amino acid residues of the N-terminal region of human Aβ and two other antibodies specific for Aβ1–40 and Aβ1–42. Since the epitope is close to the N-terminal truncated end (starting at residue 11) there may be some capturing of truncated Aβ in addition to intact full-length Aβ. Hence, hereafter Aβ40 and Aβ42 indicate full-length forms and possible truncated Aβ forms. The Aβ40 and Aβ42 levels were calculated by comparison with a standard curve of synthetic human Aβ1–40 and 1–42. For each Aβ species quantified, all samples from the three AD groups and controls were run in the same ELISA. The Aβ levels were expressed as pg/mg tissue, calculated from the original brain weight measurement before homogenization.
Data analysis
The relationship between Aβ40 and Aβ42 and between Aβ and age were obtained by regression analysis where the Pearson's product-moment correlation coefficient was calculated. For group comparisons, data are given as mean values ± SE and analyzed by one-factor ANOVA followed by Fisher's PLSD post-hoc test.
Results
Distribution of insoluble (guanidine-extractable) and soluble A β 40 and A β 42 in familial and sporadic AD cases
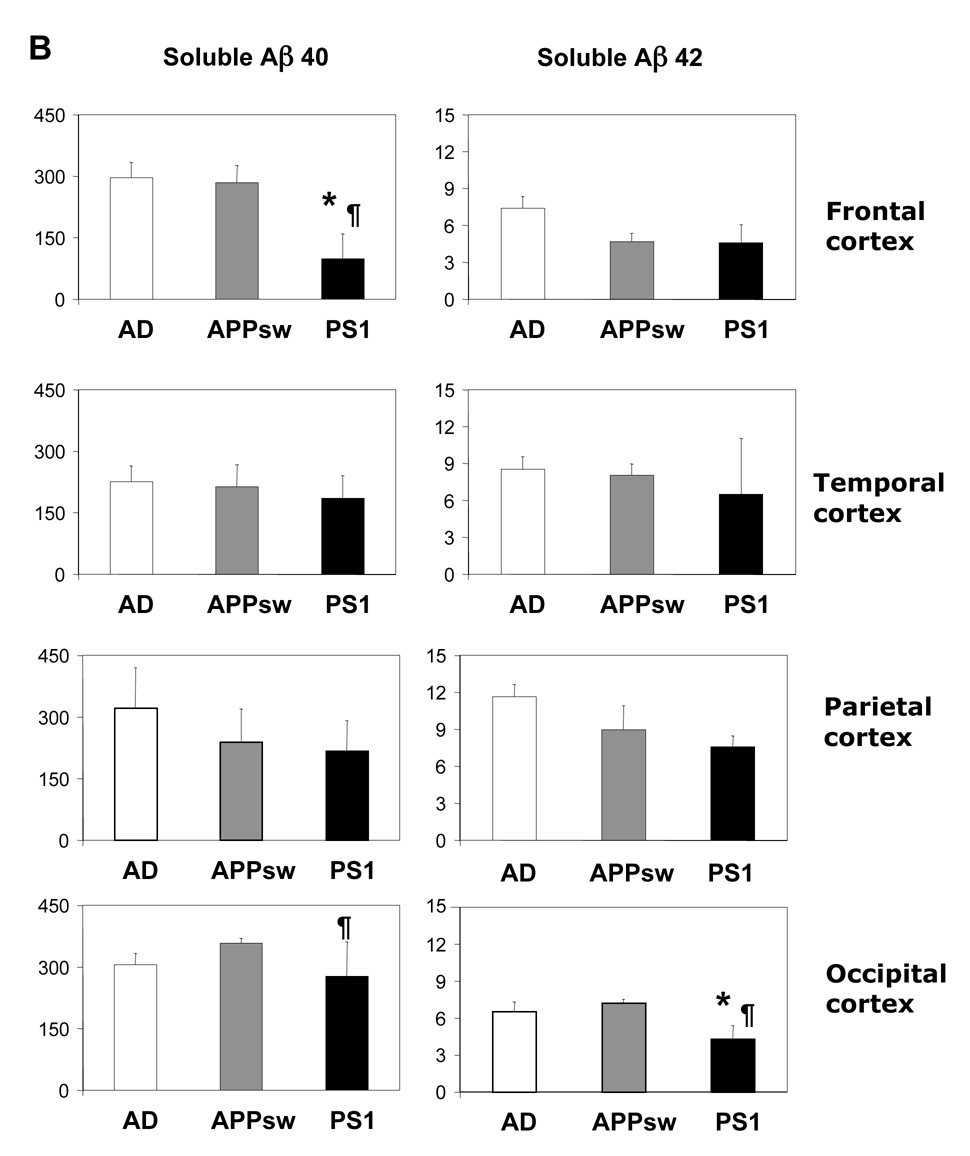
In both SAD cases and patients carrying the APPsw mutation the highest levels of insoluble Aβ40 were found in the frontal cortex followed by occipital cortex having just slightly lower Aβ40 levels (Figure 1A, Table 2). There was no significant difference in mean Aβ40 levels between the four cortical regions in SAD, but in APPsw brain the level in temporal cortex was significantly lower (P<0.05) compared to frontal and occipital cortex. In PS1 brain insoluble Aβ40 levels were highest in occipital cortex and the mean level was approximately 3-fold higher compared to frontal cortex (P<0.05) and 4.5-fold higher than parietal cortex (P<0.05). The highest levels of insoluble Aβ42 were in all three AD groups detected in frontal and occipital cortex, whereas the lowest Aβ42 levels were found in the parietal cortex. Soluble Aβ40 and Aβ42 was almost equally distributed between the four cortical regions except for the frontal cortex of PS1 brain, in which the levels of soluble Aβ40 were markedly lower.
