Abstract
Purpose
CTLA-4 blockade is being explored in numerous clinical trials as an immune based therapy for different malignancies. Our group conducted the first pre-operative clinical trial with the anti-CTLA-4 antibody ipilimumab in 12 patients with localized urothelial carcinoma of the bladder.
Experimental Design
Six patients were treated with 3mg/kg/dose of anti-CTLA-4 and six patients were treated with 10mg/kg/dose of antibody. Primary endpoints of the study were safety and immune monitoring.
Results
Most drug-related adverse events consisted of grade 1/2 toxicities. All patients had measurable immunologic pharmacodynamic effects, consisting of an increased frequency of CD4+ICOShi T cells in tumor tissues and the systemic circulation. To determine if CD4+ICOShi T cells could be a correlative marker for clinical outcome after treatment with anti-CTLA-4, a cohort of metastatic melanoma patients was studied retrospectively for frequency of CD4+ICOShi T cells and survival. Data from this small cohort of patients indicated that an increased frequency of CD4+ICOShi T cells, sustained over a period of 12 weeks of therapy, correlates with increased likelihood of clinical benefit consisting of overall survival.
Conclusions
Our trial demonstrates that anti-CTLA-4 therapy has a tolerable safety profile in the pre-surgical setting and that a pre-operative model can be used to obtain biological data on human immune responses, which can efficiently guide the monitoring of patients treated in the metastatic disease setting.
Introduction
Cytotoxic T lymphocyte associated antigen (CTLA-4) plays a critical role in the regulation of T cell activation (1-4). Blockade of CTLA-4 has led to enhanced T cell activation in animal models (5, 6) and mechanistic studies have shown that anti-CTLA-4 treated animals have an increased ratio of effector to regulatory T cells, which correlates with tumor regression (7). Moreover, the concept of CTLA-4 blockade, termed checkpoint blockade, has been used in the clinical setting and has shown promise in the induction of anti-tumor responses in patients with melanoma, prostate cancer, and lymphoma (8-15).
Prior clinical trials with anti-CTLA-4 therapy enrolled patients with metastatic disease, who rarely undergo surgical biopsies or procedures; therefore, there were limitations in accessing sufficient tumor tissues for phenotypic and functional immunologic studies. Laboratory studies from these prior trials focused primarily on assessing immune responses in peripheral blood; however, these studies have not led to the identification of immunologic markers that clearly correlate with clinical outcome. To circumvent these issues, we designed a clinical trial using anti-CTLA-4 in the pre-operative setting so that we may obtain tumor tissues for immunologic studies and attempt to identify biomarkers in peripheral blood that might correlate with those in tumor tissues. The primary aim of our study was to establish the safety and feasibility of using anti-CTLA-4 in the pre-operative setting.
Prior clinical trials reported adverse events associated with anti-CTLA-4 therapy consisting of tissue specific inflammatory conditions termed immune-related adverse events (irAEs), which have included dermatitis, hepatitis, colitis, pancreatitis, hypophysitis, inflammatory myopathy and uveitis (16-19). In our pre-surgical study, we found that anti-CTLA-4 had a tolerable safety profile without an increase in peri-operative complications after a short course of antibody. We observed grade 1/2 rash and diarrhea as the most common drug-related side effects.
The overall purpose of our research effort was to examine the immunologic profile in peripheral blood and corresponding tumor tissues of treated patients in order to identify clinically useful biomarkers. We found that CTLA-4 blockade led to an increased frequency of CD4+ICOShi T cells in tumor tissues that could be correlated with an increased frequency of these cells in the peripheral blood.
Inducible costimulator (ICOS), a T cell specific molecule that is a close homologue of CD28 and CTLA-4, has been predominantly thought of as a marker of T cell activation associated with follicular helper T cells (20-22). However, we previously demonstrated that CD4+ICOShi T cells from peripheral blood samples (18) and tumor tissues (23) contained a population of cells that could produce interferon gamma (IFN-γ) and recognize the NY-ESO-1 tumor antigen. We now report that increases in CD4+ICOShi T cells were more pronounced after treatment with 10 mg/kg/dose of antibody, with concomitant increases in CD8+ICOShi T cells, which were not observed after treatment with 3 mg/kg/dose of antibody.
To determine if CD4+ICOShi T cells could be a correlative marker for clinical outcome after treatment with anti-CTLA-4, a cohort of metastatic melanoma patients were studied retrospectively for frequency of CD4+ICOShi T cells and survival. Data from this small cohort of patients indicated that an increased and sustained frequency of CD4+ICOShi T cells correlated with improved overall survival.
Results
Study Population Characteristics
The study population consisted of 12 patients (10 males and 2 females) with localized urothelial carcinoma of the bladder who provided informed consent to participate on an institutional research board (IRB) approved clinical trial in which the anti-CTLA-4 antibody ipilimumab was administered in the pre-operative setting. All patients were candidates for radical cystectomy as treatment for their disease. Demographics and patient characteristics are detailed in Table 1. Patients had pT1-T2 urothelial carcinoma, high-grade, as observed on initial biopsies (Table S1). Pre-therapy and post-therapy blood samples as well as surgical samples were obtained as per protocol (Figure S1).
Table 1.
Clinical characteristics of patients with localized urothelial carcinoma who received anti-CTLA-4.
| Patient | Sex | Age | Prior Therapy | Adjuvant Therapy | Drug-Related irAEs | Surgery Delay (wks) | Follow-Up (mos) | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 66 | BCG | None | Rash, Gr 1 | None | 33.37 | NED Alive |
| 2 | M | 75 | None | Cis, Gem, Ifos chemo | None | 5.1 (due to cardiac eval) | 32.67 | NED Alive |
| 3 | M | 71 | BCG | None | Amylase & Lipase Increased, Gr 1 | None | 28.83 | NED Alive |
| Uveitis, Gr 2 Ischemic Papillitis, Gr 3, Diarrhea Gr 1 | ||||||||
| 4 | M | 60 | None | MVAC chemo | None | None | 27.3 | NED Alive |
| 5 | M | 55 | None | None | Rash, Gr 1 | None | 24.9 | NED Alive |
| 6 | M | 75 | BCG | None | Rash, Gr 2 | None | 23.1 | NED Alive |
| 7 | M | 76 | None | None | Rash, Gr 1 | None | 7.7 | NED Deceased |
| Testicular swelling/Epididymitis, Gr 2 | ||||||||
| 8 | F | 69 | None | None | Rash, Gr 1 | 4.0 (due to irAE) | 17.5 | NED Alive |
| Transaminitis, Gr 3 | ||||||||
| Diarrhea, Gr 2 | ||||||||
| 9 | M | 63 | None | None | Diarrhea, Gr 2 | None | 17.03 | NED Alive |
| 10 | F | 68 | None | None | Diarrhea, Gr 3 (Received only one dose of antibody) | 10.3 (due to irAE and cardiac & GI eval) | 12.23 | NED Alive |
| 11 | M | 71 | BCG | Ifos-Adria-Gem chemo | Rash, Gr 1, Diarrhea, Gr 3 | N/A * | 9.27 | Metastatic Disease Alive |
| 12 | M | 66 | None | Gem-Cis chemo | Diarrhea, Gr 2 | None | 8.33 | Metastatic Disease Alive |
UC=Urothelial Carcinoma, Dx=Disease, BCG = Bacillus Calmette-Guerin given as intravesical therapy Cis=Cisplatin, Gem=Gemcitabine, Ifos=Ifosfamide, MVAC=Methotrexate, Vinblastine, Adriamycin, Cisplatin, Adria=Adriamycin, NED=No Evidence of Disease, irAE=immune related adverse event GI=gastrointestinal, Gr=Grade.
Surgery cancelled.
Surgical Delays
The initial cohort of 6 patients received 3 mg/kg/dose of ipilimumab for two doses, with a 3-week interval between doses, and were scheduled for surgery 4 weeks after the last dose of antibody. In this cohort, one patient had a delay in surgery due to pre-operative cardiac workup, but there were no significant surgical delays due to irAEs in the 3 mg/kg/dose cohort (Table 1).
A second cohort of 6 patients received 10 mg/kg/dose of antibody for one (N=1) or two doses (N=5) prior to surgery. Surgical delays occurred in two cases as a result of development of irAEs. One patient (Patient #8, Table 1) had a 4-week delay due to grade 2 diarrhea that occurred after the second dose of antibody and subsequently resolved with steroid therapy. Another patient (Patient #10, Table 1) had a 10-week delay due to multiple factors, including grade 3 diarrhea after the first dose of antibody, which subsequently resolved with steroid therapy, as well as pre-operative cardiac and gastrointestinal workup that were necessary due to multiple prior abdominal surgeries. A third patient (Patient #11, Table 1) in the 10mg/kg/dose cohort had an initial delay in surgical planning due to grade 3 diarrhea, which resolved with steroid therapy, but this patient did not undergo surgery due to enlarging pulmonary nodules on CT scans with subsequent biopsy indicating metastatic disease.
Safety Profile and Adverse Events
Grade 1/2 adverse events at 3 mg/kg dosing consisted of three cases of rash, one episode of diarrhea, one episode of uveitis, and one episode of increased amylase and lipase, suggestive of pancreatitis but without clinical symptoms (Table 1). The three cases of rash occurred in 3 separate patients and resolved with symptomatic treatment consisting of diphenhydramine and topical hydrocortisone. The episode of diarrhea, increased amylase/lipase and uveitis all occurred in the same patient (Patient #3, Table 1). Patient #3 was also the only patient to develop a grade 3 irAE after treatment with two doses of antibody at 3 mg/kg/dose. This patient developed a grade 3 irAE consisting of ischemic papillopathy and optic neuritis, which resulted in a loss of vision and required treatment with high dose intravenous steroids, as well as brief courses of infliximab and mycophenolate mofetil for immune suppression (19). The patient's visual acuity has subsequently returned to 20/20 on the right and 20/25 on the left. Of note, this patient's irAE was associated with a high pre-therapy level of the Th2 cytokine IL-10, which decreased after treatment with anti-CTLA-4 (19).
Grade 1/2 drug-related adverse events at the 10mg/kg dosing consisted of three cases of rash, one episode of testicular swelling/orchitis, and three cases of diarrhea. Grade 3 drug-related adverse events at the 10mg/kg dose level consisted of two episodes of diarrhea and one case of elevated transaminases without clinical symptoms. These events were deemed to be irAEs and were treated with intravenous and/or oral steroids.
One patient (Patient #10, Table 1) developed grade 3 diarrhea after the first dose of ipilimumab and, as per protocol, did not receive a second dose of antibody. After treatment with intravenous and oral systemic steroids, the patient's diarrhea resolved. The patient eventually proceeded to surgery after a 10-week delay, due to multiple factors, including the occurrence of grade 3 diarrhea. The final pathology after surgery revealed no evidence of disease within the specimen. The patient remains without any evidence of disease on post-operative computed tomographic (CT) scans.
Another patient (Patient #11, Table 1) had grade 3 diarrhea that lasted approximately 9 weeks after receiving the second dose of antibody. His diarrhea also resolved after treatment with intravenous and oral systemic steroids; however, the patient's surgery was aborted due to the presence of metastatic disease. This patient was noted to have sub-centimeter pulmonary nodules on baseline CT scans, which prompted repeat imaging studies one-month later, prior to the patient enrolling onto the anti-CTLA-4 study. Repeat CT scans indicated stable number and size of pulmonary nodules, which were thought to be benign, and the patient was deemed eligible for the pre-operative clinical trial protocol with anti-CTLA-4. Unfortunately, on repeat CTs that were completed after the administration of 2 doses of anti-CTLA-4 at 10 mg/kg/dose as per protocol, the patient was found to have enlarging pulmonary nodules consistent with metastatic disease.
One other patient (Patient #12) was found to have metastatic disease. The patient completed both doses of anti-CTLA-4 therapy at 10 mg/kg/dose and proceeded to surgery (Table 1). The patient's surgical pathology revealed urothelial carcinoma with sarcomatoid and micropapillary components. Pathology also revealed metastatic disease within lymph nodes removed at the time of surgery (Table S1). Post-operative follow-up CT scans indicated new metastatic disease in the lungs and the patient was started on systemic chemotherapy.
