Abstract
Background
The increasing use of fluoroscopy-based surgical procedures and the associated exposure to radiation raise questions regarding potential risks for patients and operating room personnel. Computer-assisted technologies can help to reduce the emission of radiation; the effect on the patient’s dose for the three-dimensional (3-D)-based technologies has not yet been evaluated.
Questions/purposes
We determined the effective and organ dose in dorsal spinal fusion and percutaneous transsacral screw stabilization during conventional fluoroscopy-assisted and computer-navigated procedures.
Patients and Methods
We recorded the dose and duration of radiation from fluoroscopy in 20 patients, with single vertebra fractures of the lumbar spine, who underwent posterior stabilization with and without the use of a navigation system and 20 patients with navigated percutaneous transsacral screw stabilization for sacroiliac joint injuries. For the conventional iliosacral joint operations, the duration of radiation was estimated retrospectively in two cases and further determined from the literature. Dose measurements were performed with a male phantom; the phantom was equipped with thermoluminescence dosimeters.
Results
The effective dose in conventional spine surgery using 2-D fluoroscopy was more than 12-fold greater than in navigated operations. For the sacroiliac joint, the effective dose was nearly fivefold greater for nonnavigated operations.
Conclusion
Compared with conventional fluoroscopy, the patient’s effective dose can be reduced by 3-D computer-assisted spinal and pelvic surgery.
Level of Evidence
Level II, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Computer-assisted surgery (CAS) systems have been used in orthopaedic operations since the early 1990s. For the lumbar and the thoracolumbar junction, image-guided procedures appear superior to the conventional approach concerning pedicle screw misplacements [2, 14, 22, 26]. Another expected advantage is the reduction of radiation for operating room personnel when using computer-navigated systems, which has been reported for different types of procedures [4, 6, 9, 11].
Fractures of the lumbar spine are common and posterior stabilization with pedicle screws is one possible treatment depending of the type of fracture [3, 20]. Injuries of the pelvis frequently are treated with percutaneous transsacral screw stabilization using minimally invasive techniques, but proper placement of implants is challenging owing to the complex anatomy of the region [7]. Although navigation systems are used where available, conventional approaches using conventional fluoroscopy are likely most widely used [1]. Furthermore, these operations must be performed using conventional techniques when navigation systems malfunction. Both types of operations are being performed by orthopaedic surgeons with and without navigation systems, depending on the equipment of the hospital and the training of the surgeon.
The newest development in navigation systems is 3-D navigation, which requires a special C-arm that can perform orbital rotation around the object of interest and creates a CT-like data set, which is used for the navigation (Fig. 1). For nonnavigated fusions at the thoracolumbar spine, most surgeons use fluoroscopy to control the position of the screws and to improve orientation in AP and lateral views (Fig. 2) [20].
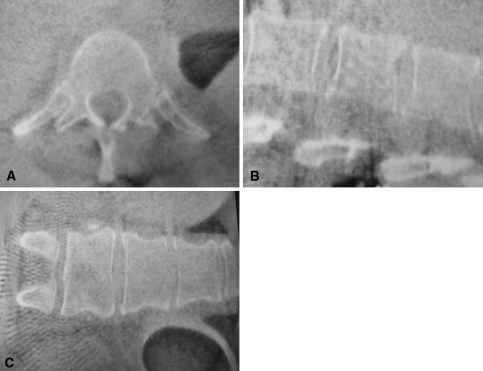
Fig. 1A–C.
(A) Transverse, (B) sagittal, and (C) coronal planes of a 3-D scan of the lumbar spine are being reconstructed of 100 single 2-D images. To acquire these images, the C-arm rotates 190° around the patient. The single images then are processed by the attached work station and the CT-like data set then is sent to the navigation system.
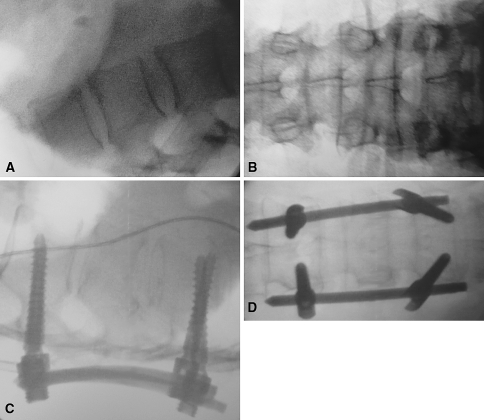
Fig. 2A–D.
The preoperative (A) lateral and (B) AP views of the lumbar spine are being made with the same C-arm as are the 3-D scans. The postoperative control (C) lateral and (D) AP views after spinal fusion show the correct placed pedicle screws. In both groups (navigated and conventional) these images usually are acquired after surgery and in both groups these images have been applied to the phantom.
The use of radiation for medical procedures and the associated exposure to radiation raises questions regarding potential risks for operating room personnel and for the patient. Medical doses are now greater than those arising from the natural background and radon and are likely to continue to increase [8]. Several studies evaluating the emission of radiation during pedicle screw implantation [10, 18, 19] showed potential danger to the health of patients and surgeons owing to the emission of ionizing radiation, especially for spinal fusion procedures [19] (Table 1). Other studies comparing the emission of radiation of navigated versus nonnavigated procedures at the spine [6, 9, 11, 24, 25] (Table 2) and on the pelvis [29] reported a reduction of the emission of radiation when using computer navigated techniques. These studies focus mainly on the radiation dosages to operating room personnel and the different study designs make the data difficult to compare.
Table 1.