Figure 1.
Comparison of insoluble (guanidine-extractable) (A) and soluble (B) Aβ40 and Aβ42 levels in the cortex of sporadic AD patients with patients carrying the APPsw and PS1 mutations. Results are given as mean values ± SE and expressed as pg/mg tissue.
* Significant different from sporadic AD cases, P≤ 0.05
¶ Significant different from APPsw mutation carriers, P≤ 0.05
Table 2.
Rank order of Aβ 40 and Aβ 42 levels in cortical regions of AD brain.
| Group | Order of brain regions | Group | Order of brain regions |
|---|---|---|---|
| Insoluble Aβ 40 | Insoluble Aβ 40 | ||
| SAD | F≈O>P>T | Controls, 83–94 ys | F>>O>P≈T |
| (3819; 3751; 2979; 2326) | (249; 55; 15; 13) | ||
| APPsw | F>O>P>T | Controls, 58–74 ys | F>>O≈T≈P |
| (4095; 3799; 2085; 1767) | (32; 4.1; 3.4; 2.4) | ||
| PS1 | O>>T≈F>P | Controls, 41–49 ysa | F>T≈P |
| (1624; 582; 525; 353) | (2.0; 1.4; 1.4) | ||
| Soluble Aβ 40 | Soluble Aβ 40 | ||
| SAD | P≈O≈F>T | Controls, 83–94 ys | T≈F>P>O |
| (322; 306; 296; 225) | (9.6; 9.5; 6.4; 4.3) | ||
| APPsw | O>F>P≈T | Controls, 58–74 ys | T>P>F≈O |
| (358; 284; 238; 231) | (3.9; 1.8; 0.5; 0.5) | ||
| PS1 | O>P>T>F | Controls, 41–49 ysa | T>P≈F |
| (277; 217;185; 99) | (0.5; 0.2; 0.1) | ||
| Insoluble Aβ 42 | Insoluble Aβ 42 | ||
| SAD | F>O≈T>P | Controls, 83–94 ys | F>>T>P>>O |
| (1379; 1117; 1094; 707) | (203; 122; 91; 8.0) | ||
| APPsw | O>F>T>P | Controls, 58–74 ys | F>>T>P>O |
| (809; 708; 575; 432) | (219; 128; 16.9; 8.2) | ||
| PS1 | F>O≈T>P | Controls, 41–49 ysa | T>P>F |
| (942; 504; 465; 260) | (13.2; 9,1; 4.3) | ||
| Soluble Aβ 42 | Soluble Aβ 42 | ||
| SAD | P>T>F>O | Controls, 83–94 ys | T>F≈P>O |
| (11.6; 8.5; 7.4; 6.5) | (8.6; 3.5; 3.5; 0.7) | ||
| APPsw | P≈T≈O>F | Controls, 58–74 ys | T>P>F>O |
| (8.9; 8.0; 7.2; 4.7) | (4.5; 3.5; 2.1; 0.6) | ||
| PS1 | P≈T>F≈O | Controls, 41–49 ysa | T>P≈ F |
| (7.6; 6.5; 4.6; 4.3) | (0.9; 0.1; 0.0) | ||
Mean values are given in parenthesis as pg/mg tissue. F, frontal: T, temporal; P, parietal, O, occipital cortex
Occipital cortex from the youngest control group was not available
Comparison of insoluble (guanidine-extractable) and soluble Aβ 40 and Aβ 42 levels in familial and sporadic AD cases
The mean levels of insoluble Aβ40 in the brain of APPsw mutation carriers did not differ much from the levels detected in SAD (Figure 1A), whereas the levels of insoluble Aβ42 were significant lower in the frontal and temporal cortex of patients carrying the APPsw mutation compared with SAD. The levels of both insoluble Aβ40 and Aβ42 were significant lower in all four cortical regions of PS1 mutation carriers compared with SAD brain, and insoluble Aβ40 was also significantly lower in frontal cortex and occipital cortex compared with APPsw brain. There were less striking differences between the three AD groups in the levels of soluble Aβ (Figure 1B).
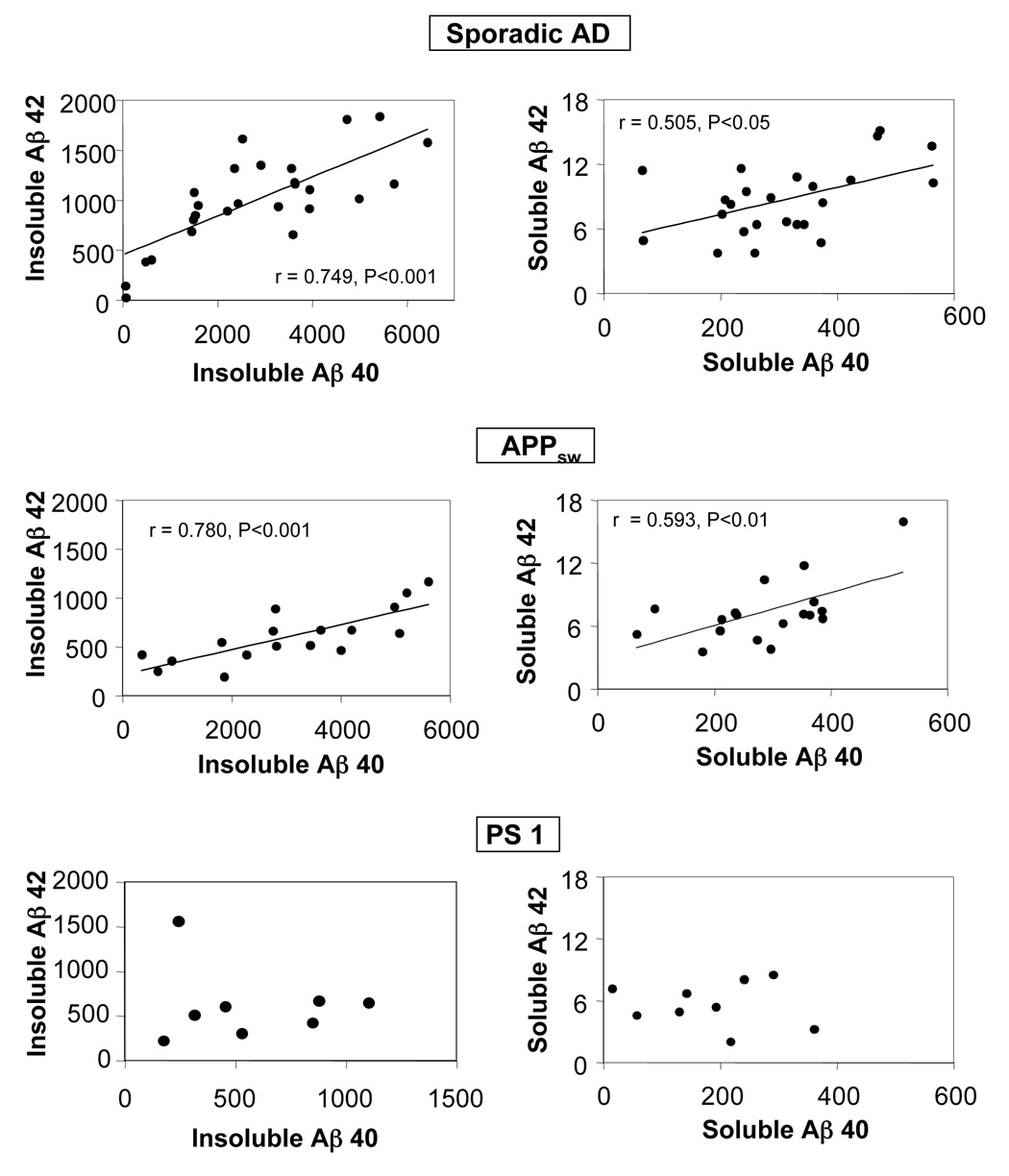
A positive correlation between Aβ40 and Aβ42 levels in both SAD and APPsw brain was observed when data from all four regions were included (Figure 2). In contrast, no tendency of a relationship between these Aβ species was observed in the brain of PS1 mutation carriers.
Figure 2.
Relationship between Aβ40 and Aβ42 levels in the cortex of sporadic AD patients and patients carrying the APPsw and PS1 mutations. Data from all four cortical regions are included in the regression analysis. r indicates Pearson's product-moment correlation coefficient. The Aβ levels are expressed as pg/mg tissue
Ratio of Aβ 42/Aβ 40
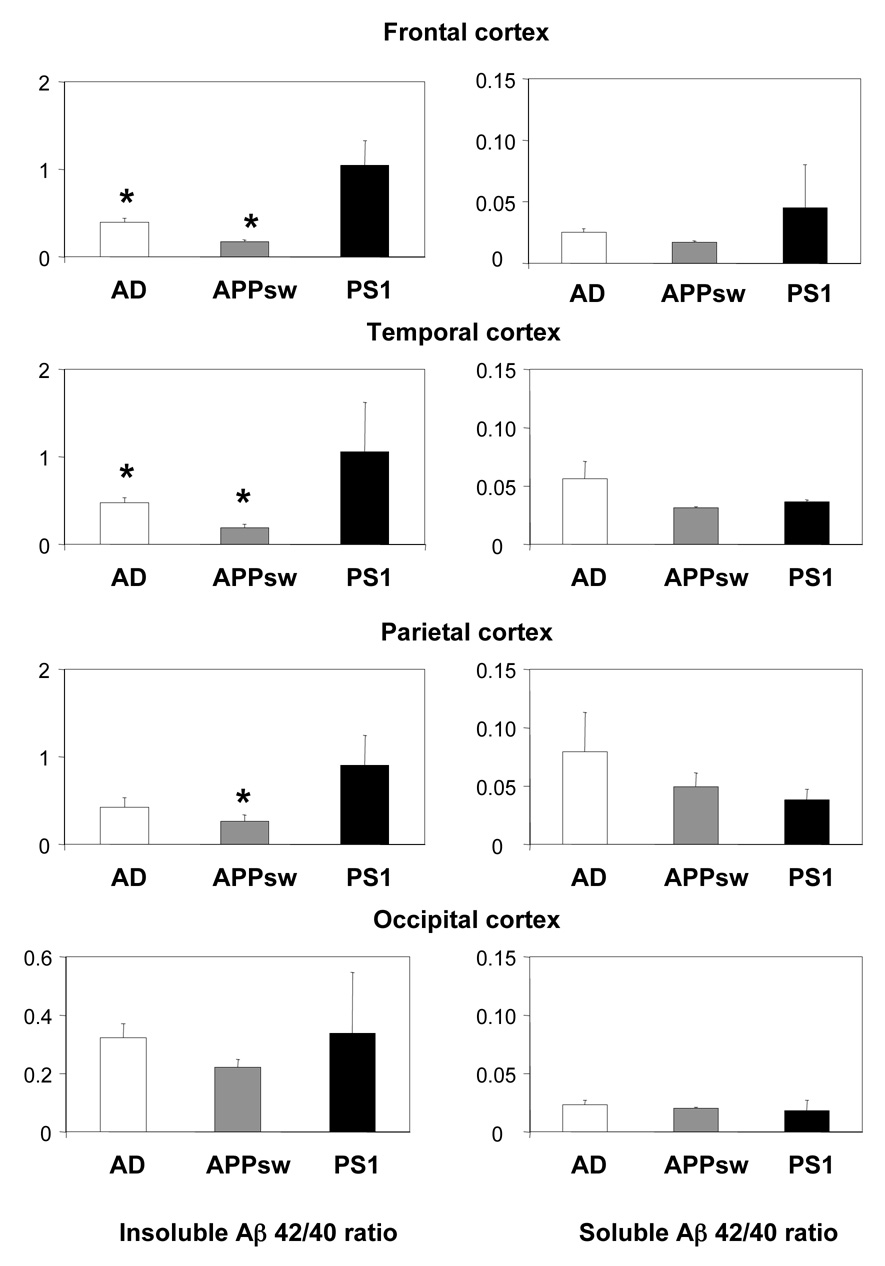
The ratio of insoluble Aβ42/40 did not differ between SAD and APPsw. However, in PS1 mutation carriers this ratio was significantly higher in the frontal and temporal cortex compared to SAD and APPsw, and also significantly higher in parietal cortex compared with APPsw (Figure 3). No significant differences were found in the soluble Aβ42/40 ratio between the three AD groups (Figure 3).
Figure 3.
Comparison of Aβ40/Aβ42 ratio in the cortex of sporadic AD patients with patients carrying the APPsw and PS1 mutations. Results are given as mean values ± SE.
* Significant different from patients carrying the PS1 mutation, P≤ 0.05
Distribution and levels of Aβ 40 and Aβ 42 in non-demented control individuals