Post-operative complications that occurred on study, but were not thought to be attributable to anti-CTLA-4, included wound dehiscence as well as enterocutaneous fistula in one patient who had multiple prior abdominal surgeries (Patient #10) and urinary tract infections in five patients. One patient, who completed the clinical trial and who was without any evidence of disease on post-operative CT scans, subsequently died due to causes that were not related to malignancy or anti-CTLA-4. Drug-related grade 4 adverse events or death did not occur as a result of CTLA-4 blockade on this clinical trial. In total, all 12 patients received at least one dose of antibody, with 11/12 patients receiving both doses of antibody; 11 patients completed surgery; 4 patients did not have any evidence of disease noted on the surgical pathology sample; and 10 patients remained disease-free during the follow-up period after surgery.
Surgical Pathology and Urine Cytology
Surgical pathology was evaluated from transurethral resections obtained prior to anti-CTLA-4 and in radical cystectomy specimens obtained after treatment with anti-CTLA-4. Eight patients were found to have a lower stage of disease on their surgical specimen obtained after anti-CTLA-4 administration as compared to transurethral resection specimens obtained prior to anti-CTLA-4. Since transurethral resection of disease may have accounted for this downstaging effect, we also assessed urine samples for malignant cells by cytology and chromosomal abnormalities associated with urothelial carcinoma by fluorescent in situ hybridization (FISH) (Table S1). Urine cytology and FISH analyses of urine samples from treated patients were performed after transurethral resections but prior to anti-CTLA-4 (baseline sample) and after antibody treatment (post-therapy sample), without any other intervention between the baseline and post-therapy analyses. We found that 4 patients demonstrated a change from a positive urine cytology and/or FISH analysis to negative urine cytology and/or FISH analysis after treatment with anti-CTLA-4 (Table S1). These data suggest that treatment with anti-CTLA-4 antibody leads to a therapeutic effect in the setting of urothelial carcinoma, which will need to be explored in future clinical studies.
Increased ICOS expression is detected on CD4 and CD8 T cells after treatment with 10mg/kg/dose of anti-CTLA-4 antibody
We previously analyzed tumor tissues and peripheral blood of patients treated with anti-CTLA-4 at 3 mg/kg/dose for immunological changes that might correlate with administration of anti-CTLA-4 antibody. Markers such as CD4, CD8, HLA-DR, CD69 and CD45RO and CD45RA were monitored, but showed no consistent change in tumor tissues after treatment. However, a homolog of CD28 and CTLA-4 known as inducible co-stimulator (ICOS) was found to have increased expression on CD4 T cells within tumor tissues after treatment with anti-CTLA-4 antibody. These cells were shown to contain a population of effector T cells that recognized the tumor-associated antigen NY-ESO-1. Peripheral blood samples from patients treated with 3 mg/kg/dose of antibody also showed a 2 to 7-fold increase in CD4+ICOShi T cells (18). CD4+ICOShi T cells from post-therapy peripheral blood samples were shown to produce higher levels of IFN-γ than CD4+ICOSlow T cells or T cells from pre-therapy blood samples (18). In addition, CD4+ICOShi T cells from post-therapy blood samples were capable of recognizing the NY-ESO-1 tumor antigen expressed on 3 patients' tumor samples (18). The increased frequency of CD4+ICOShi T cells, which consisted of a population of effector T cells, was provocative as an immunological marker that could be used for immune monitoring of patients treated with anti-CTLA4 therapy.
At 10 mg/kg/dose of antibody, CD4+ICOShi T cells were similarly increased in tumor tissues and, in addition, CD8+ICOShi T cells were detectable in tumor tissues (Figure 1A), which were not detected in tumor samples from untreated patients or patients treated with 3 mg/kg/dose of antibody. Figure 1B demonstrates that the frequencies of CD4+ICOShi and CD8+ICOShi T cells were significantly higher in tumor tissues (p=0.004 and p=0.002, respectively) after treatment with anti-CTLA-4 (N=5) as compared to untreated tumor tissues (N=10). Furthermore, 2/5 tumor samples demonstrated increased infiltrating cells into blood vessels after patients received treatment with 10 mg/kg/dose of anti-CTLA-4 antibody as compared to 0/11 tumor samples obtained prior to therapy and 0/6 patients who received treatment with 3 mg/kg/dose of antibody (Figure 2, Upper Panel). The infiltrating cells were positive for CD3, CD8, CD4 and granzyme but predominantly negative for CD20 and CD56 staining (Figure 2, Lower Panel).
Figure 1. Increased frequency of CD4 +ICOShi and CD8+ ICOShi T cells in tumor tissues after treatment with 10mg/kg/dose of anti-CTLA-4.
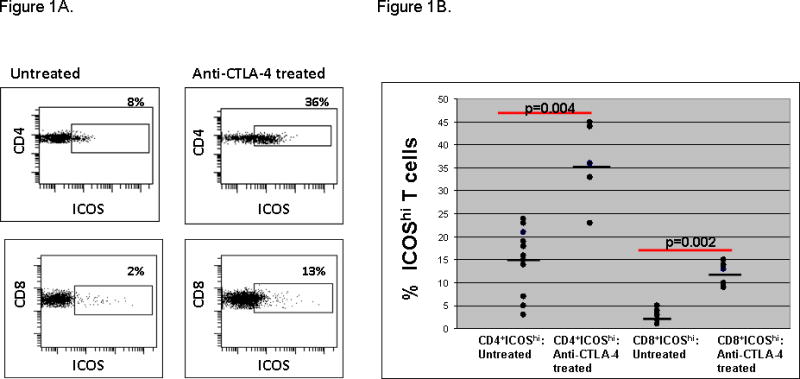
Representative patient samples demonstrating that CD4+ICOShi (Upper Panel) and CD8+ICOShi (Lower Panel) T cells were increased in frequency in tumor tissues after treatment with 10 mg/kg/dose of anti-CTLA-4 as compared to untreated tumor tissues (A). Compilation of data showing statistically significant increased frequencies of CD4+ICOShi and CD8+ICOShi T cells in tumor tissues from anti-CTLA-4 treated patients (N=5) as compared to tumor tissues obtained from untreated patients (N=10) (B).
Figure 2. Perivascular infiltration of cells into tumor tissues after treatment with 10 mg/kg/dose of anti-CTLA-4.
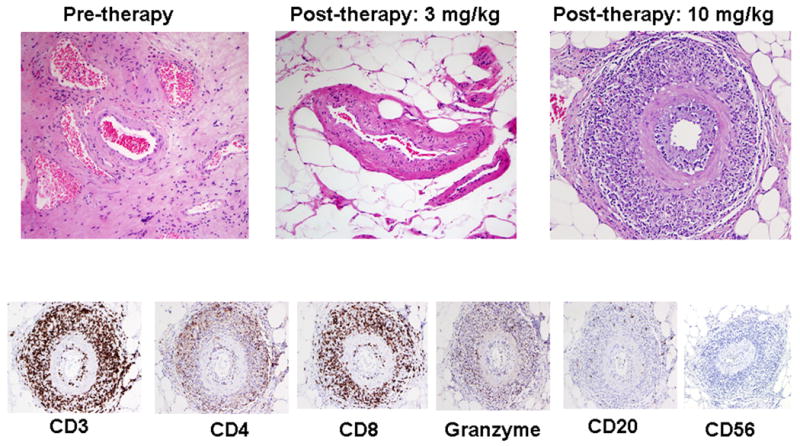
Representative pictures demonstrating an absence of perivascular infiltration of cells in untreated tumor tissues (0/11) and tumor tissues obtained from patients treated with 3 mg/kg/dose of anti-CTLA-4 (0/6) as compared to the presence of perivascular infiltration noted in tumor tissues obtained from patients treated with anti-CTLA-4 at 10 mg/kg/dose (2/5) (Upper Panel). Immunohistochemistry revealed that the infiltrating cells were positive for CD3, CD8, CD4 and granzyme but predominantly negative for CD20 and CD56 (Lower Panel).
Within peripheral blood samples, we observed an approximately 5 to 10-fold increase in CD4+ICOShi T cells (Figure 3A, Upper Panel), which was approximately 2-fold higher than that seen with the 3 mg/kg/dose of antibody (18). Samples from Patient #9 (Table 1) showed an increase in CD4+ICOShi T cells from 3% at baseline to 10% at week 3 (after dose #1) and 40% at week 7 (after dose #2). Similarly, at 10 mg/kg/dose of antibody we observed an increase in frequency of CD8+ICOShi T cells within the systemic circulation (Figure 3A, Lower Panel), which was not detectable after patients received treatment with 3 mg/kg/dose of antibody. The data for all 6 patients treated with anti-CTLA-4 at 10 mg/kg/dose is provided in Figures 3B and 3C for frequency of CD4+ICOShi (Figure 3B) and CD8+ICOShi T cells (Figure 3C) at pre-therapy, post-therapy week 3 and post-therapy week 7 time points. As shown, there is no statistical difference between CD4+ICOShi and CD8+ICOShi T cell average frequencies in healthy donors (HD, N=10) as compared to pre-therapy values from the bladder cancer patients (N=6); however, after treatment with anti-CTLA-4 at 10 mg/kg/dose there is a statistically significant increase in CD4+ICOShi (Figure 3B, p=0.036 at weeks 3 and 7 as compared to pre-therapy values) and CD8+ICOShi T cells (Figure 3C, p=0.031 and 0.036 at weeks 3 and 7, respectively, as compared to pre-therapy values).
Figure 3. Increased frequency of CD4+ICOShi and CD8+ICOShi T cells in the peripheral blood after treatment with 10 mg/kg/dose of anti-CTLA-4.
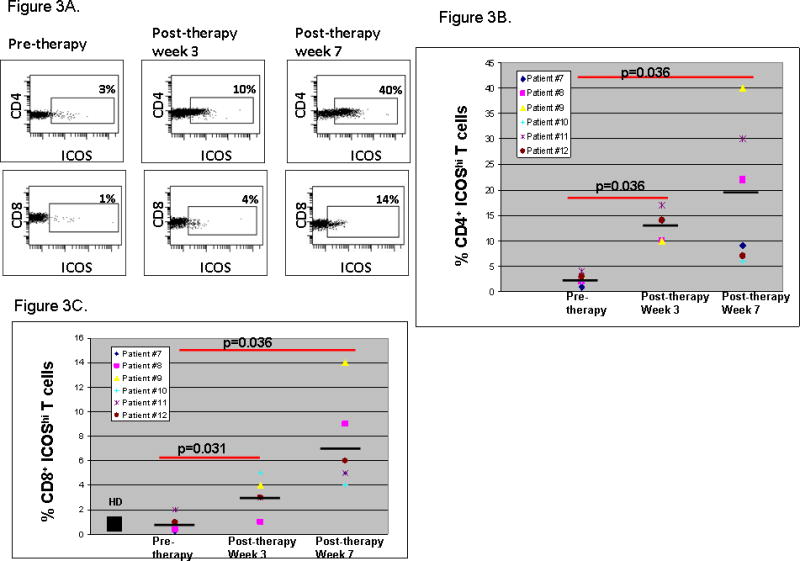
Representative peripheral blood samples taken after the first dose of anti-CTLA-4 at week 3 and after the second dose of anti-CTLA-4 at week 7 showed an increased frequency of CD4+ICOShi (Upper Panel) and CD8+ICOShi T cells (Lower Panel) as compared to baseline (pre-therapy) samples (A). Compilation of data showing pre-therapy values for frequency of CD4+ICOShi T cells (B) and CD8+ICOShi T cells (C) in bladder cancer patients (N=6) and average frequency in healthy donors (HD, N=10) with statistically significant increases in frequencies of ICOShi T cells at weeks 3 and 7 after treatment with anti-CTLA-4.
Correlation of frequency of CD4+ICOShi T cells with clinical benefit in a cohort of metastatic melanoma patients
We performed multiparametric flow cytometric analyses on fresh peripheral blood mononuclear cell (PBMC) samples from healthy donors and a group of 14 melanoma patients prior to and at various time points after anti-CTLA-4 therapy (ipilimumab) at 10mg/kg/dose of antibody. We also analyzed frozen PBMC samples from control patients with advanced melanoma who received other treatments. Of note, we did not notice significant differences in ICOS expression on T cells from frozen samples as compared to fresh samples (Figure S2). Healthy donors, control melanoma patients and the 14 melanoma patients prior to anti-CTLA-4 therapy all had low but detectable expression of CD4+ICOShi cells of approximately 2-3% of the CD4+ population. There was no significant difference between each of these groups. Following therapy with anti-CTLA-4, the majority of patients had increase in frequency of CD4+ICOShi T cells at weeks 7 (p=0.0012) and 12 (p=0.0006) following dose #2 and dose #4 of anti-CTLA-4 antibody, respectively, compared to baseline values (Figures 4A and 4B). By week 24, the frequency of CD4+ICOShi T cells had returned to approximately the baseline values.
Figure 4. Increased frequency of CD4+ ICOShi T cells is detected after patients with metastatic melanoma are treated with anti-CTLA-4.
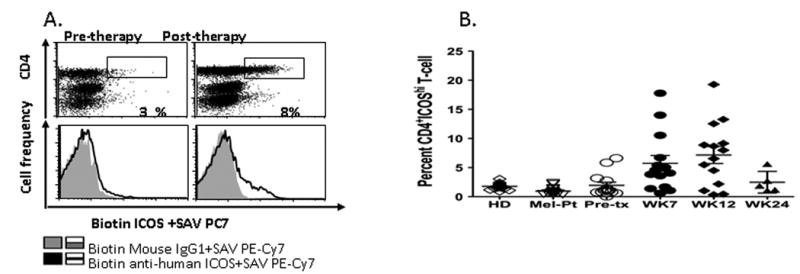
A representative dot plot of ICOS staining in a melanoma patient at baseline and after anti-CTLA-4 therapy, as well as the mouse IgG1 isotype control (A). All samples analyzed for % CD4+ICOShi expression in the different donor and patient groups at different time points (B) (HD, healthy donor; Mel-Pt, control melanoma patients; Pre-tx, pre-treatment; SAV, streptavidin).
In addition, we also examined FOXP3 expression in CD4+ cells for the above groups of donors and patients as a marker of regulatory T cells. The data are shown in Supplementary Figures 3A and 3B. Healthy donors and control melanoma patients had relatively low frequency of CD4+FOXP3+ T cells, averaging 2-3%. Our cohort of 14 anti-CTLA-4 treated patients had a frequency of CD4+FOXP3+ T cells of approximately 5% at baseline, which was not statistically different from what was observed in the healthy donor and control melanoma patient samples. The frequency of CD4+FOXP3+ T cells did not change during weeks 7 and 12 of anti-CTLA-4 therapy. By week 24, the frequency of CD4+FOXP3+ T cells had decreased to levels approximating those of the healthy donors and control melanoma patients. We also assessed co-expression of ICOS and FOXP3 in CD4 T cells. We noted that <20% of the CD4+ICOShi population was FOXP3+ (Figure S3C). A similar proportion of CD4+FOXP3+ cells were ICOShi (Figure S3D), suggesting that only a minority of cells was positive for both markers after treatment with anti-CTLA-4 therapy, as previously reported for patients with urothelial carcinoma (18).
We then correlated changes in ICOS expression in the 14 treated patients with clinical outcome. Specifically, we examined changes in the CD4+ICOShi expression from baseline to week 7 and 12 of anti-CTLA-4 therapy. Eight of 14 patients (IDs 5, 8, 9, 10, 11, 12, 13, and 14) were noted to have a persistent increase in CD4+ICOShi expression, defined as a ≥2-fold increase in % CD4+ICOShi expression at week 7 or 12 over baseline that was sustained at week 12. The other six patients (IDs 1, 2, 3, 4, 6, 7) either had a <2-fold increase in CD4+ICOShi expression at either week 7 or 12 over baseline or had a ≥2-fold increase in CD4+ICOShi expression at week 7 that had declined by week 12 from the week 7 value. Of the eight patients with persistent increase in CD4+ICOShi expression, seven had evidence of clinical benefit at week 24 consisting of stable disease for 6 months, partial responses and complete responses as defined by immune related response criteria (24) (Figure 5A). Of the six patients without persistent increase in CD4+ICOShi expression, none had evidence of clinical benefit at week 24 (Figure 5A). The proportion of patients with persistent CD4+ICOShi increase who had clinical benefit at week 24 was significantly higher than those without persistent CD4+ICOShi expression (p=0.004).
Figure 5. Correlation of clinical outcome with frequency of CD4+ICOShi T cells after patients were treated with anti-CTLA-4.
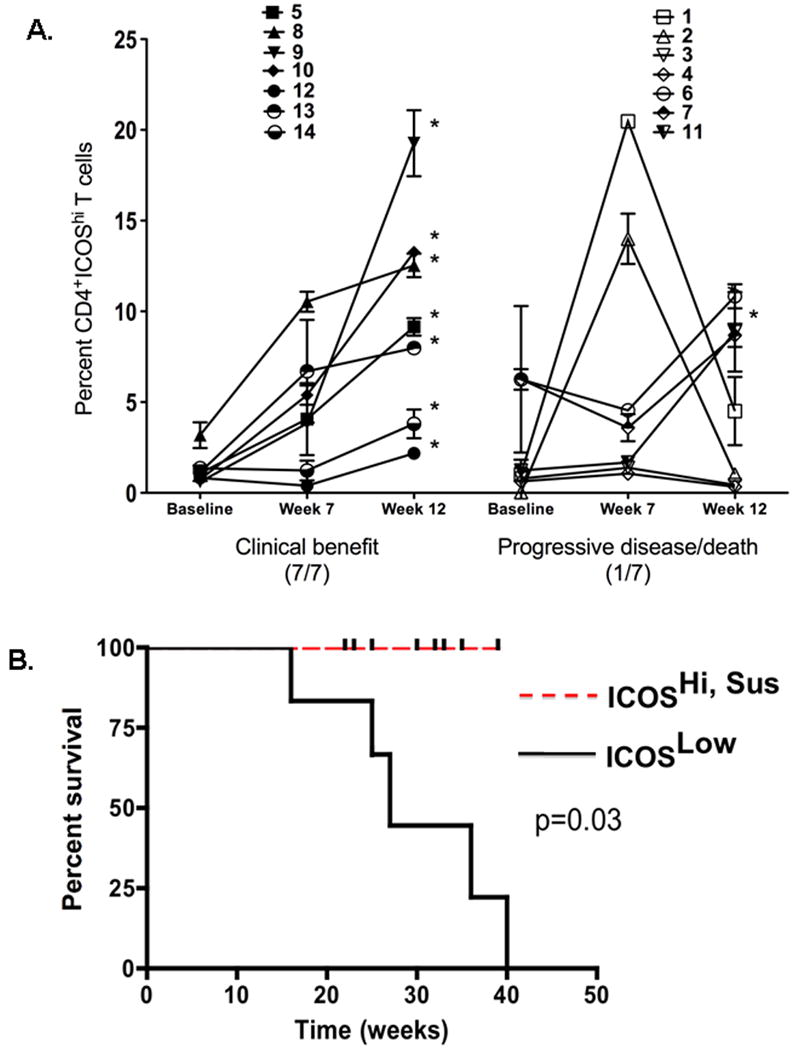
Multiparameter flow cytometric analyses were performed on fresh patient PBMCs at baseline, week 7 and 12. Seven of seven patients with clinical benefit at week 24 had persistent elevation in % of CD4+ICOShi cells, defined as a ≥2-fold increase in % CD4+ICOShi expression at week 7 or 12 over baseline that was sustained at week 12. In comparison, one of seven patients without clinical benefit, i.e. with progressive disease or death at week 24, had persistent elevation in % of CD4+ICOShi cells. (* denotes patients with persistent CD4+ICOShi increase) (A). Kaplan-Meier curve showing the difference in OS for patients with and without persistent % CD4+ICOShi increase is statistically significant (median OS not reached vs. 27 weeks, p=0.03) (B).
We also examined the correlation between the absolute number of CD4+ICOShi cells and clinical benefit and found that a similar trend existed. A persistent increase in the absolute number of CD4+ICOShi cells was defined as a ≥2-fold increase in the number of CD4+ICOShi cells at week 7 or 12 over baseline that was sustained at week 12. Eight of the 14 patients had a persistent increase in the number of CD4+ICOShi cells. Of these, seven had clinical benefit at week 24. Again, the proportion of patients with persistent increase in CD4+ICOShi cell number who had clinical benefit at week 24 was significantly higher than those without persistent CD4+ICOShi increase (p=0.03).
Finally, we noted a statistically significant difference in overall survival (OS) when the data was stratified by persistent versus non-persistent % CD4+ICOShi increase (median OS not reached vs. 27 weeks, p=0.03). A Kaplan-Meier survival curve is shown in Figure 5B.
Discussion
We determined the immunodulatory effects following a brief exposure of anti-CTLA-4 in patients with urothelial carcinoma of the bladder requiring surgery. This study, to our knowledge, is the first analysis of CTLA-4 blockade in the setting of urothelial carcinoma and in the setting of a pre-surgical clinical trial. The study population consisted of 12 patients, with 6 patients receiving 3mg/kg/dose of anti-CTLA-4 and another 6 patients receiving 10mg/kg/dose for 2 doses prior to surgery. The treatment was found to be tolerable in our cohort of patients with 11/12 patients receiving both doses of antibody. Grade 1-2 diarrhea and rash were the most common drug-related side effects. Of relevance, the only noted grade 3 irAEs were ischemic papillopathy and diarrhea, which were both responsive to treatment with steroids. Patients have been followed for a median of 20 months with 9/12 patients continuing to remain without evidence of disease. In addition, the pre-operative approach in this study overcomes a major hindrance of biological studies, in that it provides for analyses of tumor tissues. The availability of cystectomy specimens provided sufficient tissue to perform detailed immunological analyses, which could be correlated with biomarkers in peripheral blood.
Data from our preliminary results on the first 6 patients treated with 3mg/kg/dose of anti-CTLA-4, as well as the current analyses from the 6 patients treated at 10mg/kg/dose demonstrated that treatment with anti-CTLA-4 antibody leads to an increase in the frequency of CD4+ICOShi cells, which comprise a population of effector T cells. This population of CD4+ICOShi cells, which was found to be increased in both tumor tissues and peripheral blood, represented a potential biomarker that could be used for correlation with clinical outcome in patients with metastatic disease who receive treatment with anti-CTLA-4. A retrospective analyses of peripheral blood samples obtained from patients with metastatic melanoma who were treated with anti-CTLA-4 antibody at 10mg/kg/dose demonstrated that a sustained increase in CD4+ICOShi T cells at week 12, after 4 doses of therapy, correlated with improved overall survival.
Our clinical trial does have limitations. The analyses of untreated bladder tumor tissues were performed on samples obtained from a separate group of patients since cystectomy samples consisting of the entire bladder were necessary in order to obtain sufficient numbers of cells for immunological analyses. Therefore, specimens from stage matched untreated patients were utilized for comparison in flow cytometry studies. This does allow for confounders in that we could not account for unknown factors that may impact our data. The general trend in the group of patients suggests that although there may be unknown factors that could account for differences between patients in the two separate groups, the increased frequency of CD4+ICOShi cells after treatment serves as a marker of CTLA-4 blockade that supersedes minor contributions from unknown variables. Another limitation is that our small sample size limits the statistical power of the immunological correlates. More patients will need to be analyzed in larger studies.
In our current study, we report that anti-CTLA-4 is tolerable in the pre-operative setting, which provides an opportunity to obtain immunological data from both tumor tissues and the peripheral blood. Our data, consisting of an increased frequency of CD4+ICOShi T cells, were obtained in a cohort of patients with localized urothelial carcinoma of the bladder but nonetheless may be relevant in patients with other types and stages of malignancies, such as metastatic melanoma, as we report here. Our data support the concept that a sustained increased frequency of CD4+ICOShi T cells may serve as a biomarker of anti-CTLA-4 activity and/or as a clinical benefit for patients who are being treated with this novel agent. We have shown that anti-CTLA-4 can induce similar immunological changes in patients with different types of malignancies and in different tumor tissues (25). Future studies with additional patients will need to be conducted in order to validate CD4+ICOShi T cells as a biomarker to optimize anti-CTLA-4 therapy and/or indicate potential clinical benefit thus warranting continued therapy.
Ours are the first studies to demonstrate alterations in ICOS expression on T cells during anti-tumor responses. It is of considerable interest to determine the role of ICOS and its interactions with its ligand in these responses. ICOS is expressed on both effector and regulatory T cells and has a very complex biology, having been variously implicated in T/B cell interactions, germinal center formation, and regulation of Th1 and Th2 cytokines (26-28). A key fact may be that it has the potential to signal via the PI3 kinase pathway and may enhance T cell survival. Mechanistic studies are ongoing to understand how the ICOS pathway affects anti-tumor responses.
In summary, we have shown that anti-CTLA-4 can be delivered in the pre-operative setting without an increase in surgical complication. The previously reported toxicities associated with anti-CTLA 4 therapy were observed and appropriately managed. Furthermore, the pre-operative model can serve as a powerful discovery platform to efficiently identify biomarkers that can be applied to the metastatic disease setting to establish the relevance of experimental findings. Our identification of ICOS is the first immunologic marker to be identified in both tumor tissues and the peripheral blood of patients treated with anti-CTLA-4 thus providing a relevant biomarker on which to build future immune monitoring strategies.
Materials and Methods
Bladder Cancer Patients
Patients with localized (T1-T2, NO, MO) urothelial carcinoma who were candidates for radical cystectomy were consented on an M.D. Anderson Cancer Center Institutional Review Board (IRB) approved protocol (2006-0080) to receive two doses of anti-CTLA-4 antibody (Ipilimumab) prior to undergoing surgery. Six patients completed analyses for safety at 3mg/kg/dose before six additional patients were enrolled to receive 10mg/kg/dose. Surgery was scheduled on or about week seven of the protocol. Healthy donor blood and additional tissue samples from untreated patients with localized urothelial carcinoma of the bladder were obtained for comparison as per an IRB-approved laboratory protocol (2005-0027).
Metastatic Melanoma Patients
Eligible patients had a diagnosis of unresectable stage III or metastatic/recurrent stage IV melanoma and had experienced PD or intolerance to at least one prior systemic therapy. All pathology was confirmed at Memorial Sloan-Kettering Cancer Center (MSKCC). Patients were ≥18 years of age, had normal hematologic and organ function and an Eastern Cooperative Oncology Group status of 0 or 1. Exclusion criteria included any other prior invasive malignancy, autoimmune disease or active infection or pregnant or lactating women. All patients signed an informed consent approved by the MSKCC Institutional Review Board. Additional blood draws were obtained for investigational purposes after patients gave further informed consent. Healthy donors also provided blood samples after signing an informed consent. At baseline, patients underwent a complete history and physical examination, laboratory evaluation and radiographic imaging appropriate for tumor evaluation. The same imaging modality was used for initial screening and post-therapy evaluation. Initial clinical responses were evaluated at week 12 and onward and adjudicated using modified WHO criteria.
Collection, preparation and cryopreservation of PBMCs
Whole blood from patients was collected in Vacutainer or Cell Preparation Tubes (CPT) containing sodium heparin (BD Vacutainer, Franklin Lakes, NJ). PBMCs were isolated from whole blood by centrifuging the CPT tubes at 800g for 25 min. The plasma was collected and stored frozen at -80 °C for subsequent cytokine detection experiments. The interface cells were harvested and washed twice with PBS with 10% fetal calf serum at 500g and 450g for 10 minutes respectively. PBMCs were then resuspended in complete RPMI 1640 with 10% autologous plasma or pooled human serum and 10% DMSO.
ICOS and FOXP3 phenotype staining
One million PBMCs were washed with 2 ml FACS buffer (phosphate buffered saline (PBS) containing 1% bovine serum albumin and 0.05 mM EDTA). The cells were resuspended in 50 μl FACS buffer and stained with 0.375 μL ICOS-biotin antibody (eBioscience, San Diego, CA) for 20 min at 4°C before washing again with 2 ml FACS buffer. The following antibodies were then added for 30 min at room temperature: 0.3 μL strepavidin-PE-Cy7 (eBioscience), 3 μL CD3-Pacific Blue (eBioscience), 1 μL CD4-ECD (Beckman Coulter Inc., Fullerton, CA) and 3 μL CD25-APC-Cy (BD Bioscience, San Jose, CA). After re-washing with FACS buffer, the cells were fixed and permeabilized with 250 μl 1× Fixation/Permeabilization solution (eBioscience) for 30 min at 4°C before being washed with 2 ml 1× Permeabilization buffer (eBioscience). 5 μL FOXP3-APC antibody (eBioscience) was then added for 60 min at 4°C before a final washing with 1× Permeabilization buffer. The cells were then resuspended in 400 μL FACS buffer and acquired on a CyAn ADP flow cytometer with Summit software (DakoCytomation California Inc., Carpinteria, CA). Analysis was performed using FlowJo software (version 8.1; TreeStar, Inc., Ashland, OR). Isotype controls included the appropriate biotin or fluorochrome conjugated mouse IgG1a or IgG2a (Dako).
Immunohistochemistry
Tissue was obtained from untreated urothelial carcinoma specimens, as well as from patient specimens after treatment with either 3mg/kg of Ipilimumab or 10mg/kg of Ipilimumab. After standard fixation, paraffin-embedded samples were stained with hematoxylin and eosin, as well as with antibodies for CD3 (Dako, Carpinteria, CA), CD4 (Novocastra, Leica Microsystems, Bannockburn, IL), CD8 (Labvision, Fremont, CA), Granzyme B (Cell Sciences, Canton, MA), CD20 (Dako, Carpinteria, CA) and CD56 (Invitrogen, Carlsbad, CA).
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH) of cells recovered from voided urine/urinary tract washings was performed using the Vysis UroVysion Kit, 4-color probe mixture of DNA sequences from specific regions of chromosomes 3 (D3Z1), 7 (D7Z1), 17 (D17Z1) and 9p21 (p16). A positive result is defined as the detection of four or more cells with greater than two signals (polysomy) for at least two probes for chromosomes 3, 7, 17 or 9p21 and/or 12 cells with no signal for chromosome 9. The UroVysion Kit is designed and has been FDA cleared to detect aneuploidy for chromosomes 3, 7, 17, and loss of 9p21 locus in transitional cell carcinoma urine specimens.
Statistical Analyses
Comparisons between groups were made using Wilcoxon's rank test. Survival was analyzed by the Kaplan-Meier method. Correlation was analyzed using Spearman's rank test.
Supplementary Material
Anti-CTLA-4 antibody was given at 3 mg/kg/dose or 10 mg/kg/dose. Blood tests were done at time of study entry, at week 3 before the second dose of antibody (post-therapy week 3) and at week 7 before surgery (post-therapy week 7). Tumor tissues were collected at the time of surgery.
ICOS expression was analyzed by flow cytometry on CD4 T cells from eight matched fresh and frozen samples. There were four pre-therapy samples and four post-therapy (week 12) samples. There was no statistically significant difference noted in the frequency of CD4+ICOShi cells in fresh and frozen samples before (pre-therapy) and after (post-therapy) CTLA-4 blockade.
A representative dot plot of FOXP3 staining in a melanoma patient at baseline and after anti-CTLA-4 therapy, as well as the rat isotype control (A). Percentages of CD4+FOXP3+ expression in the different donor and patient groups at different time points. (HD, healthy donor; Mel-Pt, control melanoma patients; Pre-tx, pre-treatment) (B). A representative dot plot where the CD4+ICOShi population was gated for FOXP3, with the numerical values indicating the mean value ± standard deviation. Approximately 15% of CD4+ICOShi cells are also FOXP3+ (C). A representative dot plot where the CD4+FOXP3+ population was gated for ICOS. Again, only approximately 15% of the CD4+FOXP3+ cells are ICOShi (D).
Specimen pathology, urine cytology and fluorescence in situ hybridization results before and after patients with localized urothelial carcinoma were treated with anti-CTLA-4.
Acknowledgments
Grant Support: BCC was supported in part by an NIH T32 Training grant (NIH 5 T32 CA009666) awarded to M. D. Anderson Cancer Center. JDW was supported in part by a Melanoma Research Alliance Established Award. This work was also supported in part by a UTMDACC Physician-Scientist Program Award, a Gillson Longenbaugh Foundation Award, a Carl C. Anderson Sr. & Marie Jo Anderson Charitable Foundation Award, a Melanoma Research Alliance Young Investigator Award and a Doris Duke Clinical Scientist Development Award (P.S.). Bristol-Myers Squibb (BMS) sponsored and funded the clinical trial of pre-operative Ipilimumab in patients with localized urothelial carcinoma of the bladder. Immune monitoring done at MSKCC was supported by the Ludwig Trust and the Goodwin-Commonwealth Fund for Cancer Research.
Footnotes
Conflict of Interest Disclosure: P.S., J.W., C.J.L., and J.P.A. have served on BMS advisory boards and received honorariums for their services. JPA holds the patent for anti-CTLA-4, which is currently being developed as Ipilimumab by BMS.
Statement of Translational Relevance: Anti-tumor responses, including durable complete regression of disease, have been observed in ∼10% of patients who receive anti-CTLA-4 therapy. Why some patients respond to therapy while others do not remains unknown. Most clinical trials of CTLA-4 blockade have been conducted in the metastatic disease setting, which allow for correlation of treatment with clinic outcomes but do not allow for detailed immunologic studies on tumor-infiltrating lymphocytes to evaluate how the antibody impacts human immune responses. To overcome this challenge we conducted the first pre-operative trial with anti-CTLA-4. Our trial focused on safety as well as on identifying immunological changes within the systemic circulation that correlated with those found in tumor tissues. We present data demonstrating safety of the pre-surgical trial and identification of a blood-based biomarker that reflects changes within tumor tissues thus making it useful for immune monitoring in the metastatic disease setting, where access to tumor tissues is limited.
References
- 1.Harding FA, McArthur JG, Gross JA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 3.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The Journal of Experimental Medicine. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. The Journal of Experimental Medicine. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 6.van Elsas A, Sutmuller RP, Hurwitz AA, et al. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. The Journal of Experimental Medicine. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quezada SA, Peggs KS, Curran MA, et al. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. The Journal of Clinical Investigation. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korman A, Yellin M, Keler T. Tumor immunotherapy: preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Investig Drugs. 2005;6:582–91. [PubMed] [Google Scholar]
- 11.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–5. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 14.Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–53. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–81. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 16.Dillard T, Yedinak CG, Alumkal J, et al. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 17.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. The New England journal of medicine. 2009;361:211–2. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 18.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Schiffman J, Raghunath A, et al. Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun. 2008;8:9. [PMC free article] [PubMed] [Google Scholar]
- 20.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature Immunology. 2009;10:167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasheed AU, Rahn HP, Sallusto F, et al. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. European Journal of Immunology. 2006;36:1892–903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 22.Akiba H, Takeda K, Kojima Y, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–8. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Gnjatic S, Jungbluth AA, et al. Frequency of NY-ESO-1 and LAGE-1 expression in bladder cancer and evidence of a new NY-ESO-1 T-cell epitope in a patient with bladder cancer. Cancer Immun. 2003;3:19. [PubMed] [Google Scholar]
- 24.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7116–8. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Liakou CI, Kamat A, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2729–34. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Curr Opin Immunol. 2010 Jan 28; doi: 10.1016/j.coi.2010.01.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Kohyama M, Sugahara D, Sugiyama S, et al. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4192–4197. doi: 10.1073/pnas.0400214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gigoux M, Shang J, Pak Y, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20371–6. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-CTLA-4 antibody was given at 3 mg/kg/dose or 10 mg/kg/dose. Blood tests were done at time of study entry, at week 3 before the second dose of antibody (post-therapy week 3) and at week 7 before surgery (post-therapy week 7). Tumor tissues were collected at the time of surgery.
ICOS expression was analyzed by flow cytometry on CD4 T cells from eight matched fresh and frozen samples. There were four pre-therapy samples and four post-therapy (week 12) samples. There was no statistically significant difference noted in the frequency of CD4+ICOShi cells in fresh and frozen samples before (pre-therapy) and after (post-therapy) CTLA-4 blockade.
A representative dot plot of FOXP3 staining in a melanoma patient at baseline and after anti-CTLA-4 therapy, as well as the rat isotype control (A). Percentages of CD4+FOXP3+ expression in the different donor and patient groups at different time points. (HD, healthy donor; Mel-Pt, control melanoma patients; Pre-tx, pre-treatment) (B). A representative dot plot where the CD4+ICOShi population was gated for FOXP3, with the numerical values indicating the mean value ± standard deviation. Approximately 15% of CD4+ICOShi cells are also FOXP3+ (C). A representative dot plot where the CD4+FOXP3+ population was gated for ICOS. Again, only approximately 15% of the CD4+FOXP3+ cells are ICOShi (D).
Specimen pathology, urine cytology and fluorescence in situ hybridization results before and after patients with localized urothelial carcinoma were treated with anti-CTLA-4.