Studies examining the emission of radiation for pedicle screw insertion
| Study | Study design | Materials and methods | Results/conclusion |
|---|---|---|---|
| Rampersaud et al. [19] | In vitro study on cadavers, dorsal spondylodesis | Badge measurement and TLD | Highest dose ipsilateral on the side of the beamer (53.3 mREM), spine surgeons receive greater radiation than others |
| Jones et al. [10] | 140 patients with fusion of lumbar spine | Digital dosimeter for skin (patient) and scatter (surgeon) radiation | Duration of radiation: 1.4 minutes per case; recommendation for source-superior position of C-arm |
| Perisinakis et al. [18] | 20 cases with dorsal spondylodesis analyzed regarding duration of radiation and position of fluoroscope | RANDO-Phantom with TLD | Effective doses: AP beam: 3.31 mS Lateral beam: 1.22 mS |
TLD = thermoluminescence dosimeter.
Table 2.
Studies comparing the effect on emission of radiation navigated versus nonnavigated spine operations
| Study | Study design | Materials and methods | Results/conclusion |
|---|---|---|---|
| Izadpanah et al. [9] | 30 navigated versus 30 conventional balloon kyphoplasties in osteoporotic fractures | Measurement of radiation time and dose are product | Radiation exposure of surgeon and patient could be reduced significantly |
| Kim et al. [11] | Combined human and cadaveric study, comparing minimally invasive transforaminal lumbar interbody fusion | 18 cadavers, 10 patients, navigated versus conventional, compared fluoroscopy time and dose measurements | Fluoroscopy shorter for navigated operations, no emission detected for navigated, 12.4 mREM surgeon dose |
| Smith et al. [25] | Cadaver study comparing 3-D navigation versus conventional lumbar spinal fusion | Badge measurements above apron of surgeon | 4.33 mREM torso exposure without and 0.33 mREM with navigation |
| Slomczykowski et al. [24] | 20 cases with lumbar spinal fusion analyzed regarding duration of radiation and position of fluoroscope, comparison of CT-based navigation and fluoroscopy-assisted nonnavigated surgery | REMAB and RANDO-Phantom used, testing of CT protocols, measurement of organ and effective doses of patients | Effective doses: Fluoroscopy: 1 mS Sequential CT: 4.1 mS Spiral CT optimized: 3.0 mS Sequential CT optimized: 2.4 mS |
| Gebhard et al. [6] | 38 patients, comparison of different types of navigation for pedicle screw placement | TLD on patient, source and receiver, spinal fusion, four screws | Lowest emission in 3-D navigation, highest in conventional fusion |
TLD = thermoluminescence dosimeter.
There are several ways to measure the emission of radiation intraoperatively. Most of the studies noted above used skin dosimeters that allow determining the amount of radiation only for the exposed body part. The risks deriving from ionizing radiation are best related to organ doses and the effective dose. The organ dose is defined as the total energy (εT) imparted in a tissue or organ divided by the mass (mT) of the tissue (DT = εT/mT). The effective dose is the sum of organ doses (HT) multiplied with the appropriate weighting factors (wT; Table 3) (E = ΣT wT*HT, ΣT wT = 1). The effective and organ doses for patients were determined in three other studies for lumbar spinal fusion for conventional and CT-based spinal fusion [10, 18, 24] (Table 4), but there are no published data showing the effective dose for 3-D–based navigation and percutaneous screw fixation of the spine and sacroiliac joint. As 3-D imaging-based techniques are more widely used in comparison to the older CT-based techniques for navigated procedures [5], knowing the effective dose to the patients undergoing these procedures is important to determine the specific risks for the patients and to compare various current and future techniques.
Table 3.
Tissue weighting factors according to recommendations of the International Commission on Radiological protection (ICRP) 1990 [17]
| Tissue or organ | Tissue weighting factor wT |
|---|---|
| Gonads | 0.20 |
| Bone marrow (red) | 0.12 |
| Colon | 0.12 |
| Lung | 0.12 |
| Stomach | 0.12 |
| Bladder | 0.05 |
| Breast | 0.05 |
| Liver | 0.05 |
| Esophagus | 0.05 |
| Thyroid | 0.05 |
| Skin | 0.01 |
| Bone surface | 0.01 |
| Remainder | 0.05 |
Table 4.
Literature comparison of effective dose for pedicle screw insertions using phantom measurements based on clinical observations of real operations
| Study | Examined technique | Fluorotime for conventional technique | Effective doses |
|---|---|---|---|
| Slomczykowski et al. [24] | Conventional fluoroscopy for lumbar spinal fusion; Sequential CT for CT-based navigation; Spiral CT optimized; Sequential CT optimized |
63 seconds per screw | 1. 1.0 mSv 2. 4.1 mSv 3. 3.0 mSv 4. 2.4 mSv |
| Perisinakis et al. [18] | Gender-averaged effective dose for pedicle screw internal fixation | 1.2 minutes AP and 2.1 minutes lateral view per procedure (4.8 screws) | 1.5 mSv |
| Jones et al. [10] | Recording of entry and scatter doses, review of records for fluoroscopy time, estimation of effective dose | 1.4 minutes per case (lumbar spine) | Source-superior position: 2.3 mSv Source-inferior position: 6.9 mSv |
| Current study | Conventional fluoroscopy for lumbar spinal fusion; 3-D navigated procedure; 3-D navigated SI screw; Conventional SI screw |
105 seconds per procedure (4 screws) | 1. 5.03 mSv 2. 0.4 mSv 3. 0.51 mSv 4. 2.5 mSv |
SI = sacroiliac.
We therefore quantified the organ and effective doses for patients for four different surgical procedures and compared the standard operation with the navigated one.
Patients and Methods
To evaluate the most representative intraoperative condition for use of conventional 2-D fluoroscopy, 20 consecutive patients with single vertebra fractures of the lumbar spine receiving posterior stabilization with the Universal Spine System (Synthes, Oberdorf, Switzerland) were observed; each was stabilized with four pedicle screws. The four examined surgical procedures were: (1) nonnavigated lumbar dorsal spinal fusion (four screws) using a fluoroscope (20 patients); (2) 3-D–navigated lumbar dorsal spinal fusion (four screws) (20 patients); (3) nonnavigated percutaneous transsacral screw stabilization for sacroiliac joint injuries of the dorsal ring of the pelvis (one screw) (two patients and literature review), and (4) 3-D–navigated percutaneous transsacral screw stabilization for sacroiliac joint injuries (one screw) (20 patients). The total fluoroscopy time was registered, the position of the C-arm during radiation (AP and lateral views) was recorded, and the settings of the xray tube were recorded in kilovolts and milliamperes (Table 5).
Table 5.
Conventional spine group
| Patient number | Age (years) | Gender | Fracture | Level | Number of screws | Exposure (seconds) | kV | mA |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 | M | L 1 | T 12/L 1 | 4 | 63 | 86 | 6 |
| 2 | 52 | M | L 3, L 4 | L 2/L 5 | 4 | 90 | 80 | 5 |
| 3 | 49 | F | L 2 | L 1/L 3 | 4 | 57 | 90 | 4 |
| 4 | 45 | M | L 1 | T 12/L 2 | 4 | 126 | 80 | 3.5 |
| 5 | 49 | M | L 1 | T 12/L 2 | 4 | 138 | 90 | 3.8 |
| 6 | 35 | M | L 1 | T 12/L 2 | 4 | 144 | 110 | 5 |
| 7 | 68 | M | L 2 | L 1/L 3 | 4 | 114 | 94 | 5 |
| 8 | 42 | M | L 1 | T 12/L 2 | 4 | 108 | 100 | 4.5 |
| 9 | 40 | M | L 1 | T 12/L 2 | 4 | 102 | 96 | 5 |
| 10 | 28 | M | L 1 | T 12/L 2 | 4 | 96 | 110 | 3 |
| 11 | 67 | M | L 3 | L 2/L 4 | 4 | 104 | 76 | 3.9 |
| 12 | 70 | M | L 1 | T 12/L 2 | 4 | 112 | 110 | 5 |
| 13 | 57 | F | L 3 | L 2/L 4 | 4 | 162 | 70 | 3.5 |
| 14 | 50 | M | L 1 | T 12/L 2 | 4 | 54 | 90 | 4 |
| 15 | 49 | M | L 3 | L 2/L 4 | 4 | 102 | 87 | 3.8 |
| 16 | 58 | F | L 1 | T 12/L 2 | 4 | 102 | 90 | 3.3 |
| 17 | 54 | F | L 2 | L 1/ L 3 | 4 | 96 | 88 | 6 |
| 18 | 37 | F | L 2 | L 1/L 3 | 4 | 138 | 76 | 4.3 |
| 19 | 39 | F | L1 | T 12/L 2 | 4 | 76 | 88 | 6 |
| 20 | 48 | M | L 1 | T 12/L 2 | 4 | 116 | 90 | 3.5 |
To determine dosages from the C-arm during 3-D–navigated procedures, 20 consecutive patients with single vertebra fractures of the lumbar spine receiving posterior stabilization with the Universal Spine System (Synthes) were observed; the fracture in each patient was stabilized with four pedicle screws (Table 6). To evaluate dosages from the C-arm for 3-D–navigated percutaneous transsacral screw stabilization for sacroiliac joint injuries, 20 patients were observed and the number of scans and additional use of the fluoroscope were recorded. For conventional iliosacral joint operations, no prospective patients could be enrolled because all cases at our institute have been navigated since the introduction of the CAS technique. The duration of radiation was analyzed in two retrospective cases in which the data were available and a review of the literature was performed to define the typical use of the C-arm in nonnavigated sacroiliac screw insertions [7, 21, 23, 29].
Table 6.
3-D navigation group
| Patient number | Age (years) | Gender | Fracture | Level | Number of screws | Exposure (seconds) | kV | mA |
|---|---|---|---|---|---|---|---|---|
| 1 | 57 | F | L 1 | T 12/L 2 | 4 | 67 | 87 | 4 |
| 2 | 68 | F | L 1 | T 12/L 2 | 4 | 102 | 67 | 2.3 |
| 3 | 70 | F | L 2 | L 1/L 3 | 4 | 65 | 70 | 3.5 |
| 4 | 47 | M | L 1 | T 12/L1 | 4 | 72 | 82 | 2.9 |
| 5 | 60 | M | L 1 | T 12/L 1 | 4 | 64 | 80 | 3.8 |
| 6 | 27 | M | L 2 | L 1/L 3 | 4 | 74 | 67 | 7 |
| 7 | 70 | F | L 1 | T 12/L 2 | 4 | 84 | 98 | 5.5 |
| 8 | 48 | M | L 1 | T 12/L 2 | 4 | 63 | 89 | 5 |
| 9 | 66 | M | L 1 | T 12/L 2 | 4 | 64 | 64 | 2.9 |
| 10 | 73 | F | L 4 | T 12/L 2 | 4 | 75 | 101 | 5.4 |
| 11 | 64 | F | L 4 | L 3/L 5 | 4 | 65 | 84 | 3.9 |
| 12 | 52 | M | L 2/L 3 | L 1/L 4 | 4 | 72 | 90 | 4.2 |
| 13 | 37 | M | L 2 | L 1/L 3 | 4 | 65 | 90 | 3.5 |
| 14 | 70 | F | L 1 | T 12/L 2 | 4 | 104 | 80 | 3.5 |
| 15 | 64 | F | L 4 | L 3/L 5 | 4 | 65 | 90 | 4 |
| 16 | 25 | F | L 1 | T 12/L 2 | 4 | 67 | 70 | 3.5 |
| 17 | 61 | F | L 2 | L 1/L 3 | 4 | 63 | 90 | 4.5 |
| 18 | 73 | F | L 1 | T 12/L 2 | 4 | 67 | 80 | 3.5 |
| 19 | 40 | F | L 1 | T 12/L 2 | 4 | 64 | 69 | 3.7 |
| 20 | 47 | M | L 1 | T 12/L 2 | 4 | 72 | 95 | 4.5 |
Handling of the fluoroscope always was performed by the same operating room personnel. For each operation, the same C-arm was used (ARCADIS Orbic 3D®; Siemens AG Healthcare Sector, Erlangen, Germany) with a Brainlab® navigation module (Brainlab®, Feldkirchen, Germany) mounted at any time. For AP views, the source-inferior position of the C-arm is used. For all navigated procedures, the Brainlab VectorVision®-Software (Brainlab®) and the Brainlab® navigation system (Brainlab®) was used. For 3-D navigation the C-arm rotates 190° around the patient and acquires 100 single 2-D images which are processed by the attached computer to a CT-like 3-D image set.
To simulate the average case scenario, scan protocols were developed based on the acquired clinical data for each of the four procedures in the previously described method (Table 7). These protocols reflect the typical average use of a C-arm for the respective procedure.
Table 7.
Radiation protocol for the spine and SI joint
| C-arm settings | 3-D navigated | Conventional |
|---|---|---|
| Spine | ||
| Duration | 72 seconds | 105 seconds |
| Position of C-arm | 1 × 3-D scan (60 seconds, high quality), 6 seconds AP, 6 seconds lateral |
79 seconds AP 26 seconds lateral |
| Sacroiliac joint | ||
| Duration | 87 seconds | 120 seconds |
| Position of C-arm | 1 × 3-D scan (60 seconds, high quality), 14 seconds AP, 7 seconds lateral, 3 seconds inlet, 3 seconds outlet |
60 seconds AP, 30 seconds lateral, 15 seconds inlet, 15 seconds outlet |
SI = sacroiliac.
For each of the four procedures, one Alderson-Rando Phantom was radiated according to the developed scan protocols using the same setup that was used for the actual operations that were performed. An anthropomorphic male Alderson-Rando Phantom (Alderson Research Laboratories, Long Island City, NY) consists of 35 axial segments containing a human skeleton and material with similar properties to soft tissues, including lungs (Fig. 3). The phantom was equipped with 126 thermoluminescence dosimeter (TLD) 100 readers (thermoluminescence dosimeters; Harshaw Thermo Fisher Scientific, LiF; 1 × 1 × 6 mm). The principle of this procedure is based on the ability of certain crystals to store energy (here in the form of ionizing radiation). During irradiation, electrons are freed from their bonds and can be captured by so-called ion traps in states of higher energy. These ion traps can be emptied by heating. The energy previously absorbed by x-radiation then is radiated away as visible light. The amount of light emitted can be measured in analyzing units. From this, it is possible to calculate back to the xray dose originally incident on the detector crystal. The TLD-100 is able to measure radiation from any direction independent of the axis of radiation and is widely used for dosimetry for fluoroscopy and tomography. The wide variety of TLD materials and their different physical forms allow the determination of different radiation qualities at dose levels from microGy to kGy [13, 16].
Fig. 3.
An anthropomorphic male Alderson-Rando Phantom consists of 35 axial segments containing human skeleton and material with similar properties to soft tissues.
The TLDs were placed in 42 positions in the phantom representing the organs. Three TLDs were placed to obtain reliable results at each place of measurement. The following organs were represented: gonads, red bone marrow, colon (sigmoid, rectum), lung, stomach, bladder, liver, esophagus, thyroid gland, bone surface, and the so-called remainder (small intestine, colon [ascending, descending, transverse], thymus, spleen, kidney, adrenals, pancreas, and brain). The organs were sorted according to the standards of the International Commission on Radiological Protection depending on their sensitivity beginning with the most sensitive [17]. According to the manufacturer, the useful range amounts from 0.01 mGy to 10 Gy. The calibration of the dosimeters was performed with an exposure to 80 mGy absorbed dose to water at 120 kV with a calibrated radiography machine (maximum error 3%). The dosimeters were preselected in subsets with similar sensitivities (variation in subset, less ± 2%). A TLD reader with a maximum error of 2% was used for readout (TLD reader 505A; National Panasonic, Hamburg, Germany). All readouts were made the day after irradiation of the TLDs. An annealing process was performed after readout with a TLD annealing oven (TLDO annealing oven; PTW-Freiburg, Freiburg, Germany). Calibration and readout were performed by a laboratory assistant. Each organ dose was weighted by multiplication with the weighting factor provided by the 1990 Commission on Radiological Protection [17]. The effective dose was calculated by summarizing the weighted organ doses (Table 3).
The phantom was positioned like a patient to simulate the respective procedure (Fig. 4).
Fig. 4.
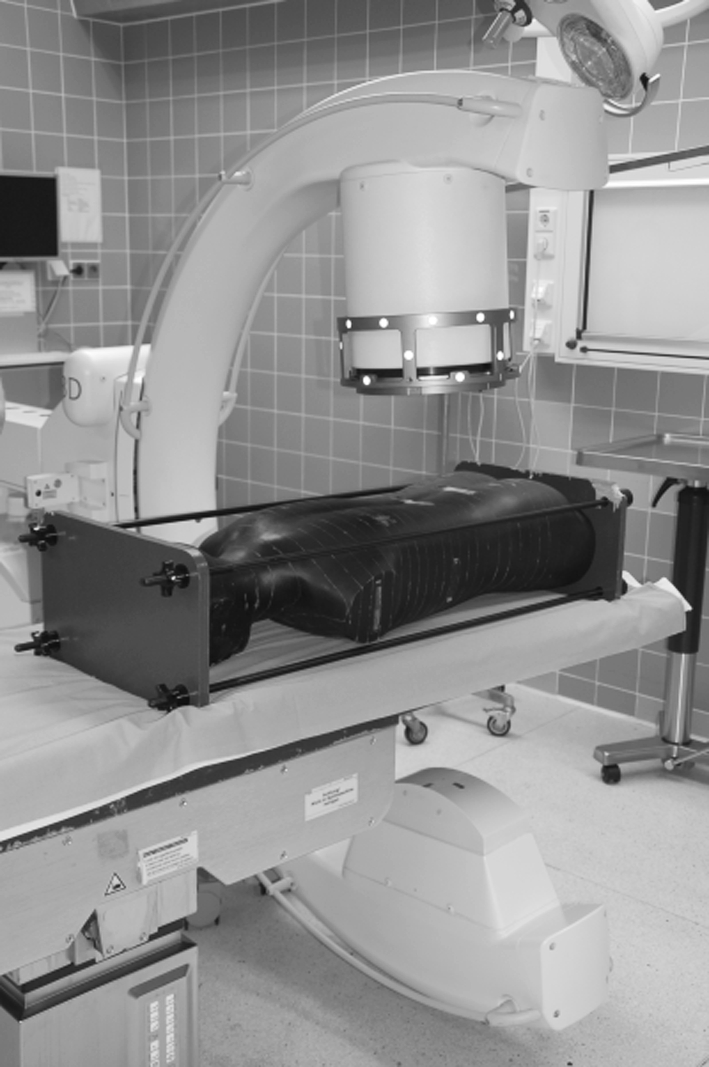
The Phantom was irradiated according to the scan protocols. The real use of the C-arm was simulated on the phantom, which was positioned in the same manner as a patient on the operating room table.
The setup and data were recorded in an operating room using standard hospital equipment (Fig. 5). In all cases, we used the same operating table (Maquet Carbon Table, Rastatt, Germany). The radiated phantom was returned to Siemens Healthcare to read out the TLDs and to reset the phantom for the next measuring cycle.
Fig. 5.
Standard hospital equipment was used for irradiation of the phantom. The same equipment was used for the real operations.
Differences in the transection until suture time and radiation time between navigated and nonnavigated operations were determined with the Wilcoxon signed rank test (StatView®; SAS, Version 5.0, Cary, NC).
Members of Siemens Healthcare had no influence on the readout or evaluation of any study data. The Institution’s Committee on Ethics approved the study.
Results
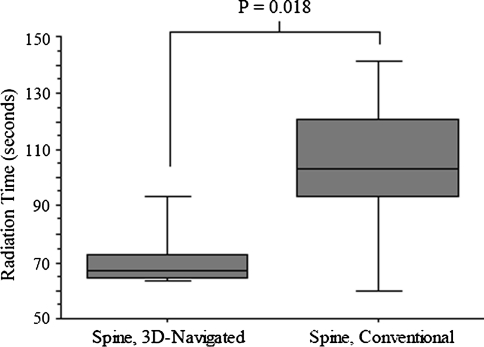
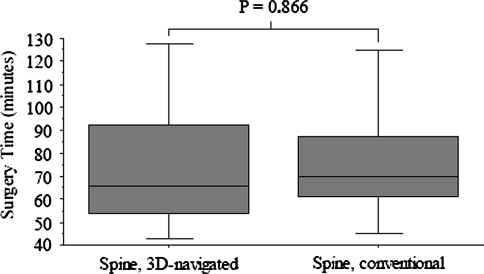
For spinal lumbar fusion, the mean duration of radiation was decreased (p = 0.018) for navigated operations (n = 20; 72 seconds ± 12; range, 63–104 seconds) in comparison to nonnavigated operations (n = 20; 105 seconds ± 29; range, 54–162 seconds) (Fig. 6). For nonnavigated operations, the position of the C-arm was mainly in an AP view (79 seconds ± 24; range, 24–121 seconds) in comparison to the lateral position (26 seconds ± 14; range, 7–60 seconds). The time from transection until suture for navigated operations (76 minutes ± 32; range, 35–145 minutes) was comparable (p = 0.86) to time for nonnavigated spinal lumbar fusions (76 minutes ± 25; range, 45–130 minutes) (Fig. 7). The exposure factors are slightly higher (kV, p = 0.03; mA, p = 0.26) for nonnavigated operations (90 kV ± 11; range, 70-110 kV; 4.4 mA ± 1 mA; range, 3–6 mA) in comparison to the 3-D–navigated spinal fusion (82 kV ± 11; range, 64–101 kV; 4 mA ± 1; range, 2.3–7 mA).
Fig. 6.
Radiation time is reduced by 3-D navigation compared with the conventional approach for lumbar spinal fusion.
Fig. 7.
The total duration of surgery is relatively similar comparing 3-D navigation with conventional lumbar spinal fusion.
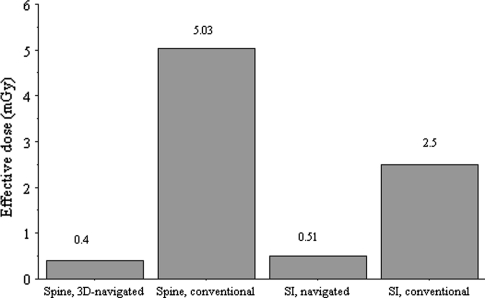
The effective dose, determined with the Alderson-Rando Phantom for 3-D–navigated spinal fusions, was 0.4 mSv in comparison to 5.03 mSv for conventional operations (Fig. 8). All organ doses were greater for nonnavigated operations (Table 8).
Fig. 8.
The effective dose is less for 3-D–navigated operations at the spine and at the pelvis. SI = sacroiliac.
Table 8.
Organ dose for different procedures (in order of sensitivity)
| Organ dose (mGy) for different procedures | ||||
|---|---|---|---|---|
| Organs in order of sensitivity | 3-D navigated spinal fusion | Conventional fluoroscopy- based spinal fusion | 3-D navigated SI screw | Conventional SI screw |
| Gonads | ||||
| Ovaries | 0.03 | 0.97 | 0.45 | 4.59 |
| Testes | 0.04 | 0.83 | 0.16 | 0.79 |
| Bone marrow (red) | 0.61 | 22.48 | 2.31 | 14.39 |
| Sigmoid/rectum | 0.05 | 2.22 | 1.23 | 3.24 |
| Lung | 0.03 | 0.17 | 0.04 | 0.14 |
| Stomach | 1.55 | 8.28 | 0.08 | 0.23 |
| Bladder | 0.07 | 3.47 | 0.32 | 1.88 |
| Liver | 1.18 | 3.30 | 0.07 | 0.33 |
| Oesophagus | 0.02 | 0.09 | 0.04 | 0.08 |
| Thyroid gland | 0.00 | 0.09 | 0.01 | 0.02 |
| Bone surface | 0.46 | 1.68 | 0.16 | 0.79 |
| Remaining total | 1.02 | 10.47 | 0.38 | 1.14 |
| Adrenals | 1.15 | 4.68 | 0.10 | 0.40 |
| Brain | 0.00 | 0.02 | 0.00 | 0.03 |
| Small intestine | 1.43 | 31.14 | 0.28 | 0.92 |
| Kidneys | 1.79 | 13.26 | 2.35 | 0.74 |
| Pancreas | 1.09 | 4.79 | 0.09 | 0.30 |
| Spleen | 2.59 | 16.96 | 0.16 | 0.43 |
| Thymus | 0.00 | 0.06 | 0.02 | 0.06 |
| Colon (ascending, descending, transverse) | 1.16 | 22.32 | 2.07 | 2.82 |
SI = sacroiliac.
For the 3-D–navigated sacroiliac screw insertion (n = 20), the mean duration of radiation (87 seconds ± 10; range, 72–112 seconds) was reduced (p = 0.179) in comparison to conventional sacroiliac screw insertion (n = 2) (Table 7) (120 seconds; Case 1: 109 seconds, Case 2: 131 seconds). The time from transection until suture for navigated sacroiliac screw insertions was 33 minutes ± 16 minutes (range, 15–60 minutes). For conventional sacroiliac screw operations, the time from transection until suture was 25 minutes for Case 1 and 34 minutes for Case 2. The exposure factors for navigated sacroiliac screw insertions (85 kV ± 15; range, 63–110; 3 mA ± 0.5 mA; range, 2–3.5 mA) could not be compared with conventional operations; the data were not documented for the two retrospective cases.
The effective dose for 3-D–navigated sacroiliac screw insertions was 0.51 mSv and for conventional sacroiliac screw insertions 2.5 mSv (Fig. 8). All organ doses except for the kidneys were less in the navigated group (Table 8).
Discussion
The emission of radiation for medical purposes is increasing [8]. New technologies have been introduced and probably can be helpful in reducing the emitted and absorbed doses during surgery [5, 6, 9, 11, 25]. The purpose of this study was to evaluate the typical use of the C-arm and the effective and organ doses for patients having 3-D–navigated and conventional lumbar spinal fusion procedures and, as a second group, for 3-D–navigated and conventional sacroiliac screw insertions.
We acknowledge limitations to our study. First, we did not measure absorbed doses of the operating room personnel, therefore no conclusion can be made regarding reduction for the staff when using computer-navigated systems. However, the less radiation emitted, the better it is for staff working in an environment in which radiation is used, more or less, on a daily basis. Second, the control group for the navigated cases was based on a review of the literature, as all our cases had been done using navigation after the introduction of 3-D navigation at our institute. We had only two retrospective cases in which the relevant data were available. The quality of retrospective determined data cannot be compared with the prospective design of the spinal fusion procedure. Third, the different positions of the C-arm during irradiation of the phantom were based on techniques described in the literature and not on our data. To determine the average length of use of a fluoroscope for conventional insertion of SI screws, we analyzed several studies (Table 9) describing the position during irradiation. Fourth, the phantom measurements in our study show the average effective dose for patients enrolled in this study and can differ considerably depending on the size of the patient, experience of the surgeon, the personnel who work the C-arm, and many other factors. Our findings therefore must be interpreted as trend and may not be valid for each case. Fifth, the results depend on the described surgical technique and the equipment used. In other places the technique may be different than ours, and therefore the emission of radiation also may be different. In this case a similar study with a modified scan protocol is necessary to show the effect of the differing technique on the effective dose.
Table 9.
Studies examining emission of radiation for conventional SI screw insertions
| Study | Study design | Materials and methods | Results/conclusion |
|---|---|---|---|
| Schep et al. [23] | 48 nonnavigated versus 48 navigated screws | Comparison of radiation time | Navigation: 0.7 minutes Conventional: 1.8 minutes |
| Zwingmann et al. [29] | Comparison navigated versus nonnavigated operations | 26 navigated versus 35 conventional screws | Navigation: 63 seconds Conventional: 141 seconds |
| Hilgert et al. [7] | Measuring radiation time for conventional screw insertion | 24 screws | Conventional: 1.6 minutes per screw |
| Current study | 20 clinical 3-D-navigated cases and literature review | 20 navigated versus conventional approach (literature) | 3-D–navigated effective dose: 0.51 mSv Conventional: 2.5 mSv |
There are limitations comparing observations from different studies because many factors can influence the outcome of the measurement [8, 13, 16]. One factor with an incalculable effect on the emission of radiation is the size of the patient. With increasing thickness of the patient, exposure to the patient and staff also is increasing [15, 27]. Fluoroscopes automatically increase the exposure factors (kV, mA) when more mass must be screened. This cannot be influenced by the surgeon, but navigation may be an option to reduce the emission of radiation, especially when bad image quality and size of the patient force extensive use of radiation. This needs to be evaluated in additional studies; we did not evaluate the influence of body mass in this study. Another factor that influences the comparability of the studies is the evaluated duration of radiation for the respective procedure. Slomczykowski et al. [24] used 378 seconds in different positions to the phantom; the position of the source was not stated. Jones et al. [10] used 84 seconds and compared the effective dose with the position of the source (superior 2.3 mSv, inferior 6.9 mSv). In all studies, different C-arms were used and positions during radiation were different. Position of the source especially seems to have a major influence on the effective dose of the patient [10]. Jones et al. [10] reported a reduction of the patient’s effective dose for the source-superior position; the surgeon was exposed to a greater dose for this C-arm position. Another influencing effect is loading of the phantom with TLD. There is no standardized procedure to equip the phantom, and even the number of TLDs used varies among studies. Directly comparable results are delivered only by the same study protocol. Slomczykowski et al. [24] reported a higher effective dose for the patient when CT-based navigation was used; 3-D navigation did not exist at the time of their study.
3-D–navigated procedures for pedicle screw insertion can reduce the duration of radiation and the effective and organ doses of patients with a comparable duration of the surgical procedure. We identified three studies in which comparable methods were used to examine the same issue (Table 4). First, Slomczykowski et al. [24] used a phantom to evaluate the organ and effective doses of patients for pedicle screw insertion using a conventional approach and compared the conventional approach with CT-based navigation. As in our study, they measured the use of the C-arm for different techniques and applied the average radiation on the phantom. They reported a greater effective dose for the patient when using the CT-based navigated technique. Slomczykowski et al. included the preoperative CT scan in the measurement, which is essential for this type of navigation. In comparison to our study, the effective dose for conventional operations was 1 mSv, which is only one-fifth of the dose we measured. Two other studies also quantified the effective dose for conventional operations with doses ranging from 1.5 mSv to 6.9 mSv [10, 18]. Compared with published data, the results of our study are in the normal range. Second, Perisinakis et al. evaluated the radiogenic risks for cancer induction after pedicle screw fixation and found an induction rate of 110 per million [18]. This and the incalculable risks of deterministic stochastic effects deriving from ionizing radiation should support every means to reduce the burden for patients and operating room personnel [12, 28]. Therefore, Perisinakis et al. recommended use of computer navigation at the spine only for certain procedures to keep the effective dose of the patient as low as possible. In contrast to CT-based navigation, the relatively new 3-D navigation can reduce radiogenic risks for patients for pedicle screw insertions and therefore we believe should be the preferred approach; otherwise the high image quality of the CT scan is needed for safe navigation. Third, Jones et al. [10] used an anthropomorphic phantom to estimate the effective dose for lumbar pedicle screw insertion and showed an effective dose for the patient of 2.3 mSv for the source-superior position and an effective dose of 6.8 mSv for the source-inferior position, the mean radiation time was 1.4 minute per case. Compared with our results, Jones et al. reported a higher effective dose for conventional screw insertion in either position of the source in comparison to the navigated approach. The average effective dose can be compared with our dose results for the conventional method. Jones et al. described greater exposure to the surgeon in the source-superior position and therefore recommended this position, although the effective dose of the patient increases. As a conclusion the best results for patient and surgeon regarding the exposure to radiation may be the use of a navigation system and the source-inferior position for the control-images.
The second examined procedure is minimally invasive screw fixation of the sacroiliac joint. Published radiation times (Table 9) and those of our two cases for conventional sacroiliac screw insertion are greater than the evaluated time for navigated sacroiliac screw procedures [7, 23, 29]. As a result, the patient’s effective dose also is reduced. Similar results regarding radiation time and emission of radiation were published in a prospective study that compared navigated versus nonnavigated sacroiliac screw insertion [29]. As a result of the study design, no effective doses of patients were evaluated, but the total radiation time for navigated operations (63 ± 15 seconds; range, 36–84 seconds; 822 ± 164 cGy/cm2; range, 542–1145 cGy/cm2) was reduced compared with nonnavigated procedures (141 ± 69 seconds; range, 42–252 seconds; 1843 ± 1052 cGy/cm2; range, 600–3811 cGy/cm2). In another study, the radiation time for navigated operations was 42 seconds and for nonnavigated sacroiliac screw insertions 108 seconds [23]. These studies and our current results allow us to conclude that the emission of radiation is reduced with computer-navigated surgery.
Based on our data, we recommend the use of the 3-D–navigated techniques whenever they are available to reduce the emission of radiation and decrease the patient’s effective dose. When using navigated techniques, the surgeon still must be able to perform the operation the conventional way; the more sophisticated the systems are, the more often a technical failure may occur. The surgeon then must be able to continue the operation without computer guidance.
Footnotes
This study was funded in part by Siemens AG Healthcare Sector (Erlangen, Germany). Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Carbone JJ, Tortolani PJ, Quartararo LG. Fluoroscopically assisted pedicle screw fixation for thoracic and thoracolumbar injuries: technique and short-term complications. Spine (Phila Pa 1976). 2003;28:91–97. [DOI] [PubMed]
- 2.Fu TS, Chen LH, Wong CB, Lai PL, Tsai TT, Niu CC, Chen WJ. Computer-assisted fluoroscopic navigation of pedicle screw insertion: an in vivo feasibility study. Acta Orthop Scand. 2004;75:730–735. doi: 10.1080/00016470410004102. [DOI] [PubMed] [Google Scholar]
- 3.Gaines RW., Jr The use of pedicle-screw internal fixation for the operative treatment of spinal disorders. J Bone Joint Surg Am. 2000;82:1458–1476. doi: 10.2106/00004623-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Gebhard F, Kraus M, Schneider E, Arand M, Kinzl L, Hebecker A, Bätz L. [Radiation dosage in orthopedics: a comparison of computer-assisted procedures] [in German] Unfallchirurg. 2003;106:492–497. doi: 10.1007/s00113-003-0606-9. [DOI] [PubMed] [Google Scholar]
- 5.Gebhard F, Weidner A, Liener UC, Stockle U, Arand M. Navigation at the spine. Injury. 2004;35(suppl 1):S-A35–45. [DOI] [PubMed]
- 6.Gebhard FT, Kraus MD, Schneider E, Liener UC, Kinzl L, Arand M. Does computer-assisted spine surgery reduce intraoperative radiation doses? Spine (Phila Pa 1976). 2006;31:2024–2027; discussion 2028. [DOI] [PubMed]
- 7.Hilgert RE, Finn J, Egbers HJ. [Technique for percutaneous iliosacral screw insertion with conventional C-arm radiography] [in German] Unfallchirurg. 2005;108(954):956–960. doi: 10.1007/s00113-005-0967-3. [DOI] [PubMed] [Google Scholar]
- 8.Huda W, Nickoloff EL, Boone JM. Overview of patient dosimetry in diagnostic radiology in the USA for the past 50 years. Med Phys. 2008;35:5713–5728. doi: 10.1118/1.3013604. [DOI] [PubMed] [Google Scholar]
- 9.Izadpanah K, Konrad G, Sudkamp NP, Oberst M. Computer navigation in balloon kyphoplasty reduces the intraoperative radiation exposure. Spine (Phila Pa 1976). 2009;34:1325–1329. [DOI] [PubMed]
- 10.Jones DP, Robertson PA, Lunt B, Jackson SA. Radiation exposure during fluoroscopically assisted pedicle screw insertion in the lumbar spine. Spine (Phila Pa 1976). 2000;25:1538–1541. [DOI] [PubMed]
- 11.Kim CW, Lee YP, Taylor W, Oygar A, Kim WK. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008;8:584–590. doi: 10.1016/j.spinee.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures. Part 2: review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13–20. doi: 10.2214/ajr.177.1.1770013. [DOI] [PubMed] [Google Scholar]
- 13.Kron T. Thermoluminescence dosimetry and its applications in medicine. Part 1: physics, materials and equipment. Australas Phys Eng Sci Med. 1994;17:175–199. [PubMed] [Google Scholar]
- 14.Merloz P, Troccaz J, Vouaillat H, Vasile C, Tonetti J, Eid A, Plaweski S. Fluoroscopy-based navigation system in spine surgery. Proc Inst Mech Eng H. 2007;221:813–820. doi: 10.1243/09544119JEIM268. [DOI] [PubMed] [Google Scholar]
- 15.Miller DL, Kwon D, Bonavia GH. Reference levels for patient radiation doses in interventional radiology: proposed initial values for U.S. practice. Radiology. 2009;253:753–764. doi: 10.1148/radiol.2533090354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscovitch M, St John TJ, Cassata JR, Blake PK, Rotunda JE, Ramlo M, Velbeck KJ, Luo LZ. The application of LiF:Mg, Cu, P to large scale personnel dosimetry: current status and future directions. Radiat Prot Dosimetry. 2006;119:248–254. doi: 10.1093/rpd/nci692. [DOI] [PubMed] [Google Scholar]
- 17.Mountford PJ, Temperton DH. Recommendations of the International Commission on Radiological Protection (ICRP) 1990. Eur J Nucl Med. 1992;19:77–79. doi: 10.1007/BF00184120. [DOI] [PubMed] [Google Scholar]
- 18.Perisinakis K, Theocharopoulos N, Damilakis J, Katonis P, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Estimation of patient dose and associated radiogenic risks from fluoroscopically guided pedicle screw insertion. Spine (Phila Pa 1976). 2004;29:1555–1560. [DOI] [PubMed]
- 19.Rampersaud YR, Foley KT, Shen AC, Williams S, Solomito M. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine (Phila Pa 1976). 2000;25:2637–2645. [DOI] [PubMed]
- 20.Reinhold M, Knop C, Beisse R, Audigé L, Kandziora F, Pizanis A, Pranzl R, Gercek E, Schultheiss M, Weckbach A, Bühren V, Blauth M. [Operative treatment of traumatic fractures of the thorax and lumbar spine. Part II: surgical treatment and radiological findings] [in German] Unfallchirurg. 2009;112:149–167. doi: 10.1007/s00113-008-1538-1. [DOI] [PubMed] [Google Scholar]
- 21.Routt ML, Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9:207–214. doi: 10.1097/00005131-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Sagi HC, Manos R, Park SC, Von Jako R, Ordway NR, Connolly PJ. Electromagnetic field-based image-guided spine surgery part two: results of a cadaveric study evaluating thoracic pedicle screw placement. Spine (Phila Pa 1976). 2003;28:E351–E354. [DOI] [PubMed]
- 23.Schep NW, Haverlag R, Vugt AB. Computer-assisted versus conventional surgery for insertion of 96 cannulated iliosacral screws in patients with postpartum pelvic pain. J Trauma. 2004;57:1299–1302. doi: 10.1097/01.TA.0000133573.53587.2E. [DOI] [PubMed] [Google Scholar]
- 24.Slomczykowski M, Roberto M, Schneeberger P, Ozdoba C, Vock P. Radiation dose for pedicle screw insertion: fluoroscopic method versus computer-assisted surgery. Spine (Phila Pa 1976). 1999;24:975–982; discussion 983. [DOI] [PubMed]
- 25.Smith HE, Welsch MD, Sasso RC, Vaccaro AR. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J Spinal Cord Med. 2008;31:532–537. doi: 10.1080/10790268.2008.11753648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjardes T, Shafizadeh S, Rixen D, Paffrath T, Bouillon B, Steinhausen ES, Baethis H. Image-guided spine surgery: state of the art and future directions. Eur Spine J. 2010;19:25–45. doi: 10.1007/s00586-009-1091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vano E, Gonzalez L, Fernandez JM, Prieto C, Guibelalde E. Influence of patient thickness and operation modes on occupational and patient radiation doses in interventional cardiology. Radiat Prot Dosimetry. 2006;118:325–330. doi: 10.1093/rpd/nci369. [DOI] [PubMed] [Google Scholar]
- 28.Vlietstra RE, Wagner LK, Koenig T, Mettler F. Radiation burns as a severe complication of fluoroscopically guided cardiological interventions. J Interv Cardiol. 2004;17:131–142. doi: 10.1111/j.1540-8183.2004.09885.x. [DOI] [PubMed] [Google Scholar]
- 29.Zwingmann J, Konrad G, Kotter E, Sudkamp NP, Oberst M. Computer-navigated iliosacral screw insertion reduces malposition rate and radiation exposure. Clin Orthop Relat Res. 2009;467:1833–1838. doi: 10.1007/s11999-008-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]