Three groups (younger 41–49 years, middle aged 58–74 years, and older 83–94 years) of non-demented individuals aged-matched with PS1, APPsw and SAD, respectively, were investigated. In the oldest and middle-aged group the highest levels of both insoluble Aβ40 and Aβ42 were detected in the frontal cortex, whereas in younger controls Aβ were more equally distributed in the cortical regions (Table 2). The highest levels of both soluble Aβ40 and Aβ42 were found in the temporal cortex. In the youngest control group the levels of soluble Aβ were in all regions below 1 pg/mg tissue or undetectable.
There was an age-dependent increase of insoluble Aβ40 in control brains (Figure 4A), and regression analysis revealed a significant positive correlations between Aβ40 and age in frontal, temporal and parietal cortex (r=0.701, P< 0.01, r=0.685, P< 0.01 and r=0.621, P< 0.05, respectively), and between Aβ42 and age in the parietal cortex (r=0.679, P< 0.01). The age-dependent pattern for soluble Aβ40 (Figure 4B) was similar to that for insoluble Aβ40, whereas soluble Aβ42 increased with age in frontal and temporal cortex (r=0.632, P< 0.05 and r=0.768, P< 0.01, respectively). The Aβ levels in the control groups were significantly lower that those detected in PS1, APPsw and SAD brain.
Figure 4.
Levels of insoluble (guanidine-extractable) (A) and soluble (B) Aβ40 and Aβ42 in the cortex of normal healthy individuals. Occipital cortex was not obtained from the youngest group of controls. Soluble Aβ42 was undetectable in frontal cortex of the youngest control group. Results are given as mean values ± SE and expressed as pg/mg tissue.
* Significant different from the 83–94 ys group, P≤ 0.05
¶ Significant different from the 58–74 ys group, P≤ 0.05
Discussion
This study was undertaken to investigate differences in the quantity and distribution of Aβ in post mortem brain between SAD, APPsw and PS1 mutations carriers. In earlier studies the number of Aβ deposits has usually been quantified by immunohistochemical methodologies and very few studies have used sensitive ELISA methods to measure soluble and insoluble Aβ. In addition, neuropathological observations have been based on small series of patients or sometimes on individual cases carrying different PS1 or APP mutations. The low number of cases earlier reported and to some extent also in the present study confers limitations in drawing any firm conclusions on the pathological phenotypes. One should keep in mind that a considerable variation in Aβ has been observed even in family members with the identical PS1 mutation (Gomez-Isla et al. 1999; Mann et al 2001).
In the present study, we found that the levels of Aβ40 and Aβ42 detected in APPsw brain were not much different from those in SAD brain. In both groups the Aβ42/Aβ40 ratio, insoluble Aβ40 and soluble Aβ levels were similar. These findings are in agreement with a previous immunohistochemical study that included 3 out of the 5 cases investigated here (Mann et al. 1996b). The number of Aβ40 and Aβ42 immunoreactive amyloid deposits and the percentage area of amyloid in frontal cortex, as well as the Aβ40/42 ratio were reported to be similar in APPsw mutation carriers and SAD. Our results also fit well with data from studies on cells transfected with the APPsw mutation demonstrating several fold higher levels of secreted Aβ 40 and Aβ 42 than cells expressing wild-type APP, but with unchanged Aβ42/40 ratio (Citron et al 1992; Takeda et al. 2004).
The levels of Aβ40 were much lower in PS1 brain compared with SAD and APPsw brain, which in turn resulted in a significantly higher Aβ42/Aβ40 ratio in PS1 brain. Earlier studies have demonstrated that PS1 mutation carriers have a significant higher number of Aβ42 and Aβ40 deposits in brain compared to SAD patients, although some of the SAD brains deposited more Aβ42 and Aβ40 than some PS1 mutation carriers (Kumar-Sing, 2006; Mann et al 1996a). However, it is not entirely correct to compare the mean plaque densities based on cases of different PS1 mutations with SAD, since it is now evident that the effect on Aβ production is dependent upon the individual PS1 mutation. Mann et al. (2001) showed an increased immunostaining for Aβ42 but not Aβ40 in the frontal cortex of 54 cases carrying 25 different PS1 mutations in comparison with SAD. In one case carrying the same PS1 mutation (M146V) as investigated in our study, the percentage area of frontal cortex occupied by Aβ42 was lower in comparison with many other PS1 mutation carriers and Aβ40 was even lower than the average value for SAD. Although based on only one individual case, these results are in agreement with our present findings obtained using sensitive ELISA measurements, indicating that not all PS1 mutations result in elevated Aβ42 levels compared to SAD. Furthermore in consistence with our findings there was no correlation between the amounts of Aβ40 and Aβ42 within plaques across the 54 cases with different PS1 mutations.
Studies in cells expressing PS1 mutations have shown that the effect on Aβ generation and the extent of the increase in Aβ42/40 ratio seem to be dependent upon the individual PS1 mutation, similar to findings in brain of PS1 mutation carriers. Kumar-Sing et al. (2006) found that several PS1 mutations failed to increase Aβ 42 and some mutations caused significant reductions in the production of Aβ40. Similar, Shimojo et al. (2007) reported that PS1 mutants significantly reduced Aβ secretion, and that Aβ40 production was reduced more than Aβ42. However, caution in comparing findings in transfected cells with post mortem brain is necessary since in most cell models only soluble Aβ released into media and not intracellular Aβ, have been measured.
In normal aging, a slow progressive increase of Aβ levels is seen over decades. A predominance of Aβ42 in diffuse plaques has been found in the brains of elderly subjects, in the absence of signs of neuronal degeneration or dementia (Hellstrom-Lindahl et al. 2004, 2008; Price and Morris 1999; Thal et al. 2000). In agreement with previous findings (Funato et al. 1998; Hellstrom-Lindahl et al. 2008; Morishima-Kawashima et al. 2000) we found a strong correlation between insoluble Aβ40 levels and age in cortex of normal brain. Levels of Aβ42 seemed to reach a plateau at higher age while there was no apparent ceiling for Aβ40 accumulation confirming our previous observations in non-demented individuals (Hellstrom-Lindahl et al. 2008). Our results indicate that during normal aging insoluble Aβ40 starts to accumulate especially in frontal cortex and increases markedly with age, whereas insoluble Aβ42 is highest in the temporal cortex before 50 years and then increases dramatically with age in the frontal cortex.
In contrast to immunohistochemistry, ELISA analysis does not distinguish between Aβ from brain parenchyma and blood vessels. Cerebral amyloid angiopathy (CAA) is characterized by deposits of Aβ surrounding and inside cerebral and meningeal blood vessels. The major amyloid peptide species within blood vessels appears to be Aβ40 with lesser amounts of Aβ42 being present (Mann et al. 1996b). These deposits occur in about a third of non-demented elderly individuals, but are found in about 90% of AD patients (Ray et al. 1998). Thus, a variable extent of CAA may therefore influence the detected levels of parenchymal Aβ40 when using the ELISA methodology. Aβ 40 has been shown to be the predominant species deposited in AD brains with typically prominent CAA (Gravina et al., 1996, Kawarabayashi et al. 2001). In a recent study (Svedberg et al. unpublished) we found that CAA was highly present in most of the SAD cases investigated here, and this is probably the main reason to the somewhat unexpected finding that the detected levels of insoluble Aβ40 was higher than Aβ42. Neuropathological assessments have shown that CAA was present to a variable and only moderate degree in some of APPsw mutation carriers investigated here (Mann et al. 1996b). In PS mutation carriers the extent of CAA seems to be related to mutational position, with a heavier amyloid angiopathy in cases with mutations occurring after codon 200 (Mann et al. 2001). Accordingly, the rating of CAA in one case with the PS1 M146/V mutation was lower compared to many other PS1 mutations examined (Mann et al. 2001). Another difference between immunohistochemistry and ELISA is that immunostaining of sections may also identify N-truncated Aβ42 typically present in diffuse plaques (Dickson, 1997; Iwatsubo et al., 1996) while the ELISA method used in our study, measured mainly full-length Aβ40 and Aβ42.
Our findings indicate that the pattern of Aβ deposition in PS1 brain is apparently different from that in both APPsw and SAD, whereas the pattern in APPsw mutation carriers is more similar to that observed in SAD. The different levels of Aβ in PS1 compared with APPsw and SAD cases do not appear to be a reflection of differences in the duration of disease since this was similar in all three groups (8–10 years). The earlier age of onset and more aggressive course of PS-linked FAD in comparison to APP-linked FAD cannot solely be explained by elevated Aβ levels since APP mutations often elicit a far greater elevation in Aβ levels, evident in the present study. Even though total Aβ levels are lower in individuals carrying the PS1 M146V mutation compared to SAD and APPsw, a shift from shorter to longer Aβ species might lead to neurotoxicity in the brain of these individuals. It has been shown in transgenic mice that Aβ42 has considerable impact on both the rate of amyloid deposition and the age at which amyloid deposition first appear (Borchelt et al. 1997; Jankowsky et al. 2004). In addition to rapid fibril formation, Aβ42 has a strong propensity to form soluble Aβ oligomers (Chen and Glabe, 2006; Xia et al. 1997), which may disrupt synaptic transmission (for review, see Haas and Selkoe 2007; Walsh and Selkoe 2007). Earlier reports have shown weak or no correlation between plaque density and severity of cognitive impairment (Arrigada et al. 1992; Terry et al. 1991) while more recent studies have reported a correlation between soluble Aβ levels and the extent of synaptic loss or degree of dementia (Lue et al. 1999; Wang et al. 1999). Interestingly, we found that in PS1 brain the levels of soluble Aβ42, in contrast to insoluble Aβ42, did not differ much from those found in APPsw and SAD brain and soluble Aβ42 might therefore be related to the early onset of the disease in these PS1 mutation carriers. Recent lines of evidence have suggested that PS mutations, also could cause partial PS loss-of-function which may contribute to neurodegeneration (Bentahir et al. 2006; De Strooper 2006; Shen and Kelleher, 2007). Studies with AD transgenic mice models have indicated that Aβ40 might mediate protective functions by keeping Aβ42 from aggregating. Reduction in brain Aβ 40 levels with no change in Aβ42 levels have been shown to exacerbate amyloid plaque pathology, while increased steady-state levels of Aβ40 decreased Aβ deposition and toxicity (Deng et al. 2006; Kim et al. 2007; McGowan et al. 2005; Mucke et al. 2000). We found that the Aβ40 levels were decreased to a much higher extent than Aβ42 in brains of PS1 mutation carriers. The insoluble Aβ40 levels were in frontal, temporal and parietal cortex of PS1 brain only 12,14 and 12%, respectively, of the levels detected in SAD, while the corresponding values for Aβ42 were 68, 43 and 37%, respectively. It is therefore possible that the reduction in Aβ40 like we observed here in the PS1 L146V mutation carriers may actually worsen the disease course. Our findings suggests that the ability of PS1 mutations to cause AD does not seem to be directly related to an increase in the total load of Aβ in brain in comparison to APPsw and SAD, but the relative contribution of short and long Aβ species could be more important in promoting neurodegeneration in these PS1 mutation carriers.
Acknowledgements
This study was supported by grants from the Swedish Research Council, Loo and Hans Osterman’s Foundation, Swedish Alzheimer Foundation, KI Foundations, Gun and Bertil Stohne’s Foundation, Brain Foundation, Demensfonden and Stiftelsen för Gamla tjänarinnor.
References
- Arrigada PV, Growdon JH, Hedely WE, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Axelman K, Basun H, Winblad B, Lannfelt L. A large Swedish family with Alzheimer's disease with a codon 670/671 amyloid precursor protein mutation: A clinical and genealogical investigation. Arch. Neurol. 1994;51:1193–1197. doi: 10.1001/archneur.1994.00540240037013. [DOI] [PubMed] [Google Scholar]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horré K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can effect γ-secretase activity by different mechanisms. J. Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- Bogdanovic N, Corder E, Lannfelt L, Winblad B. APOE polymorphism and clinical duration determine regional neuropathology in Swedish APP670,671 mutation carriers: implications for late-onset Alzheimer's disease. J. Cell Mol. Med. 2002;6:199–214. doi: 10.1111/j.1582-4934.2002.tb00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratoitsky T, Prada C-M, Kim G, Seekens S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins N, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Chen YR, Glabe CG. Distinct early folding and aggregation properties of Alzheimer amyloid-β peptides Aβ 40 and Aβ 42: stable trimer or tetramer formation by Aβ 42. J. Biol. Chem. 2006;281:24414–24422. doi: 10.1074/jbc.M602363200. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Liberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser R, St. George-Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer's diease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat. Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Clark RF, Hutton M, Fuldner RA, Froelich S, Karran E, Talbot C, Crook R, Lendon C, Prihar G, He C, Korenblat K, Martinez A, Wragg M, Busfield F, Behrens MI, Myers A, Norton J, Morris J, Mehta N, Pearson C, Lincoln S, Baker M, Duff K, Zehr C, Perez-Tur J, Houlden H, Ruiz A, Ossa J, Lopera F, Arcos M, Madrigal L, Collinge J, Humphreys C, Ashwoth T, Sarner S, Fox N, Harvey R, Kennedy A, Roques P, Cline RT, Philips CA, Venter JC, Forsell L, Axelman K, Lilius L, Johnston J, Cowburn R, Viitanen M, Winblad B, Kosik K, Haltia M, Poyhonen M, Dickson D, Mann D, Neary D, Snowden J, Lantos P, Lannfelt L, Rossor M, Roberts GW, Adams MD, Hardy J, Goate A. The structure of the presenilin 1 (S 182) gene and identification of six novel mutations in early onset AD families. Nat. Genet. 1995;11:219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- Deng Y, Tarassishin L, Kallhoff V, Peethumnongsin E, Wu L, Li M-W, Zheng H. Deletion of presenilin 1 hydrophilic loop sequence leads to impaired γ-secretase activity and exacerbated amyloid pathology. J. Neurosci. 2006;26:3845–3854. doi: 10.1523/JNEUROSCI.5384-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. EMBO reports. 2006;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Duering M, Grimm MO, Grimm HS, Schröder J, Hartmann T. Mean age of onset in familial Alzheimer's disease is determined by amyloid beta 42. Neurobiol. Aging. 2005;26:785–788. doi: 10.1016/j.neurobiolaging.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta 42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Aβ amyloid peptides. Peptides. 2002;23:1285–1297. doi: 10.1016/s0196-9781(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R, Ihara Y. Quantitation of amyloid β-protein (Aβ) in the cortex during aging and in Alzheimer’s disease. Am. J. Pathol. 1998;152:1633–1640. [PMC free article] [PubMed] [Google Scholar]
- Gómez-Isla T, Growdon WB, McNamara MJ, Nochlin D, Bird TD, Arango JC, Lopera F, Kosik KS, Lantos PL, Cairns NJ, Hyman BT. The impact of different presenilin 1 and presenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuron loss in the familial Alzheimer's disease brain: Evidence for other phenotype-modifying factors. Brain. 1999;122:1709–1719. doi: 10.1093/brain/122.9.1709. [DOI] [PubMed] [Google Scholar]
- Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr., Younkin LH, Suzuki N, Younkin SG. Amyloid β protein (Aβ) in Alzheimer's disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ40 or Aβ42(43) J. Biol. Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Haltia M, Viitanen M, Sulkava R, Ala-Hurula V, Poyhonen M, Goldfarb L, Brown P, Levy E, Houlden H, Crook R, Goate A, Clark R, Korenblat K, Pandit S, Keller HD, Lilius L, Liu L, Axelman K, Forsell L, Winblad B, Lannfelt L, Hardy J. Chromosome 12-encoded Alzheimer's disease: Genetic and clinicopathological description. Ann. Neurol. 1994;36:362–367. doi: 10.1002/ana.410360307. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E, Mousavi M, Ravid R, Nordberg A. Reduced levels of Aβ 40 and Aβ 42 in brains of smoking controls and Alzheimer’s patients. Neurobiol. Disease. 2004;15:351–360. doi: 10.1016/j.nbd.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E, Ravid R, Nordberg A. Age-dependent decline of neprilysin in Alzheimer's disease and normal brain: inverse correlation with Aβ levels. Neurobiol. Aging. 2008;29:210–221. doi: 10.1016/j.neurobiolaging.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Saido TC, Mann DMA, Lee VM-Y, Trojanowsky JQ. Full-length amyloid-β-(1–42(43) and amino-terminally modified and truncated amyloid β42 (43) deposit in diffuse plaques. Am. J. Pathol. 1996;149:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ-secretase. Hum. Mol. Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Hsiao Ashe K, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta 40 inhibits amyloid deposition in vivo. J. Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Theuns J, Van Broeck B, Pirici D, Vennekens K, Corsmit E, Cruts M, Dermaut B, Wang R, Van Broeckhoven C. Mean age of onset of familial Alzheimer disease caused by presenilin mutations correlates with both increased Aβ42 and decreased Aβ 40. Hum. Mut. 2006;27:686–695. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- Lannfelt L, Bogdanovic N, Appelgren H, Axelman K, Lilius L, Hansson G, Schenk D, Hardy J, Winblad B. Amyloid precursor protein mutation causes Alzheimer's disease in a Swedish family. Neurosci. Lett. 1994;168:254–256. doi: 10.1016/0304-3940(94)90463-4. [DOI] [PubMed] [Google Scholar]
- Lleó A, Berezovska O, Growdon JH, Hyman BT. Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS-1 mutations. Am. Geriatr. Psychiatry. 2004;12:146–156. doi: 10.1097/00019442-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Saunders AM, Smith TW, Swearer JM, Drachman DA, Ghetti B, Nee L, Pulaski-Salo D, Dickson D, Robitaille Y, Bergeron C, Crain B, Benson MD, Farlow M, Hyman BT, St George-Hyslop P, Roses AD, Pollen DA. Familial and sporadic Alzheimer's disease: Neuropathology cannot exclude a final common pathway. Neurology. 1996;46:406–412. doi: 10.1212/wnl.46.2.406. [DOI] [PubMed] [Google Scholar]
- Lue L-F, Kuo Y-M, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Iwatsubo T, Cairns NJ, Lantos PL, Nochlin D, Sumi SM, Bird TD, Poorkaj P, Hardy J, Hutton M, Prihar G, Crook R, Rossor MN, Haltia M. Amyloid β protein (Aβ) deposition in chromosome 14-linked Alzheimer's disease: Predominance of Aβ 42(43) Ann. Neurol. 1996a;40:149–156. doi: 10.1002/ana.410400205. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Iwatsubo T, Ihara Y, Cairns NJ, Lantos PL, Bogdanovic N, Lannfelt L, Winblad B, Maat-Schieman MLC, Rossor MN. Predominant deposition of amyloid-β42(43) in plaques in cases of Alzheimer's disease and hereditary cerebral hemorrhage associated with mutations in the amyloid precursor protein gene. Am. J. Pathol. 1996b;148:1257–1266. [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Pickering-Brown SM, Takeuchi A, Iwatsubo T the members of the Familial Alzheimer's Disease Pathology Study Group. Amyloid angiopathy and variability in amyloid β deposition is determined by mutation position in presenilin-1-linked Alzheimer's disease. Am. J. Pathol. 2001;158:2165–2175. doi: 10.1016/s0002-9440(10)64688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutle A, Warpman U, Bogdanovic N, Lannfelt L, Nordberg A. Neuronal nicotinic receptor deficits in Alzheimer patients with the Swedish amyloid precursor protein 670/671 mutation. J. Neurochem. 1999;72:1161–1169. doi: 10.1046/j.1471-4159.2000.0721161.x. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Aβ 42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Khann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of NINCDS-ADRDA Work Group under the auspices of department of health and human services task forces on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Menédez M. Pathological and clinical heterogeneity of presenilin 1 gene mutations. J. Alz. Dis. 2004;6:475–482. doi: 10.3233/jad-2004-6503. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, Ihara Y. Effect of apolipoprotein E allele ε4 on the initial phase of amyloid β-protein accumulation in the human brain. Am. J. Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConloge L. High-level neuronal expression of Abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama O, Tomita T, Nihonmatsu N, Murayama M, Sun X, Honda T, Iwatsubo T, Takashima A. Enhancement of amyloid β 42 secretion by 28 different presenilin 1 mutations of familial Alzheimer's disease. Neurosci. Lett. 1999;2665:61–63. doi: 10.1016/s0304-3940(99)00187-1. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Ashall F, Goate AM. Molecular pathogenesis of sporadic and familial forms of Alzheimer's disease. Mol. Med. Today. 1998;4:151–157. doi: 10.1016/s1357-4310(98)01229-5. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer’s disease: Molecular understanding predicts amyloid-based therapeutics. Ann. Rev. Pharmacol. Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkai P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ. The presenilin hypothesis of Alzheimer's disease: Evidence for loss-of-function pathogenic mechanism. Proc. Natl. Acad. Sci. USA. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo M, Sahara N, Murayama M, Ichinose H, Takashima A. Decreased Aβ secretion by cells expressing familial Alzheimer's disease-linked mutant presenilin 1. Neurosci. Res. 2007;57:446–453. doi: 10.1016/j.neures.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Takeda K, Araki W, Tabira T. Enhanced generation of intracellular Aβ42 amyloid peptide by mutation of presenilins PS1 and PS2. Eur. J. Neurosci. 2004;19:258–264. doi: 10.1111/j.0953-816x.2003.03135.x. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, Fraser PE, Ishii K, Sahara N, Ozawa K, Ikeda M, Saunders AM, Komatsuzaki Y, Sherrington R, Levesque G, Yu G, Rogaeva E, Shoji S, Nee LE, Pollen DA, Hendriks L, Martin JJ, Van Broeckhoven C, Roses AD, Farrer LA, St. George-Hyslop PH, Mori H. Amyloid-β-protein isoforms in brain of subjects with PS1-linked, βAPP-linked and sporadic Alzheimer disease. Mol Brain Res. 1998;56:178–185. doi: 10.1016/s0169-328x(98)00044-8. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physiological bases of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ oligomers - decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM-Y. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 1999;58:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ. Enhanced production by Chinese hamster ovary cells stably expressing mutant presenilins. J. Biol. Chem. 1997;272:7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]