Abstract
Background
According to some current guidelines, extended thromboprophylaxis after hip and knee arthroplasties is recommended. Outpatient prophylaxis with low molecular weight heparins (LMWH) is an important part of this prophylaxis, although the rates of adherence to these regimens is not known.
Questions/purposes
We determined (1) the degree of nonadherence (NA) of patients with LMWH outpatient prophylaxis, and (2) whether specific independent factors explain NA.
Methods
NA was determined by syringe count and by indirect and direct questions to patients. We defined six different NA indicators. To identify factors explaining LMWH NA, we used three different logistic regression models.
Results
NA rates ranged between 13% and 21% depending on the indicator used for measurement. Patients who were nonadherent missed between 38% and 53% of their outpatient LMWH injections. If patients attended an outpatient rehabilitation program, the probability for their NA increased substantially. Moreover, the NA probability increased with each additional day between acute hospitalization and start of rehabilitation (linking days). NA was lower for patients who feared thrombosis or who believed antithrombotic drugs to be the most important measure in thromboprophylaxis.
Level of Evidence
Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The importance of medication-based or other forms of thromboprophylaxis after hip and knee arthroplasties (ie, major orthopaedic surgery) is well documented [4, 9, 14, 15, 27, 50, 52]. Therefore, thromboprophylaxis has been included in international guidelines [2, 13, 21, 22]. Current American College of Chest Physicians (ACCP) guidelines recommend medication-based thromboprophylaxis for at least 10 days (Evidence Grade 1A) postoperatively, but preferably for as much as 35 days (Evidence Grade 1A for THAs, Evidence Grade 2B for TKAs [2, 13, 21, 22]).
In Europe, orthopaedic thromboprophylaxis has been dominated by the use of LMWHs [39]. In Germany, there is a clear trend toward shorter hospitalization of patients after major orthopaedic surgery, leading to greater importance of outpatient thromboprophylaxis [51]. However, the question arises whether the level of LMWH prophylaxis adherence in an outpatient setting is sufficient to ensure effective treatment. This is particularly topical because the recent introduction of oral direct thrombin inhibitors provides easier administration and potentially could provide better adherence [18, 19]. Oral direct thrombin inhibitors are reportedly as effective as LMWHs [17, 19, 23, 37] but more cost-effective from a hospital’s perspective [56]. Furthermore, analysis of patients’ preferences showed they have a clear preference for oral thromboprophylaxis, especially in an outpatient setting [55].
We identified seven studies reporting NA to LMWH thromboprophylaxis after surgery, with NA rates ranging from 5% to 45% [10, 33, 35, 38, 41, 49, 54]. However, almost none of the studies measured the patients’ NA in real-world conditions: the studies were conducted in single centers or the NA measurement conditions failed to reflect real-world circumstances. Further, NA measurement was influenced by several factors such as preceding training and education programs for patients [10, 35, 38, 41, 54], use of special reporting cards for documentation of injections administered by the patients to themselves [10, 38], high frequency of visits by physicians [38], or concurrent testing of special devices for LMWH injections [33].
Following the WHO definition, medication NA in general can be defined as the extent to which a person’s drug-taking behavior corresponds with instructions from a healthcare provider [30, 45]. There is no clinically based NA threshold in thromboprophylaxis, owing to a dearth of evidence informing the number of LMWH missed injections that would result in a substantially increased risk of thrombosis or pulmonary embolism (PE). Therefore, in accordance with most of the cited publications dealing with LMWH NA, we defined a patient who was LMWH-specific nonadherent as one who missed an LMWH injection on at least one day even though the treating clinic had recommended such an injection. To deal with the uncertainty concerning the NA threshold, (1) we defined the possible threshold in terms of alternatives (2 or 3 days), and (2) we also provide the number of noninjection days for each level of NA.
Only if the exact causes of NA are addressed is it possible to formulate an effective adherence program [28, 29, 42]. Existing general medication adherence research shows a patient’s adherence is influenced by many factors ranging from sociodemographic, socioeconomic, illness-related, patient-related, and medication-specific to health system-related factors [12, 24, 36, 45, 58]. One of the tasks is to identify which of these possible causes are important in LMWH outpatient treatment.
We therefore (1) determined whether there was real-world NA of patients in LMWH outpatient prophylaxis, and (2) generated hypotheses concerning which specific independent factors can explain this NA.
Patients and Methods
We report results of a telephone survey conducted between June 2008 and February 2009. We interviewed 1495 patients who had major orthopaedic surgery at 22 different clinics in Germany (Appendix 1). NA was measured using six different indicators based on the previously mentioned different NA thresholds. NA reasons were identified based on multivariate regression models. As parts of our study were designed to be hypothesis generating, a sample size power analysis was not conducted. The study protocol was reviewed and approved by the University of Hannover ethical board.
Appendix 1.
Participating clinics and enrolled patient numbers
| Participating clinic | Type of clinic | Interviewed patients | Patients with need of outpatient prophylaxis |
|---|---|---|---|
| Universitätsklinik Friedrichsheim | Acute clinic | 24 | 11 |
| DRK Kliniken Berlin Mitte | Acute clinic | 11 | 10 |
| Universitätsklinikum Greifswald | Acute clinic | 92 | 82 |
| Charité Campus Mitte | Acute clinic | 77 | 71 |
| Remigius-Krankenhaus Opladen | Acute clinic | 117 | 100 |
| Asklepios Klinik Lindenlohe | Acute clinic | 52 | 42 |
| Dietrich Bonhoeffer Klinikum Neubrandenburg | Acute clinic | 194 | 163 |
| DRK Krankenhaus Sömmerda | Acute clinic | 23 | 15 |
| Krankenhaus Marta-Maria | Acute clinic | 46 | 42 |
| Orthopädische Klinik Universität Hannover | Acute clinic | 32 | 27 |
| Klinik am Stein Düsseldorf | Outpatient rehabilitation facility | 30 | 28 |
| Ambulantes Reha-Centrum Dresden | Outpatient rehabilitation facility | 23 | 23 |
| Reha Tagesklinik im Forum Pankow | Outpatient rehabilitation facility | 26 | 24 |
| Ortho-Mobile Hattingen | Outpatient rehabilitation facility | 86 | 84 |
| ZAR Trier | Outpatient rehabilitation facility | 16 | 15 |
| ZAR Stuttgart | Outpatient rehabilitation facility | 27 | 27 |
| Ortho-Med GmbH | Outpatient rehabilitation facility | 69 | 67 |
| ZAR Münster | Outpatient rehabilitation facility | 17 | 15 |
| Orthopädisches Zentrum Rothenburg | Inpatient rehabilitation facility | 202 | 187 |
| Asklepios Klinik Bad Schwartau | Inpatient rehabilitation facility | 58 | 55 |
| Johannesbad Raupennest | Inpatient rehabilitation facility | 196 | 170 |
| Klinik Dr. Vötisch | Inpatient rehabilitation facility | 77 | 59 |
| SUM | 1495 | 1317* |
* Two patients with insufficient data; 1315 patients’ data were used for nonadherence analysis.
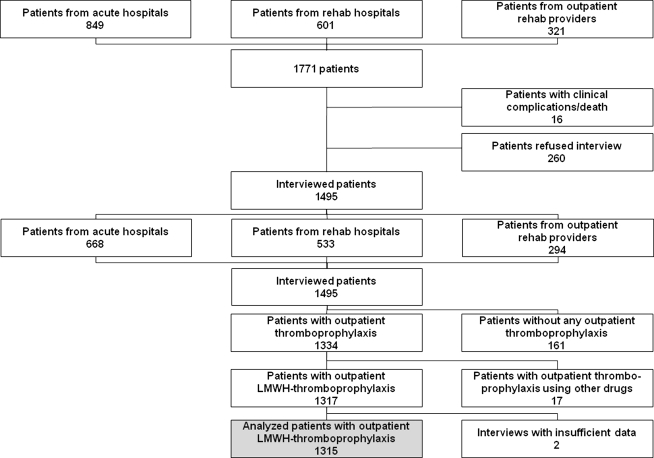
To enroll study patients, we invited 106 acute and rehabilitation hospitals and 77 outpatient rehabilitation institutions (all referred to as clinics in the following text) to participate in the study. Each of these institutions is known to treat at least 100 patients having hip or knee replacement surgery per year. Ultimately, 10 acute hospitals, four inpatient rehabilitation hospitals, and eight outpatient rehabilitation institutions identified patients who had hip or knee arthroplasty for the interviews; all other institutions refused participation because of the workload associated with patient enrollment. Our potential sample included all patients in these clinics who were scheduled for or already had hip or knee arthroplasty, and who were able to answer questions in a telephone interview. The initial sample size (1771 persons) (Fig. 1) was reduced because 16% (276 patients) refused informed consent or could not be interviewed because of poor status of health. Consequently, interviews were performed with 1495 patients (Table 1). Patients refusing interviews were older than the average for the total sample by 5 years; women refused interviews with a greater (p = 0.016) frequency than men (10.6% versus 5.0%).
Fig. 1.
A flowchart shows the structure of the sample. Analysis of patient NA was performed based on 1315 patients.
Table 1.
Descriptive statistics of the total sample and the analyzed subsample
| Variable | Total sample | Subsample (patients needing outpatient LMWH thromboprophylaxis) |
|---|---|---|
| Number of patients | 1495 (100.0%) | 1315 (100.0%) |
| Age (years)* | 67.9 | 67.6 |
| Gender | ||
| Female | 901 (60.3%) | 780 (59.3%) |
| Male | 594 (39.7%) | 535 (40.7%) |
| Surgery | ||
| Knee arthroplasty | 739 (49.4%) | 659 (50.1%) |
| Hip arthroplasty | 756 (50.6%) | 656 (49.9%) |
| Rehabilitation after acute hospital stay | ||
| Stationary rehabilitation | 1118 (74.8%) | 958 (72.9%) |
| Outpatient rehabilitation | 354 (23.7%) | 341 (25.9%) |
| Without any rehabilitation | 16 (1.1%) | 16 (1.2%) |
| Information not collected | 7 (0.4%) | |
| Linking | ||
| Yes | 843 (56.4%) | 826 (62.8%) |
| No | 645 (43.2%) | 489 (37.2%) |
| Information not collected | 7 (0.4%) | |
| Duration of linking between acute hospitalization and start of inpatient/outpatient rehabilitation (days)† | 3.9 (0–15) | 4.2 (0–16) |
| Duration of outpatient thromboprophylaxis (days)† | 8.9 (0–31) | 10.9 (1–32) |
| Duration of acute hospital stay (days)† | 11.8 (6–20) | 11.3 (6–17) |
| Time between surgery and interview date (days)† | 41.3 (34–59) | 41.0 (34–56) |
| Recommendation for the duration of thromboprophylaxis (days)† | 33.4 (21–42) | 33.3 (21–42) |
| Postoperative complications | ||
| No complications | 1143 (76.5%) | 1123 (85.4%) |
| Thromboembolic complications | 9 (0.6%) | 9 (0.7%) |
| Other complications (not specified) | 184 (12.3%) | 183 (13.9%) |
| No information available | 159 (10.6%) | |
| Number of different drugs taken per day (not including LMWH) | ||
| None | 140 (9.4%) | 138 (10.5%) |
| Up to 2 | 382 (25.5%) | 376 (28.6%) |
| 3–5 | 510 (34.1%) | 505 (38.4%) |
| 6–10 | 264 (17.7%) | 259 (19.7%) |
| > 10 | 38 (2.5%) | 37 (2.8%) |
| Information not collected | 161 (10.8%) | |
| Self-injections during outpatient thromboprophylaxis | ||
| Yes | 745 (49.9%) | 745 (56.7%) |
| No | 570 (38.1%) | 570 (43.3%) |
| Information not collected | 180 (12.0%) | |
| Chronic diseases (more than one answer possible) | ||
| None | 543 (36.3%) | 543 (41.3%) |
| Hypertension | 366 (24.5%) | 366 (27.8%) |
| Diabetes (both types) | 138 (9.2%) | 138 (10.5%) |
| Rheumatism | 38 (2.5%) | 38 (2.9%) |
| Other | 294 (19.7%) | 294 (22.4%) |
| Information not collected | 180 (12.0%) | |
| Living circumstances | ||
| Living alone | 294 (19.7%) | 294 (22.4%) |
| Living with spouse/life partner | 891 (59.6%) | 891 (67.7%) |
| Living with children | 92 (6.2%) | 92 (7.0%) |
| Other circumstances | 38 (2.5%) | 38 (2.9%) |
| Information not collected | 180 (12.0%) | |
| Highest achieved education grade | ||
| Without any formal apprenticeship | 172 (11.5%) | 172 (13.1%) |
| Completed apprenticeship | 898 (60.1%) | 898 (68.3%) |
| University degree | 179 (12.0%) | 179 (13.6%) |
| Other qualifications | 66 (4.4%) | 66 (5.0%) |
| Information not collected | 180 (12.0%) | |
Some patients with no experience of LMWH thromboprophylaxis (warfarin or direct thrombin inhibitors were used) or without obvious outpatient prophylaxis (because their defined pathway did not include outpatient prophylaxis) were not asked all interview questions; for this reason, some data are not available for these patients; *values are expressed as means; †values are expressed as means, with 95% confidence intervals in parentheses; NA = nonadherence; LMWH = low-molecular-weight heparin.
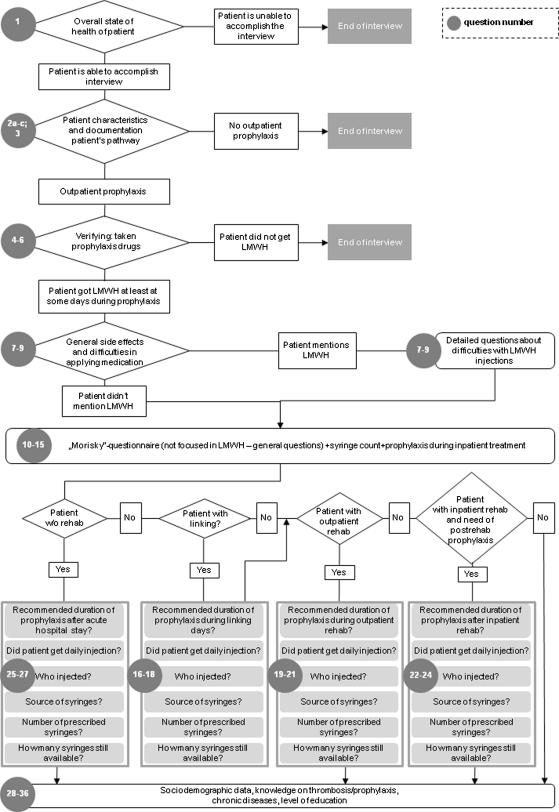
Concerning the conduct of the study, neither ward personnel in the clinics nor the patients were informed about the true objectives of the study. Patients were interviewed by five trained interviewers using a pretested electronic questionnaire (Appendices 2–4). Interviewers had to learn the content of the questionnaire; were educated in the normal treatment of a patient who had major orthopaedic surgery after release from the acute hospital; and were given detailed information regarding thrombosis medication alternatives available on the market. In addition, each of the interviewers had to conduct two to five trial interviews under supervision. The interviewers did not have medical degrees, because the level of medical knowledge necessary to conduct the interviews was not advanced. During each interview, data were documented online by the interviewers and stored in a Microsoft® Access® database (Microsoft Corp, Redmond, WA). To clarify possible incidences of data inconsistency, each interview also was saved electronically as an audio file. The average interview was conducted on the 41st day (SD, 6.8 days) after surgery; it lasted approximately 10 to 15 minutes.
Appendix 2.
Questionnaire
| No. | Questions | Possible answers | Comment |
|---|---|---|---|
| 1 | How are you today? | Fine/very critical | Fine: continue with the interview; critical: discontinue interview, poss. call emergency doctor in acc. with interview guidelines |
| 2a | When were you released from the acute hospital? | Date of release | |
| 2b | Did you stay in a rehabilitation hospital after the acute hospital phase? | Yes/No | Yes: length of stay No: continue with 2c |
| 2c | Did you receive outpatient rehab treatment after the acute hospital phase? | Yes/No | Yes: length of stay |
| 3 | Did you experience complications following the operation? (Unsupported) | No/mild/serious/severe/special thrombosis | |
| 4 | How many different medications do you take per day? (How many tablets; capsules and injection) | Number of tablets/capsules; number of injections | |
| 5 | What medications did you receive following the operation and after leaving the acute treatment hospital? | Is an NMH injection named without prompting? (Yes/No) | |
| 6 | How often and when were you given or gave yourself these medications? | Daily NMH injection named without prompting? (Yes/No); if no: ask for LMWH directly | |
| 7 | Have you noticed side effects—direct or indirect—of your medications? If so; which? | Yes/No; when yes: details noted | Yes: follow with 8; No: follow with 9. |
| 8 | Did you do anything about the side effects? | Stopped taking medications? (Yes/No) In particular, stopped using LMWH (w/o prompting)? (Yes/No) |
Yes, stopped LMWH treatment: List number of days without it |
| 9 | Were there any medications where you had problems taking/ using them? If yes, with which? What did you do? | Difficulty using LMWH injections (w/o prompting)? (Yes/No) | Yes, list the following measures and days without LMWH treatment. |
| 10 | Do you sometimes forget to take your medications? If yes, with which medicine has this happened since your release from the acute hospital? | Forgot to take medications? (Yes/No) In particular, has LMWH been forgotten (w/o prompting)? (Yes/No) |
Yes, LMWH injection forgotten: How many days without LMWH? |
| 11 | Are you sometimes careless about taking medicine? If yes, with which medicine has this happened since your release from the acute hospital? | Carelessness? (Yes/No) In particular, not used LMWH injection (w/o prompting)? (Yes/No) |
Yes, not used LMWH injection: How many days without LMWH? |
| 12 | Do you sometimes decide against taking medications if you are feeling better or well? If yes, with which medications has this happened with and how often over the past weeks? | Decided against medicine while feeling improvement? (Yes/No) In particular, decided against LMWH injection (w/o prompting)? (Yes/No) |
Yes; decided against LMWH injection: How many days without LMWH? |
| 13 | Do you sometimes decide against taking medications if you are not feeling well? If yes, with which medications has this happened with and how often over the past week? | Decided against medicine while not feeling well? (Yes/No) In particular, decided against LMWH injection (w/o prompting)? (Yes/No) |
Yes; decided against LMWH injection: How many days without LMWH? |
| 14 | How many thrombosis syringes do you still have at home? | Number of syringes | |
| 15 | Did you also receive daily thrombosis injections during your stationary rehab treatment? If so, who gave them to you? Were there any days without injections? | Daily injections? (Yes/No) By whom? (Nurse/Patient); days without injection. | Question only for patients who had been in stationary rehab treatment |
| 16 | Before going to stationary rehab, did anyone recommend you inject yourself daily? If so, who injected you? | Linking: Recommended daily injection? (Yes/No) Who gave the injection? (doctor or nurse/family member/patient) |
Question only for patients with linking between acute and stationary rehabilitations treatment |
| 17 | Who supplied you with the syringes? How many? | Linking: origin of the injections? (hospital/outpatient prescription doctor/not get a prescription/prescription is not redeemed); Number of injections | Question only for patients with linking between acute and stationary rehabilitations treatment |
| 18 | Retrospectively and in your honest opinion, if you calculate from the day you left the acute treatment hospital to the day you entered rehab treatment, did you receive an injection every day? If not, why not? On how many days did you decide not to use an injection? | Daily LMWH injection? (Yes/No); list the reasons (bruises/ache/allergic reactions/blood/changes/other); Number of days without LMWH |
Question only for patients with linking between acute and stationary rehabilitations treatment |
| 19 | During your outpatient rehab treatment, did anyone recommend you use an injection every day? | Outpatient rehab: recommendation of daily injection? (Yes/No) Who gave the injection? | Question only for patients in outpatient rehabilitation |
| 20 | Who supplied you with the syringes? How many? | Outpatient rehab: origin of the injections? (hospital/outpatient prescription doctor/not get a prescription/prescription is not redeemed); Number of injections | Question only for patients in outpatient rehabilitation |
| 21 | Retrospectively and in your honest opinion, during your outpatient rehab treatment did you receive an injection every day? If not, why not? On how many days did you decide not to use an injection? | Daily LMWH injection? (Yes/No); list the reasons (bruises/ache/allergic reactions/blood/changes/other); Number of days without LMWH |
Question only for patients in outpatient rehabilitation |
| 22 | After your rehabilitation, did anyone recommend you use an injection every day? For how many days? Who injected you? | After rehabilitation: recommendation of daily injection? (Yes/No) Who gave the injection? (doctor or nurse/family member/patient) | Question only for patients with thrombosis prophylaxis need after inpatient rehabilitation |
| 23 | Who supplied you with the syringes? How many? | After rehabilitation: origin of the injections? (hospital/outpatient prescription doctor/not get a prescription/prescription is not redeemed); Number of injections | Question only for patients with thrombosis prophylaxis need after inpatient rehabilitation |
| 24 | Retrospectively and in your honest opinion, after your inpatient rehab treatment did you—despite mobility being good—receive a daily injection until the last recommended day? If not, why not? On how many days didn′t you receive an injection? | Daily LMWH injection? (Yes/No); list the reasons (bruises/ache/allergic reactions/blood/changes/other); Number of days without LMWH |
Question only for patients with thrombosis prophylaxis need after inpatient rehabilitation |
| 25 | After your acute hospital treatment: did anyone recommend you use an injection every day? For how many days? Who injected you? | Without rehab: recommendation of daily injection? (Yes/No) Who gave the injection(doctor or nurse/family member/patient) | Question only for patients without rehabilitation |
| 26 | Who supplied you with the syringes? How many? | Without rehabilitation: origin of the injections? (hospital/outpatient prescription doctor/not get a prescription/prescription is not redeemed); Number of injections | Question only for patients without rehabilitation |
| 27 | Retrospectively and in your honest opinion, after your acute hospital treatment did you—despite perhaps mobility being good—receive a daily injection until the last recommended day? If not, why not? On how many days didn’t you receive an injection? | Daily LMWH injection? (Yes/No); list the reasons (Bruises/ache/allergic reactions/blood/changes/other); Number of days without LMWH |
Question only for patients without rehabilitation |
| 28 | Following your operation were you afraid of suffering from a thickening of the blood or a thrombosis? | Extremely, very, moderately, not much, not at all | |
| 29 | In your opinion, how much does having thrombosis restrict a person in daily life? | Extremely, a lot, moderately, not much, not at all | If the answer to question 3 is especially thrombosis: asked for experience |
| 30 | In your opinion, how long does the treatment of thrombosis take? | Very long, long, moderate amount of time, little time, very little time | |
| 31 | Do you believe a person can avoid thrombosis by drinking a lot of liquids and moving a lot? | Extremely, very, moderately, not much, not at all | |
| 32 | Do you believe that medications against thrombosis give you good protection? | Extremely, very, moderately, not much, not at all | |
| 33 | What do you think is the more important measure against thrombosis: moving and drinking or taking/using medications? | Moving/drinking, medications, both (equally) | |
| 34 | Do you suffer from chronic diseases or handicaps? | Yes/No | Yes: Details |
| 35 | Do you live alone? | Alone/with partner/with children/other | |
| 36 | What education have you had? | University/vocational training/no postschool education/other |
Appendix 4.
Clinical parameters were not documented, except for thrombosis or embolus-related or unspecified complications the patients faced after surgery. Complications were not documented for patients who refused the interview or were unable to participate in the study because of clinical complications. Consequently, it is likely the number of general and thrombosis or embolus-related complications is underestimated.
The specific thromboprophylaxis pathway was identified for each patient. This was done to allow detailed comparison of the theoretically achievable and actual adherence profiles. Pathway stages were identified by reference to the normal course of clinical treatment. Consequently, each patient’s postoperative pathway contained as many as six stages during which prophylaxis could be administered: (1) acute hospitalization, (2) inpatient stationary rehabilitation, (3) outpatient rehabilitation, and/or (4) at home between acute hospitalization and inpatient or outpatient rehabilitation (linking days), (5) after rehabilitation if prophylaxis is still needed, and (6) on an outpatient basis without any rehabilitation. The average desired duration of postsurgery thromboprophylaxis for each clinic and therefore per patient group was defined based on the recommendation of the clinic that enlisted the patient in the study. These recommendations were provided by the clinics; all clinics confirmed that they recommended daily injections. The duration of prophylaxis recommended by the patient’s clinic was taken to be the minimum possible actual number of days of recommended treatment. This figure was used as a comparison with patients’ responses, unless a patient reported that in his special case more injection days had been recommended.
For NA analysis, the 1315 patients who underwent outpatient thromboprophylaxis for at least 1 day were included (Fig. 1; Table 1). Generally, a patient’s NA can be measured using direct and indirect approaches [1, 25, 30, 32, 45, 46, 48, 58]. Possible direct methods are face-to-face observation of patients, and/or measurement of clinical parameters related to drug use. Indirect methods can include various instruments ranging from medication event measurement systems, patients’ diaries, methods based on counting prescriptions, pills or syringes, or prescription refills, and indirect and direct questioning of patients regarding NA [11].
A face-to-face observation of 1315 patients is neither advisable (as it would likely lead to more adherence and create observational bias) nor can it be implemented in any real-world study design. Measurement of clinical parameters (blood testing for anticoagulation effects) was excluded for conceptual (observation bias), clinical, and practical reasons. The use of a medication event measurement system (MEMS), patients’ diaries, and prescription and refill counts were seen as impracticable. Because of the number of participating clinics and patients, and because of the diversity of patient pathways, the use of electronically prepared syringes (MEMS) was virtually impossible. The use of diaries runs the risk of behavioral change. Regarding prescription counts, patients in Germany have free choice of the pharmacy to which to take their prescriptions; prescription count from that side is extraordinarily complex. Administrative data regarding number of prescription refills issued could not be obtained from healthcare insurance providers either.
We used all three remaining defined methods for NA measurement; syringe counting, indirect, and direct questioning. On this basis, six NA indicators were defined (Table 2). This multiple indicator concept is in line with current recommendations given for adherence measurement [40, 45]. However, there is a certain hierarchy of NA categories in terms of their predictive capacity (Table 2).
Table 2.
Definitions of six indicators for patients’ nonadherence and an example patient
| NA category/ indicator | Patient confessed deliberately not having self-injected during linking days | Patient confessed having forgotten some injections during linking days | Syringe count showed surplus: patient was prescribed five syringes (3 linking days + 2 days after rehabilitation); during interview patient mentioned still having two syringes | Thromboprophylaxis not in line with recommendations given by clinics and general practitioners (if applicable); patient mentioned having been told by a general physician to self-inject for 5 days (3 linking days + 2 days after rehab) but claimed not to have received any injections | Thromboprophylaxis not in line with clinic recommendations, but in line with the patient-reported general practitioner’s recommendation; hospital recommended outpatient prophylaxis until 35th day after surgery; patient claimed not to have been advised to self-inject after rehabilitation (2 days) | Patient was asked by the interviewer to list all postsurgery medications received but did not mention the LMWH injections |
|---|---|---|---|---|---|---|
| NA I | Yes | |||||
| NA II | Yes | |||||
| NA III | Yes | |||||
| NA IVa | Yes | |||||
| NA IVb | Yes | |||||
| NA V | Yes | |||||
| Qualitative assessment: strength of indicator | Very probable NA: patients directly confessed nonadherence | Probable NA: syringe surplus as rather objective indicator | Possible NA: Patients did not directly confess to NA but their answers indirectly indicate NA. | |||
All indicators are based on answers given by the patients during the interviews; to clarify indicator definition, an example of a typical patient is given; many patients fell into more than one NA category. Example patient: clinic recommended postsurgery thromboprophylaxis for 35 days; patient spent 10 days in acute hospital, 3 linking days at home before stationary inpatient rehabilitation, 20 days at rehabilitation clinic, 2 remaining prophylaxis days at home after rehabilitation; interview date: 37 days after surgery. NA = nonadherence; LMWH = low-molecular-weight heparin.
In the current literature regarding NA, five main variable groups are discussed as factors potentially influencing a patient’s adherence [36, 45, 58]. Variables representing each of these categories were included in the multivariate analysis in this study. Age and gender, general living circumstances, and highest education grade attained were used as sociodemographic characteristics. Illness-related factors used were the type of surgery and the existence and number of chronic diseases. The patients’ views regarding thrombosis and thromboprophylaxis were used as variables representing patient-related influences. The medication-specific factors used for multivariate design were the number of different drugs (subdivided into those in syringes and others) taken by the patient and the duration of the different steps in the patients’ pathways. Finally, as healthcare or system-related factors, the specific clinic, type of clinic enlisting the patient, and pathway-specific details were used as explanatory variables.
To measure NA (primary study objective), we computed descriptive statistics and reported the results in percentages. The number of noninjection days is reported as a series of arithmetic means and the variation in a group of patients as standard deviations.
The secondary study outcome was determined using the aforementioned six NA indicators (Table 2). In total, three multivariate logistic regression models with different dependent variables were used; the existence of a strongly probable NA measured by the categories NA I and/or NA II (Model 1), the existence of a probable NA by Model 1 plus NA III (Model 2), the existence of a possible NA by Model 2 plus NA IVa, NA IVb, and NA V (Model 3). Patients were included in Model 3 only if all of the three remaining NA categories (NA IVa, IVb and V) applied. Multivariate logistic regression analysis was performed using backward stepwise elimination to parsimoniously determine the factors most associated with NA, as follows. The initial models included all patient-reported factors (Table 1). In the backward stepwise iterations, regression terms at a significance level of p > 0.10 were successively eliminated. To reduce multicolinearity, the duration of linking was centered, by subtracting the mean from each value. The logistic regression analyses were adjusted for age, gender, living circumstances, education, and clinic. All statistical analyses were performed using STATA® 10 statistical software (StataCorp LP, College Station, TX). All tests were two-tailed.
Results
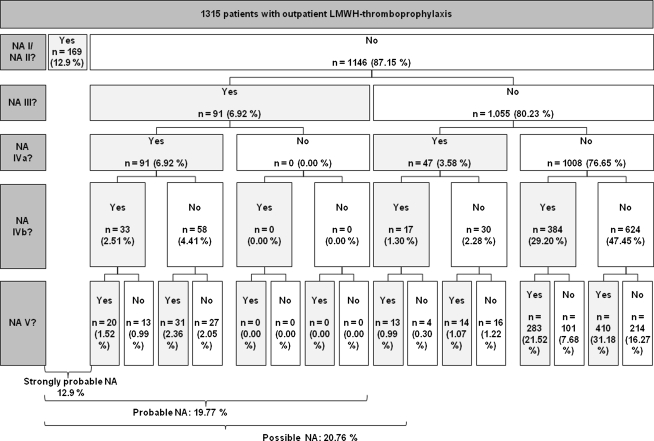
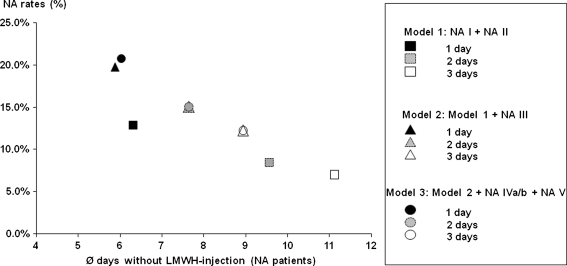
Our data show that there is LMWH NA in outpatient thromboprophylaxis. The LMWH NA rates found in our sample range from 12.9% to 20.8% depending on the NA indicator used (Fig. 2; Table 3). Approximately 12.9% of patients reported that they decided not to inject themselves or had forgotten at least one injection (NA I or NA II). If patients were nonadherent in these categories, they missed 6.4 injection days (SD, 7.77 days) on average (43.1% of all outpatient prophylaxis days). The NA rates based on the other indicators show there was probably more NA that patients did not acknowledge directly in the interviews. For approximately 19.8% of patients, NA could be evaluated as probable, either because of NA I/NA II or because of a surplus of syringes (NA III). On average, these patients missed 5.9 injection days (SD, 6.63 days), ie, 38.5% of all outpatient prophylaxis days. Almost 21% (20.8%) of patients showed at least a possible NA (either previous NA indicators and/or all remaining NA indicators apply) with an average of 6.0 missed injection days (SD, 6.54 days), which corresponds to 39.6% of all outpatient prophylaxis days. If the NA threshold as a minimum number of noninjection days is increased to 2 or 3 days, NA rates naturally decrease. However, the number of missed injection days for these patients increases (Fig. 3).
Fig. 2.
A flowchart shows the NA ratios based on different NA indicators. The NA indicators are ordered from the top to the bottom related to their theoretical strength regarding prognosis of a NA. All percentages relate to 1315 patients.
Table 3.
Nonadherence in different patient pathways
| Patient pathway (during LMWH thrombo-prophylaxis) | Number of patients | NA I | NA II | NA III | NA IVa | NA IVb | NA V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Mean days* | SD | Number of patients | Mean days* | SD | Number of patients | Mean days* | SD | Number of patients | Mean days* | SD | Number of patients | Mean days* | SD | Number of patients | ||
| AHS-INPR | |||||||||||||||||
| AHS-LINK-INPR | 412 (31.3%) | 39 (3.0%) | 5.6 | 5.990 | 0 (0.0%) | 18 (1.4%) | 1.8 | 0.732 | 49 (3.7%) | 3.7 | 2.576 | 83 (6.3%) | 1.9 | 3.099 | 272 (20.7%) | ||
| AHS-LINK-INPR-PRTP | 88 (6.7%) | 13 (1.0%) | 1.8 | 1.463 | 1 (0.1%) | 2.0 | 2 (0.2%) | 2.0 | 0.000 | 4 (0.3%) | 2.3 | 0.500 | 55 (4.2%) | 3.3 | 2.817 | 64 (4.9%) | |
| AHS-INPR-PRTP | 457 (34.8%) | 9 (0.7%) | 4.6 | 3.240 | 1 (0.1%) | 1.0 | 18 (1.4%) | 3.5 | 2.167 | 28 (2.1%) | 3.9 | 2.730 | 22 (17.0%) | 4.0 | 3.398 | 333 (25.3%) | |
| AHS-OPR | 15 (1.1%) | 0 (0.0%) | 0 (0.0%) | 3 (0.2%) | 3.3 | 1.528 | 3 (0.2%) | 3.3 | 1.528 | 6 (0.5%) | 5.5 | 6.411 | 9 (0.7%) | ||||
| AHS-LINK-OPR | 256 (19.5%) | 90 (6.8%) | 7.8 | 9.349 | 7 (0.5%) | 1.9 | 1.690 | 59 (4.5%) | 5.2 | 3.803 | 72 (5.5%) | 5.9 | 4.819 | 118 (9.0%) | 7.3 | 6.648 | 143 (10.9%) |
| AHS-LINK-OPR-PRTP | 70 (5.3%) | 16 (1.2%) | 5.4 | 6.150 | 0 (0.0%) | 17 (1.3%) | 6.9 | 3.269 | 21 (1.6%) | 7.8 | 3.945 | 54 (4.1%) | 12.0 | 9.166 | 42 (3.2%) | ||
| AHS-OPR-PRTP | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 13.0 | 1 (0.1%) | |||||||||
| AHS-NORE | 16 (1.2%) | 2 (0.2%) | 8.0 | 4.243 | 0 (0.0%) | 6 (0.5%) | 8.7 | 4.502 | 7 (0.5%) | 12.7 | 7.931 | 5 (0.4%) | 9.8 | 11.628 | 9 (0.7%) | ||
| AHS only | |||||||||||||||||
| Sum | 1315 (100.0%) | 169 (12.9%) | 6.4 | 7.844 | 9 (0.7%) | 1.8 | 0.972 | 123 (9.4%) | 4.8 | 3.613 | 184 (14.0%) | 5.4 | 4.446 | 545 (41.4%) | 5.2 | 5.867 | 873 (66.4%) |
All percentages are related to the number of patients in subsample 2 (1315 patients); the table shows how many injections an average patients in each NA category missed because of NA; because NA V does not apply to specific thromboprophylaxis days, this information is not given for NA; One patient can belong to more than one NA category; *mean days without LMWH injection. NA = nonadherence; LMWH = low-molecular-weight heparin. AHS = Acute hospital stay; LINK = linking days between acute hospital stay and rehabilitation; INPR = inpatient rehabilitation in a rehabilitation clinic; OPR = outpatient rehabilitation in a rehabilitation center; NR = no rehabilitation; PRTP = postrehabilitation thromboprophylaxis; NORE = no rehabilitation.
Fig. 3.
A scatter diagram shows NA rates (percentage of nonadherent patients based on 1315 patients) using different NA thresholds (minimum of noninjection days). Moreover, it shows number of average missed injection days for every indicator.
The strongest predictor of NA in all three models was the type of rehabilitation (Table 4). Compared with inpatient rehabilitation, patients in outpatient rehabilitation treatment in Model 1 were approximately 5 times more likely to have poor adherence (Model 2: 8 times more likely; Model 3: 7 times more likely). Differences also existed with the centered duration of linking in reported logistic regression models. Therefore, if the duration of linking increased by 1 day above the mean (from 4.24 to 5.24 days), the probability of NA would be 1.05 times (95% CI, 1.02–1.08) greater than for patients with an average number of linking days. Patients reporting fear of thrombosis or who believed in the ability of drugs to prevent thrombosis were less likely to be nonadherent. However, these factors were significant only in Model 1 (strongly probable NA).
Table 4.
Logistic regression models of factors associated with low or poor adherence*
| Factor | Number of patients | Model 1 (NA based on categories NA I, NA II) | Model 2 (NA based on Model 1 + NA III) | Model 3 (NA based on Model 2 + NA IVa/IVb, NA V) | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI)† | p value | OR (95% CI)† | p value | OR (95% CI)† | p value | ||
| Rehabilitation form | |||||||
| Inpatient | 958 | 1.00‡ | 1.00‡ | 1.00‡ | |||
| Outpatient | 357 | 4.72 (3.24–6.88) | < 0.001 | 8.10 (5.80–11.67) | < 0.001 | 6.83 (4.93–9.45) | < 0.001 |
| Duration of linking (days) | 1315 | 1.05 (1.02–1.08) | 0.002 | 1.04 (1.01–1.07) | 0.011 | 1.05 (1.02–1.08) | < 0.001 |
| Fear of thrombosis | |||||||
| No | 1087 | 1.00‡ | |||||
| Yes | 228 | 0.58 (0.35–0.98) | 0.041 | ||||
| Prevention of thrombosis by drugs§ | |||||||
| No | 38 | 1.00‡ | |||||
| Yes | 1277 | 0.38 (0.17–0.86) | 0.020 | ||||
| p Value (Hosmer and Lemeshow’s goodness of fit chi square statistic) | 0.254 | 0.020 | 0.767 | ||||
* A backward elimination method was used retaining all variables considered to be potentially important; variables with p < 0.1 remained in the final models whereas variables with p > 0.1 were removed in the stepwise backward process; †odds ratio (OR) and 95% confidence interval (CI) derived from the three logistic models adjusting for age, gender, living circumstances, education, and clinic; ‡reference group; §patients were asked how much they believe drugs protect from thrombosis.
Discussion
The existence of guidelines for medication-based thromboprophylaxis is no guarantee of outpatient LMWH adherence. This study, involving 1495 interviewed patients and 22 clinics, is the largest empirical investigation so far to deal with LMWH outpatient NA. Our study was conducted to investigate the extent of existence of NA (primary study objective) and to generate hypotheses concerning which specific independent factors can explain this NA (secondary study objective). We were able to confirm the existence of NA and to generate hypotheses that appear to be a promising basis for identifying the responsible factors.
We acknowledge limitations to the study. First, we analyzed only an injectable medication-based thrombosis prophylaxis with LMWH after hip and knee arthroplasties. It is possible oral thromboprophylaxis medications would result in lower NA. However, in Europe LMWH prophylaxis is still the dominant form of prophylaxis [39]. Second, we used only NA indicators that could be identified with the help of patients. For this reason we are open to the risk of response bias [8, 34, 59]. However, previous research suggests an assessment of patients’ adherence based on indirect or direct questioning on this topic is a good predictor of adherence [31, 43]. This technique regularly overestimates adherence by a factor of 2 [26, 29, 36, 44]. This means that although our results show that at least 12.9% of patients to be nonadherent (Model 1), the real percentage could be 20% to 30%. This matches our Model 3 where 20.8% of patients showed NA. Third, there is no clinically based NA threshold or NA model that should be used. That is why, in accordance with most of the previous publications dealing with LMWH NA, we defined a patient who was LMWH-specific nonadherent as one who missed an LMWH injection on at least one day even though the treating clinic had recommended such an injection. Although it is not possible to justify our choice of three models on the basis of clinical evidence, they have their purpose, which is to provide multiple indicators. Fourth, owing to cultural or other reasons, our analysis may be specific to Germany. Nevertheless, we suspect the results of the NA research are robust in international comparisons, for the following reason. It can be assumed drug-related outpatient prophylaxis in other countries is at least as important as it is in Germany, because most other countries do not have a comprehensive network of inpatient rehabilitation clinics. However, we have no exact knowledge concerning linking days in other countries. Fifth, there is a possibility the participating clinics did not reflect a representative sample of hip and knee arthroplasty clinics in Germany. However, the large number of clinics that participated and the large number of patients studied leads us to believe that our sample was representative. Sixth, the nonresponse rate of approximately 15.6% suggests a possible selection bias. Although there were differences in age and gender between responders and nonresponders, these variables did not influence the NA probability of a patient. Seventh, the basis for measuring patients’ NA were the recommendations made by clinics. However it is possible that in some cases the general practitioners responsible for LMWH prescriptions in the outpatient setting might have given different recommendations, eg, because of drug budget constraints. In Germany, this probably is not often the case for linking days but could have been the case during outpatient rehabilitation, days without any rehabilitation, or prophylaxis days after rehabilitation. Consequently, there is some probability that we did not measure patients’ NA but less prophylaxis days caused by general practitioners modifying the clinic recommendations [20].
We found a relatively high rate of NA. This finding is in line with published studies regarding LMWH NA l [10, 33, 35, 38, 41, 49, 54], bearing in mind our possible overestimation of adherence [29]. If patients are nonadherent, they miss approximately 40% to 50% of their entire outpatient thromboprophylaxis. Empirical research shows, after major orthopaedic surgery, clinically relevant deep venous thrombosis and PE occur at a median of 20 and 21 days after TKA and 12 and 34 days after THA [3, 53]. Our data show the linking period to be a critical phase. For an average patient in our survey, the linking period started 11.37 days (SD, 2.9 days) after surgery. For the first quartile, this period started 8.1 days after the surgery. Whether this means that NA necessarily occurs during a clinically critical phase was not further investigated by us.
Although our study suggests some initial indications concerning the factors that could explain patients’ LMWH NA, it is, in this respect, a hypothesis-generating study. We believe these factors can contribute to the general discussion regarding NA causes although the findings in NA research are controversial [16, 45]. A more detailed understanding of explanatory factors is essential for development of sophisticated patient adherence programs. In particular, factors such as the detailed recommendations given by general practitioners [20], the patient-doctor relationship [38], the patients’ education regarding thrombosis risk [41], and psychological factors describing patients’ behavior and thinking [5, 47] require more detailed investigation [12, 57]. Furthermore, because patients have a preference for oral prophylaxis [55] and because earlier studies report some adherence advantages associated with medication regimens that are perceived to be easy to follow [29], in contrast to the more complex regimens commonly associated with some drugs [6, 7, 36, 48], it could be assumed patients’ adherence to oral thromboprophylaxis regimens is greater than is the case with LMWH prophylaxis. Whether this is the case remains a matter for future research.
Acknowledgments
We thank the participating clinics. We also thank Stephan Obert for his contributions to this study and, above all, for his idea to initiate the study. Finally, we thank Mrs. Dallas Reese for proofreading and correcting the manuscript.
Appendices
Appendix 3.
Data collected in the survey
| Category | Variable | Interviewee without outpatient pathway (n = 161) | Interviewee with outpatient pathway but oral anticoagulation instead of LMWH (n = 17) | Interviewee with outpatient pathway and LMWH (n = 1315) |
|---|---|---|---|---|
| Sociodemographic data | Age | + | + | + |
| Gender | + | + | + | |
| Chronic disease | − | − | + | |
| Social environment | − | − | + | |
| Level of education | − | − | + | |
| Data regarding the LMWH-thromboprophylaxis pathway | Type of surgery | + | + | + |
| Occurrence of complications | − | + | + | |
| Day of operation | + | + | + | |
| Day of discharge from acute hospital | + | + | + | |
| Type of rehabilitation after stay in acute hospital | + | + | + | |
| Beginning and duration of rehabilitation | + | + | + | |
| Data regarding medication, thromboprophylaxis, and LMWH nonadherence | Medicine(s) taken postsurgery | − | + | + |
| Frequency and form of application | − | + | + | |
| Side effects of medicine | − | − | + | |
| Special side effects of LMWH including resulting days without LMWH | − | − | + | |
| Difficulties in using medicines | − | − | + | |
| Special difficulties in using LMWH including resulting days without LMWH | − | − | + | |
| Morisky questionnaire including documentation of resulting days without LMWH | − | − | + | |
| Balance of medicine | − | − | + | |
| Recommendation for duration of using LMWH during respective pathway | − | − | + | |
| Using daily injection during respective pathway | − | − | + | |
| Person administering syringes (self-injection or not) | − | − | + | |
| Days without LMWH during respective pathway | − | − | + | |
| Source of syringes during respective pathway | − | − | + | |
| Preference capsule | − | − | + | |
| Improvement of nonadherence by capsule | − | − | + | |
| Variables that could explain LMWH nonadherence | Fear of thrombosis | − | − | + |
| Assessment of limitation by thrombosis in daily life | − | − | + | |
| Estimated duration of thrombosis treatment | − | − | + | |
| Assessment of thrombosis prevention through movement and drinking | − | − | + | |
| Rating prevention of thrombosis by drugs | − | − | + | |
| Comparison of (1) movement/drinking with (2) drugs regarding the importance of thromboprophylaxis | − | − | + |
Footnotes
Two of the authors (Thomas Wilke, Andreas Kurth) are receiving funding from Boehringer Ingelheim Pharma GmbH & Co KG, Ingelheim, Germany. Boehringer Ingelheim Pharma GmbH & Co manufactures the oral direct thrombin inhibitor Dabigatranetexilate that is currently approved for thromboprophylaxis after knee or hip arthroplasty surgery. Matthias Pfannkuche is employed by Boehringer Ingelheim Pharma GmbH & Co KG.
The authors certify that this study was approved by an ethical board. All investigations were conducted in conformity with ethical principles of research. Informed consent for participation in the study was obtained.
This work was performed at the Institut für Pharmakoökonomie und Arzneimittellogistik.
References
- 1.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 2.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Prophylaxe der venösen Thromboembolie (VTE). Available at: http://www.uni-duesseldorf.de/awmf/ll/003-001.htm. Accessed March 24, 2009.
- 3.Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88:386–391. doi: 10.1302/0301-620X.88B3.17207. [DOI] [PubMed] [Google Scholar]
- 4.Böttner F, Sculco TP, Sharrock NE, Westrich GH, Steinbeck J. [Prevention of thrombosis in hip prosthesis implantation] [in German] Orthopäde. 2001;30:890–896. doi: 10.1007/s001320170025. [DOI] [PubMed] [Google Scholar]
- 5.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Bukstein DA, