Abstract
Background/rationale
Although TKA reliably reduces pain from knee osteoarthritis, full recovery of muscle strength and physical function to normal levels is rare. We presumed that a better understanding of acute changes in hamstrings and quadriceps muscle performance would allow us to enhance early rehabilitation after TKA and improve long-term function.
Questions/purposes
The purposes of this study were to (1) evaluate postoperative quadriceps and hamstrings muscle strength loss after TKA and subsequent recovery using the nonoperative legs and healthy control legs for comparison, and (2) measure hamstrings coactivation before and after TKA during a maximal isometric quadriceps muscle contraction and compare with nonoperative and healthy control legs.
Methods
We prospectively followed 30 patients undergoing TKA at 2 weeks preoperatively and 1, 3, and 6 months postoperatively and compared patient outcomes with a cross-sectional cohort of 15 healthy older adults. Bilateral, isometric strength of the quadriceps and hamstrings was assessed along with EMG measures of hamstrings coactivation during a maximal isometric quadriceps contraction.
Results
There were no differences in strength loss or recovery between the quadriceps and hamstrings muscles of the operative leg throughout the followup, although differences existed when compared with nonoperative and healthy control legs. Hamstrings muscle coactivation in the operative leg during a maximal quadriceps effort was elevated at 1 month (144.5%) compared to the nonoperative leg.
Conclusions
Although quadriceps dysfunction after TKA typically is recognized and addressed in postoperative therapy protocols, hamstrings dysfunction also is present and should be addressed.
Clinical Relevance
Quadriceps and hamstrings muscle strengthening should be the focus of future rehabilitation programs to optimize muscle function and long-term outcomes.
Introduction
More than 500,000 TKAs are performed each year in the United States [2] and future projections indicate by 2030, 3.48 million will be performed each year to alleviate pain and disability associated with knee osteoarthritis (OA) [18]. Although TKA reliably reduces pain and improves function in older adults with knee OA, the recovery of muscle strength and function to normal levels is rare, which predisposes patients to future disability with increasing age [32, 39, 44].
The loss of quadriceps strength after TKA has been studied extensively and is a result of a combination of insults, including preexisting quadriceps weakness characteristic of knee OA [11, 20, 33, 40], surgical trauma during TKA [8, 37], and age-related limitations in the recovery of muscle function [12, 23, 45]. One month after surgery, quadriceps strength decreases to 60% of preoperative levels despite initiating postoperative rehabilitation within 24 hours after surgery [26, 41]. Even 6 to 13 years after surgery, quadriceps weakness persists [13], walking performance remains approximately 20% to 30% less than that of healthy age-matched older adults [29, 44], and more physically demanding tasks such as stair climbing are almost 50% less than those of healthy age-matched adults [44].
Although quadriceps strength has been investigated extensively [5–7, 24–28, 41, 42], less investigation has focused on the strength of other lower extremity muscles before and after TKA [1]. In particular, few studies have evaluated hamstrings muscle strength [5, 21, 39, 44], and those that measured hamstrings strength did so months to years after surgery [39, 44]. Some of these studies suggest hamstrings strength recovers faster than quadriceps strength [5, 39], but others suggest the hamstrings remain comparably dysfunctional to the quadriceps years after surgery [39, 44]. Thus, we presume better understanding of acute changes in muscle strength would help guide the focus of rehabilitation early after surgery.
Both the quadriceps and hamstrings provide functional stability and shock absorption to the tibiofemoral joint [38]. In a healthy joint, coactivation of the quadriceps and hamstrings occurs to functionally reduce shear forces and strain across the tibiofemoral joint [38], but excessive coactivation also may increase compressive forces and joint loading causing extra wear and tear of articular cartilage or knee prostheses [4, 15, 19, 38]. Furthermore, although some coactivation during normal lower limb movement may improve movement efficiency by increasing joint stabilization and protection, excessive coactivation may result in impaired movement and weakness [4, 9]. Weakness with severe OA has been associated with greater coactivation of muscles surrounding the knee [14]. Therefore, profound weakness in the quadriceps and hamstrings muscles early after TKA could further increase muscle coactivation and compromise normal lower extremity function acutely, yet no investigations have explored this possibility to date. It is unclear if the hamstrings are as compromised as the quadriceps in the acute postoperative phase, but if so, both muscle groups should be targeted in aggressive postoperative rehabilitation.
Therefore, the purposes of this investigation were (1) to evaluate postoperative quadriceps and hamstrings muscle strength loss after TKA and subsequent recovery using the nonoperative legs and healthy control legs for comparison, and (2) to measure hamstrings coactivation before and after TKA during a maximal isometric quadriceps muscle contraction and compare with nonoperative and healthy control legs. We hypothesized that (1) compared with the quadriceps, the hamstrings would show less strength loss and faster recovery after TKA, but both muscle groups would be weaker than muscles of nonoperative and healthy control legs preoperatively and 6 months after TKA; and (2) hamstrings coactivation during maximal isometric quadriceps muscle contraction would be elevated compared with that of nonoperative and healthy control legs acutely (ie, 1 month after TKA) and 6 months after TKA.
Patients and Methods
We prospectively followed patients scheduled for a unilateral, primary TKA between June 2006 and August 2008. We evaluated bilateral quadriceps and hamstrings strength and hamstrings EMG coactivation at 2 weeks preoperatively and 1 month, 3 months, and 6 months postoperatively for comparisons with a cross-sectional cohort of healthy adults. We chose to examine the recovery of muscle strength and function after TKA through 6 months postoperatively because previous studies suggest strength and functional recovery typically plateau approximately 6 months after surgery [5, 16].
Sample size calculations were centered on our primary hypothesis that the hamstrings would show less strength loss after TKA compared with the quadriceps. We considered a difference of 15 percentage points to be clinically relevant because a 10% side-to-side strength difference is common yet functionally unnoticeable in healthy individuals [17, 34]. With a standard deviation of 19 (unpublished data) and assuming a two-sided Type I error protection of 0.05 and a power of 0.80, we anticipated that 27 patients were required; therefore, we enrolled 30 patients.
Thirty patients (13 women and 17 men; Table 1) were included between the ages of 50 and 85 years. The patients were control (untreated) subjects from an ongoing clinical trial examining the early effects of neuromuscular electrical stimulation after TKA. Exclusion criteria for the clinical trial included the following: (1) uncontrolled hypertension; (2) uncontrolled diabetes (HbA1C greater than > 7.0); (3) body mass index (BMI) greater than 35 kg/m2; (4) symptomatic OA in the contralateral knee (defined as patients reporting at least half of the pain on their nonoperative knee compared with their operative knee on an 11-point verbal analog scale); (5) lower extremity, unstable orthopaedic problems beyond knee OA that limited the patient’s physical function; and (6) any neurologic impairment. During the time of recruitment (June 2006 to August 2008), 504 patients underwent primary, unilateral TKA, of which 259 were screened for eligibility in the clinical trial.
Table 1.
Demographic information for patients undergoing TKA and healthy age-matched control subjects
| Patients | Number of patients | Gender (male:female) | Age (years)* | Body mass index (kg/m2)* |
|---|---|---|---|---|
| Patients undergoing TKA | 30 | 17:13 | 64.3 ± 9.2 | 29.8 ± 4.3 |
| Healthy age-matched control subjects | 15 | 9:6 | 66.5 ± 6.5 | 27.1 ± 3.5 |
| p Value | 0.34 | 0.03 |
* Values are expressed as mean ± SD.
We also recruited a convenience sample of 15 healthy control subjects from the community between December 2008 and March 2009 for comparison (Table 1). These healthy individuals had no history of knee pain and no lower extremity orthopaedic problems that limited function. All participated in at least 30 minutes of aerobic activity three times a week. There were no differences in the ages of healthy control subjects and patients undergoing TKA (Table 1). There were differences in BMI between groups, but previous investigation has shown, for BMIs less than 40 kg/m2, BMI does not influence strength or functional performance after TKA [43]. The study was approved by the Colorado Multiple Institutional Review Board. All participants provided written informed consent before participation.
TKA was performed by one of three surgeons using a medial parapatellar approach and PCL-sparing, cemented, modular fixed-bearing components. Surgery began with a medial parapatellar incision from the level of the distal tibial tubercle to approximately 6 cm proximal to the superior border of the patella. Resurfacing began with patellar resection, then proceeded with a proximal tibial osteotomy followed by resurfacing of the femur with distal, anterior, posterior, and chamfer cuts. Closure was performed using absorbable sutures for the capsule, subcutaneous tissue, and skin.
All postoperative rehabilitation was standardized using progressive exercises focused on quadriceps and hamstrings muscle strength. Inpatient rehabilitation at the University of Colorado Hospital (3–4 days) was followed by 2 weeks of home physical therapy (six to seven visits), after which patients proceeded to outpatient physical therapy for an average of 10 additional visits. All home therapists and outpatient clinics used a standardized rehabilitation protocol that was centered on an impairment-based model previously described [36, 42]. Although all impairments were treated, interventions targeted knee ROM, patellar mobility, pain control, gait deviations, and strength of the quadriceps, hamstrings, hip abductors, and plantar flexors. Open and closed-chain exercises were initiated with two sets of 10 repetitions and then progressed to three sets of 10 repetitions. Weights were increased to maintain a 10-repetition maximum targeted intensity level.
Bilateral, isometric strength of the quadriceps and hamstrings was measured before (preoperatively) and after (1, 3, 6 months) unilateral TKA. In brief, subjects were positioned in an electromechanical dynamometer (Humac Norm; CSMI, Stoughton, MA) with their knee flexed and stabilized at 60° flexion. The anatomic axis of the knee was aligned with the axis of the dynamometer. A force transducer was secured around the lower leg 2 cm above the proximal pole of the lateral malleolus. A seat belt harness was placed around the patient’s chest and waist for stabilization. Patients were asked to perform a maximal voluntary isometric contraction (MVIC) of the hamstrings followed by an MVIC of the quadriceps muscles using visual and verbal feedback. Testing for each muscle group was repeated up to three times, with 1 minute of rest between trials, until two attempts were within 5% of each other. The trial with the largest maximal volitional isometric force output was used for data analysis.
EMG was used to quantify hamstrings coactivation during a quadriceps MVIC similar to previously described methods [30]. A pair of surface EMG electrodes (20-mm diameter) was placed over the muscle belly of the biceps femoris at approximately one-third of the muscle length from the popliteal fossa. Electrodes were placed 2 cm apart over the biceps femoris and a ground electrode was placed over the medial malleolus. The surface electrodes were attached to a Biopac MP150WSW system (Biopac Systems, Inc, Goleta, CA) for data acquisition at 2 kHz. All EMG signals were digitally high-pass filtered at 10 Hz, low-pass filtered at 500 Hz, notch filtered at 60 Hz, and subsequently smoothed by a moving root mean square average with a time constant of 50 ms. After signal processing, the hamstrings (biceps femoris) peak EMG signal during a maximal quadriceps MVIC was normalized to the peak hamstrings EMG signal during a maximal hamstrings MVIC to calculate coactivation (peak hamstrings EMG during quadriceps MVIC/peak hamstrings EMG during hamstrings MVIC). The healthy control subjects were tested at one time using identical methods for comparison of muscle strength and hamstrings coactivation outcomes.
To address the first hypothesis comparing hamstrings and quadriceps muscle weakness after TKA, a two-way analysis of variance (ANOVA) (muscle group × time) was used to test for differences between the quadriceps and hamstrings in the operative leg over time (ie, recovery of strength after TKA) using preoperative strength as a covariate. Furthermore, a one-way ANOVA was used to assess differences in muscle strength between the operative legs, nonoperative legs, and legs of control subjects preoperatively and 6 months after surgery. Comparisons to healthy control subjects used the right leg of control subjects because there were no statistical differences in muscle strength or coactivation between the right and left legs of healthy control subjects.
To address the second hypothesis that hamstrings coactivation during a maximal isometric quadriceps muscle contraction would be elevated after TKA compared with nonoperative and healthy control legs, a multivariate ANOVA (leg × time) was used to test for differences in hamstrings coactivation between legs across all four times with Tukey’s post hoc testing as appropriate. All statistical analyses were performed with SPSS® for Windows®, Version 17.0 (SPSS Inc, Chicago, IL).
Results
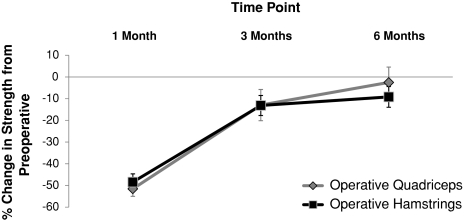
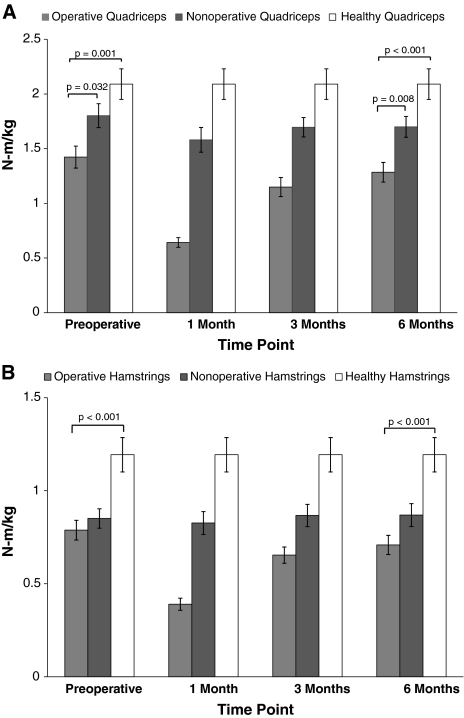
There were no differences (p = 0.17) in strength loss or recovery between quadriceps and hamstrings strength throughout the followup (Fig. 1). Preoperatively, the quadriceps strength of the operative leg was weaker than that of the nonoperative leg (21.0%; p = 0.032) and healthy control legs (32%; p = 0.001; Fig. 2A), whereas the strength of the hamstrings of the operative leg was not weaker than that of the nonoperative leg (p = 0.702; Fig. 2B), but was weaker than that of healthy control legs (34%; p < 0.001). One month after TKA, quadriceps and hamstrings muscle strength decreased 51.56% and 48.43%, respectively. Following the same pattern seen preoperatively, at 6 months after surgery, the quadriceps strength of the operative leg remained weaker than the nonoperative (24.4%; p = 0.008) and healthy control legs (38.5%; p < 0.001; Fig. 2A), whereas the hamstrings strength of the operative leg was not weaker than that of the nonoperative leg (p = 0.142), but was weaker than that of healthy control legs (40.6%; p < 0.001; Fig. 2B).
Fig. 1.
The percentage change in quadriceps and hamstrings strength in the operative leg after TKA is shown. There were no differences in strength loss or recovery between the quadriceps (dark gray) and hamstrings (light gray) muscles during the first 6 months after TKA (mean ± SE of the mean [SEM]). Statistical analyses were performed on raw strength (N-m)/body weight (kg) values, not percentage change from preoperative levels.
Fig. 2A–B.
Strength with time for the operative, nonoperative, and healthy control (A) quadriceps and (B) hamstrings are shown. Quadriceps strength in the operative leg (A) was significantly less than for the nonoperative and healthy legs preoperatively and 6 months after TKA. Hamstrings strength in the operative leg (B) was significantly less than for healthy legs, preoperatively and 6 months after TKA, yet was not significantly different from nonoperative legs at either time. Strength (N-m) is normalized to body weight (kg) (mean ± SEM).
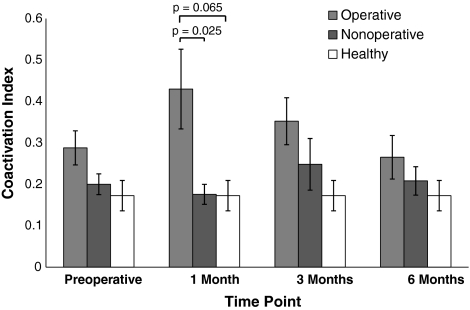
We observed differences in hamstrings coactivation during an MVIC of the quadriceps (operative, nonoperative, and healthy control legs) 1 month after TKA: there was greater coactivation in operative legs compared with nonoperative legs (144.5% elevation; p = 0.025; Fig. 3). A trend for differences between operative legs and healthy control legs also was observed at 1 month (149% elevation; p = 0.065). By comparison, before TKA, hamstrings coactivation in the operative leg was elevated 43.8% relative to the nonoperative leg and 66.8% relative to healthy control legs. Similar patterns of hamstrings coactivation persisted three and six months after TKA, but again, differences were not statistically significant.
Fig. 3.
Hamstrings EMG coactivation across time is shown. The coactivation index was defined as (peak hamstrings EMG during quadriceps maximal voluntary isometric contraction [MVIC]/peak hamstrings EMG during hamstrings MVIC). One month after TKA, hamstrings coactivation in the operative leg was significantly elevated compared with that of the nonoperative leg with a similar trend for comparisons with healthy control subjects.
Discussion
An understanding of the acute and subacute changes in hamstrings and quadriceps muscle function is important to optimize rehabilitation after TKA. Studies have shown the quadriceps lose more than 50% of preoperative strength 1 month after TKA [26, 41], which explains why rehabilitation programs currently target quadriceps strengthening and surgeons debate the optimal quadriceps-sparing surgical approach. Less information has been available regarding hamstrings muscle strength, especially within the first months after surgery. We therefore compared: (1) postoperative quadriceps and hamstrings muscle strength loss after TKA and subsequent recovery using the nonoperative legs and healthy control legs for comparison, and (2) hamstrings coactivation during a quadriceps MVIC before and after TKA compared with that of nonoperative and healthy control legs.
We acknowledge several limitations: (1) exclusion criteria; (2) potential for OA in the nonoperative leg; and (3) open-chain testing rather than closed-chain, functional testing. First, exclusion criteria such as a BMI of 35 kg/m2 or less were conservative because these patients were also control subjects for an ongoing randomized clinical trial. Yet, it is unlikely that including patients with greater BMIs (35–40 kg/m2) would change these results because BMI does not seem to impact strength or functional performance after TKA when less than 40 kg/m2 [43]. A second limitation is the potential for OA in the nonoperative leg in a subset of patients; therefore, we also included healthy adults (no knee pain) for additional comparison. During quadriceps strength testing, our patients reported average Visual Analog Scale pain levels in the operative leg of 1.9 ± 2.5 (range, 0–7) preoperatively and 2.5 ± 2.5 (range, 0–8) 1 month after TKA. Pain in the nonoperative leg was 0.2 ± 0.7 (range, 0–4) across all times, suggesting that the nonoperative leg was minimally involved in most patients. Finally, our hamstrings coactivation was assessed using an open-chain task, which may make it difficult to relate these findings to more dynamic, closed-chain tasks such as walking. We chose this approach so that we could specifically evaluate hamstrings coactivation during strength testing without introducing additional confounding variables such as movement strategies or the use of assistive devices during more dynamic tasks. However, our results suggest additional investigation is warranted to evaluate early hamstrings coactivation during more functional, closed-chain activities.
In contrast to our initial hypothesis, we found no differences in strength loss or recovery between the quadriceps and hamstrings muscles during the first 6 months after TKA. Importantly, hamstrings muscle strength in operative legs, compared with nonoperative legs, was not as compromised as quadriceps strength before and 6 months after surgery, yet the quadriceps and hamstrings muscles in these patients remain significantly weaker than healthy controls before and 6 months after TKA. Previous investigations evaluating quadriceps and hamstrings muscle strength many months to years after surgery (relative to preoperative levels, nonoperative leg, and healthy controls) generally support our findings (Tables 2, 3). Across patient groups, Perhonen et al. [35] reported a 39.4% deficit in hamstrings strength and 42.9% deficit in quadriceps strength 3 weeks after TKA, which improved to only a 15.2% hamstrings deficit and a 7.1% quadriceps deficit at 3 months. When comparing the operative with nonoperative leg, Walsh et al. [44] found larger side-to-side deficits in hamstrings muscle isokinetic strength (20.7%) than quadriceps muscle strength (11.3%) 1 year after TKA. When compared with healthy control subjects, Berman et al. [5] reported slightly larger deficits in isokinetic quadriceps strength (41.6%) than hamstrings strength (28.7%) 3 to 6 months after TKA compared with those of healthy control subjects and still a 28.9% (quadriceps) and 14.6% (hamstrings) deficit 7 to 12 months after TKA. More than 2 years after TKA, Silva et al. [39] observed a 47.1% deficit in quadriceps strength and a 55.2% deficit in hamstrings strength compared with those of healthy control subjects. Although the relative degree of hamstrings versus quadriceps muscle strength deficit varies across studies, findings consistently indicate the hamstrings and quadriceps muscles experience a compromise in strength that persists years after surgery [13, 21, 24, 35, 39, 44].
Table 2.
Comparison of published studies of absolute strength in patients undergoing TKA
| Study | Test mode | Time point | Absolute strength (N-m) | |||||
|---|---|---|---|---|---|---|---|---|
| Quadriceps | Hamstrings | |||||||
| TKA operative | TKA nonoperative | Healthy | TKA operative | TKA nonoperative | Healthy | |||
| Walsh et al. [44]† | Isokinetic 90°/s | 1.7 years | 57.2 | 64.5 | 88.3 | 33.2 | 41.8 | 48.7 |
| Silva et al. [39]‡ | Isometric 60° | 2.8 years | 93.0 (44.6)* | 175.9 (60.4) | 45.9 (16.3) | 102.4 (42) | ||
| Berth et al. [7]§ | Isometric 90° | Preoperative | 66.3 (33.4) | 81.9 (38.1) | 105 (28.4) | |||
| 2.8 years | 84.8 (35.5) | 79.4 (30.5) | ||||||
| Lorentzen et al. [21]|| | Isometric 75° | Preoperative | 66 | 87 | 24 | 32 | ||
| 3 months | 55 | 92 | 21 | 32 | ||||
| 6 months | 65 | 92 | 20 | 33 | ||||
| Berman et al. [5]¶ | Isokinetic 60°/s | Preoperative | 35.5 (9.5) | 59.8 (10.7) | 21.5 (8.4) | 32.8 (9.1) | ||
| 3–6 months | 39.1 (9.2) | 66.9 (11.0) | 26.1 (8.0) | 36.6 (9.9) | ||||
| 7–12 months | 50.4 (9.5) | 70.9 (10.7) | 30.4 (7.7) | 35.6 (9.3) | ||||
| 13–23 months | 55.8 (9.6) | 69.1 (10.6) | 28.7 (7.7) | 33.4 (9.1) | ||||
| > 23 months | 56.9 (9.9) | 68.1 (9.6) | 32.4 (9.9) | 32.6 (8.4) | ||||
| Mizner et al. [28]# | Isometric 75° | Preoperative | 183.7 | 225.6 | ||||
| 1 month | 70.7 | 222.8 | ||||||
| 2 months | 95.7 | 228.1 | ||||||
| 3 months | 148.8 | 231.7 | ||||||
| 6 months | 179.9 | 228.9 | ||||||
| Perhonen et al. [35]** | Isometric 60° | Preoperative | ~70.0 | ~33.0 | ||||
| 3 weeks | ~40.0 | ~20.0 | ||||||
| 6 weeks | ~55.0 | ~23.0 | ||||||
| 12 weeks | ~65.0 | ~28.0 | ||||||
| 24 weeks | ~75.0 | ~32.0 | ||||||
| 52 weeks | ~85.0 | ~35.0 | ||||||
| Current study | Isometric 60° | Preoperative | 121.7 (45.7) | 154.2 (52.8) | 165.3 (57.9) | 68.1 (27.9) | 72.9 (24.6) | 95.2 (32.5) |
| 1 month | 56.1 (23.2) | 136.8 (51.0) | 33.7 (15.7) | 71.9 (28.9) | ||||
| 3 months | 100.4 (46.8) | 146.8 (45.8) | 57.0 (25.5) | 74.9 (29.5) | ||||
| 6 months | 113.3 (45.9) | 145.9 (47.6) | 61.4 (26.6) | 74.2 (28.5) | ||||
* Values are expressed as mean with SD in parentheses; †TKA, n = 29 (mean age, 63.9 years); healthy age-matched, n = 40 (mean age, 62.8 years); ‡TKA, n = 32 knees (mean age, 67.3 years); healthy, n = 53 knees (mean age, 40.0 years), values were converted from foot-pounds; §TKA, n = 50 (mean age, 65.8 years); healthy age-matched, n = 50 (mean age, 63.2 years); ||TKA, n = 30 (mean age, 74.0 years); ¶TKA, n = 68 (mean age, 63.0 years), values were converted from foot-pounds; #TKA, n = 40 (mean age, 64.0 years); **TKA, n = 30 (mean age, 67.5 years); three training groups (TG1, TG2, Control) were averaged across all three groups, and values were delineated from graphs since no values were provided.
Table 3.
Comparison of published studies of percent differences in strength in patients undergoing TKA
| Study | Test mode | Time point | Percent differences (%) | |||||
|---|---|---|---|---|---|---|---|---|
| (A) Postop operative leg strength deficits: compared to preop | (B) Operative leg strength deficits: compared to nonoperative leg | (C) Operative leg strength deficits: compared to healthy individuals | ||||||
| Quadriceps | Hamstrings | Quadriceps | Hamstrings | Quadriceps | Hamstrings | |||
| Walsh et al. [44]† | Isokinetic 90°/s | 1.7 years | −11.3 | −20.7 | −35.3 | −31.9 | ||
| Silva et al. [39]‡ | Isometric 60° | 2.8 years | −47.1 | −55.2 | ||||
| Berth et al. [7]§ | Isometric 90° | Preop | −19.0 | −36.9 | ||||
| 2.8 years | 27.9 | 6.8 | −19.2 | |||||
| Lorentzen et al. [21]|| | Isometric 75° | Preop | −24 | −25 | −24.0 | −25.0 | ||
| 3 months | −16.7 | −12.5 | −40 | −34 | −40.0 | −34.0 | ||
| 6 months | −1.5 | −16.7 | −29 | −39 | −29.0 | −39.0 | ||
| Berman et al. [5]¶ | Isokinetic 60°/s | Preop | −40.6 | −34.5 | ||||
| 3–6 months | 10.1 | 21.4 | −41.6 | −28.7 | ||||
| 7–12 months | 42.0 | 41.4 | −28.9 | −14.6 | ||||
| 13–23 months | 57.2 | 33.5 | −19.2 | −14.1 | ||||
| > 23 months | 60.3 | 50.7 | −16.4 | −0.6 | ||||
| Mizner et al. [28]# | Isometric 75° | Preop | −19.0 | |||||
| 1 month | −61.5 | −68.0 | ||||||
| 2 months | −47.9 | −58.0 | ||||||
| 3 months | −19.0 | −36.0 | ||||||
| 6 months | −2.1 | −21.0 | ||||||
| Perhonen et al. [35]** | Isometric 60° | Preop | ||||||
| 3 weeks | −42.9 | −39.4 | ||||||
| 6 weeks | −21.4 | −30.3 | ||||||
| 12 weeks | −7.1 | −15.2 | ||||||
| 24 weeks | 7.1 | −3.0 | ||||||
| 52 weeks | 21.4 | 6.1 | ||||||
| Current study | Isometric 60° | Preop | −21.1 | −6.6 | −26.4 | −28.5 | ||
| 1 month | −51.6 (18.9)* | −48.4 (20.8) | −59.0 | −53.1 | −66.1 | −64.6 | ||
| 3 months | −12.0 (38.7) | −13.1 (25.3) | −31.6 | −23.9 | −39.3 | −40.1 | ||
| 6 months | −2.5 (37.5) | −9.2 (25.2) | −22.3 | −17.3 | −31.5 | −35.5 | ||
* Values are expressed as mean, with SD in parentheses; equations: (A) [(postoperative − preoperative)/preoperative] × 100; (B) [(operative − nonoperative)/nonoperative] × 100; (C) [(operative − healthy)/healthy] × 100; negative numbers indicate a deficit while positive numbers indicate an increase; †TKA, n = 29 (mean age, 63.9 years); healthy age-matched, n = 40 (mean age, 62.8 years); ‡TKA, n = 32 knees (mean age, 67.3 years); healthy, n = 53 knees (mean age, 40.0 years), values were converted from foot-pounds; §TKA, n = 50 (mean age, 65.8 years); healthy age-matched, n = 50 (mean age, 63.2 years); ||TKA, n = 30 (mean age, 74.0 years); ¶TKA, n = 68 (mean age, 63.0 years), values were converted from foot-pounds; #TKA, n = 40 (mean age, 64.0 years); **TKA, n = 30 (mean age, 67.5 years); three training groups (TG1, TG2, Control) were averaged across all three groups, and values were delineated from graphs since no values were provided; postop = postoperative; preop = preoperative.
Our study findings support our second hypothesis that the level of hamstrings coactivation in operative legs during a maximal quadriceps contraction would increase after TKA compared with nonoperative and healthy control legs. In a healthy joint, some coactivation during normal lower limb movement may improve movement efficiency by increasing joint stabilization and protection, but excessive coactivation may result in impaired movement and weakness [4, 9] and detrimentally augment stresses to articular cartilage or a knee prosthesis [4, 15, 19, 38]. Coactivation measures similar to ours have been used in previous studies with other populations. Newham and Hsiao [30] examined coactivation in 30 healthy control subjects and found hamstrings coactivation during a maximal isometric quadriceps effort in healthy control subjects was 0.23 ± 0.04 (mean ± SE of the mean), which is similar to our average value for control subjects (0.17 ± 0.04). Baratta et al. [3] reported hamstrings coactivation during an isometric quadriceps contraction ranged from 0.11 to 0.15 in young, healthy sedentary males (n = 20; mean age 22.1 years). Similarly, in 12 healthy control subjects (aged 25–59 years), antagonist hamstrings activity during a maximal isometric contraction of the quadriceps muscle was approximately 0.13 ± 0.06 [10]. We may have underestimated the level of hamstrings coactivation because the presence of hamstrings coactivation may be further magnified during weightbearing activities to increase the functional stability of the knee [4, 9, 22]. Antagonistic muscle activity of the hamstrings during open-chain, nonweightbearing exercise is less than during closed-chain, weightbearing exercise [9, 22]. For example, Busse et al. [9] found coactivation of the hamstrings during isometric knee extension was 0.12 ± 0.06 (mean ± SD) compared with 0.21 ± 0.13 during a sit-to-stand activity. Therefore, additional investigation is necessary to evaluate the degree and impact of hamstrings coactivation during weightbearing activities such as walking or sit-to-stand, especially during the acute and subacute periods after TKA. One study examining muscle coactivation using gait analysis 6 months to 2 years after TKA suggested patients still had prolonged muscle activation of antagonists to stabilize the knee during stance. This pattern of prolonged muscle activation persisted through the 2-year followup in most patients, further supporting the need for earlier evaluation of the impact of muscle coactivation during functional activities [4].
Our observations suggest that although quadriceps dysfunction after TKA typically is recognized and addressed in postoperative therapy protocols, hamstrings dysfunction also is present and should be addressed. Muscle weakness and overuse can cause tendinopathy [31], and if left untreated, hamstrings weakness and coactivation may contribute to persistent hamstrings tendinopathy and related dysfunction of the postoperative knee, resulting in increased pain. In addition to the importance of rehabilitation for the hamstrings, quadriceps muscle strength is important because persistent deficits in quadriceps muscle strength have been documented in this study and others. Therefore, quadriceps and hamstrings muscle strengthening should be the focus of future rehabilitation programs. Finally, additional investigation of quadriceps and hamstrings muscle function during dynamic, functional activities is necessary to expand on these results to better evaluate postoperative deficits.
Acknowledgments
We acknowledge Pam Wolfe, MS, for statistical consultation in preparing this manuscript.
Footnotes
One or more of the authors received funding from a New Investigator Award from the American College of Rheumatology (JSL) and National Institutes of Health Grant UL1 RR025780 (Clinical Translational Research Center).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Adili A, Bhandari M, Petruccelli D, Beer J. Sequential bilateral total knee arthroplasty under 1 anesthetic in patients > or = 75 years old: complications and functional outcomes. J Arthroplasty. 2001;16:271–278. doi: 10.1054/arth.2001.21495. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Orthopaedic Surgeons. Most commonly performed musculoskeletal-related procedures. Available at: www.aaos.org/research/stats/top_hospitalization_visits.pdf. Accessed June 27, 2009.
- 3.Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation: the role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16:113–122. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti MG, Catani F, Bilotta PW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech (Bristol, Avon) 2003;18:871–876. doi: 10.1016/S0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 5.Berman AT, Bosacco SJ, Israelite C. Evaluation of total knee arthroplasty using isokinetic testing. Clin Orthop Relat Res. 1991;271:106–113. [PubMed] [Google Scholar]
- 6.Berth A, Urbach D, Awiszus F. Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:1432–1436. doi: 10.1053/apmr.2002.34829. [DOI] [PubMed] [Google Scholar]
- 7.Berth A, Urbach D, Neumann W, Awiszus F. Strength and voluntary activation of quadriceps femoris muscle in total knee arthroplasty with midvastus and subvastus approaches. J Arthroplasty. 2007;22:83–88. doi: 10.1016/j.arth.2006.02.161. [DOI] [PubMed] [Google Scholar]
- 8.Bonutti PM, Mont MA, Kester MA. Minimally invasive total knee arthroplasty: a 10-feature evolutionary approach. Orthop Clin North Am. 2004;35:217–226. doi: 10.1016/j.ocl.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Busse ME, Wiles CM, Deursen RW. Co-activation: its association with weakness and specific neurological pathology. J Neuroeng Rehabil. 2006;3:26. doi: 10.1186/1743-0003-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JM, Mayston MJ, Newham DJ. Electrical and mechanical output of the knee muscles during isometric and isokinetic activity in stroke and healthy adults. Disabil Rehabil. 1996;18:83–90. doi: 10.3109/09638289609166022. [DOI] [PubMed] [Google Scholar]
- 11.Fisher NM, Pendergast DR, Gresham GE, Calkins E. Muscle rehabilitation: its effect on muscular and functional performance of patients with knee osteoarthritis. Arch Phys Med Rehabil. 1991;72:367–374. [PubMed] [Google Scholar]
- 12.Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13:186–190. doi: 10.1016/S0883-5403(98)90097-3. [DOI] [PubMed] [Google Scholar]
- 13.Huang CH, Cheng CK, Lee YT, Lee KS. Muscle strength after successful total knee replacement: a 6- to 13-year followup. Clin Orthop Relat Res. 1996;328:147–154. doi: 10.1097/00003086-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Hubley-Kozey C, Deluzio K, Dunbar M. Muscle co-activation patterns during walking in those with severe knee osteoarthritis. Clin Biomech (Bristol, Avon) 2008;23:71–80. doi: 10.1016/j.clinbiomech.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–298. doi: 10.1016/S0889-857X(05)70068-5. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther. 2008;88:22–32. doi: 10.2522/ptj.20070051. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol. 2009;106:769–774. doi: 10.1007/s00421-009-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 19.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorentzen JS, Petersen MM, Brot C, Madsen OR. Early changes in muscle strength after total knee arthroplasty: a 6-month follow-up of 30 knees. Acta Orthop Scand. 1999;70:176–179. doi: 10.3109/17453679909011258. [DOI] [PubMed] [Google Scholar]
- 22.Lutz GE, Palmitier RA, An KN, Chao EY. Comparison of tibiofemoral joint forces during open-kinetic-chain and closed-kinetic-chain exercises. J Bone Joint Surg Am. 1993;75:732–739. doi: 10.2106/00004623-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 23.McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1:79–93. doi: 10.1016/S0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- 24.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35:424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 25.Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32:1533–1539. [PubMed] [Google Scholar]
- 26.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty: the contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87:1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23:1083–1090. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365. [PubMed] [Google Scholar]
- 29.Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004;85:546–556. doi: 10.1016/j.apmr.2003.08.080. [DOI] [PubMed] [Google Scholar]
- 30.Newham DJ, Hsiao SF. Knee muscle isometric strength, voluntary activation and antagonist co-contraction in the first six months after stroke. Disabil Rehabil. 2001;23:379–386. doi: 10.1080/0963828001006656. [DOI] [PubMed] [Google Scholar]
- 31.Nichols AW. Achilles tendinitis in running athletes. J Am Board Fam Pract. 1989;2:196–203. [PubMed] [Google Scholar]
- 32.Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? Clin Orthop Relat Res. 2005;431:157–165. doi: 10.1097/01.blo.0000150130.03519.fb. [DOI] [PubMed] [Google Scholar]
- 33.O’Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostenberg A, Roos E, Ekdahl C, Roos H. Isokinetic knee extensor strength and functional performance in healthy female soccer players. Scand J Med Sci Sports. 1998;8:257–264. doi: 10.1111/j.1600-0838.1998.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 35.Perhonen M, Komi PV, Hakkinen K, Bonsdorf H, Partio H. Strength training and neuromuscular function in elderly people with total knee endoprosthesis. Scand J Med Sci Sports. 1982;2(4):234–243. doi: 10.1111/j.1600-0838.1992.tb00349.x. [DOI] [Google Scholar]
- 36.Petterson SC, Mizner RL, Stevens JE, Raisis L, Bodenstab A, Newcomb W, Snyder-Mackler L. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61:174–183. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 37.Scuderi GR, Tenholder M, Capeci C. Surgical approaches in mini-incision total knee arthroplasty. Clin Orthop Relat Res. 2004;428:61–67. doi: 10.1097/01.blo.0000148574.79874.d0. [DOI] [PubMed] [Google Scholar]
- 38.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 39.Silva M, Shepherd EF, Jackson WO, Pratt JA, McClung CD, Schmalzried TP. Knee strength after total knee arthroplasty. J Arthroplasty. 2003;18:605–611. doi: 10.1016/S0883-5403(03)00191-8. [DOI] [PubMed] [Google Scholar]
- 40.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinsky FD. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 41.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 42.Stevens JE, Mizner R, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004;34:21–29. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 43.Stevens-Lapsley JE, Petterson SC, Mizner RL, Snyder-Mackler L. Impact of body mass index on functional performance after total knee arthroplasty. J Arthroplasty. 2009 Oct 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 44.Walsh M, Woodhouse LJ, Thomas SG, Finch E. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther. 1998;78:248–258. doi: 10.1093/ptj/78.3.248. [DOI] [PubMed] [Google Scholar]
- 45.Watters JM, Clancey SM, Moulton SB, Briere KM, Zhu JM. Impaired recovery of strength in older patients after major abdominal surgery. Ann Surg. 1993;218:380–390. doi: 10.1097/00000658-199309000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]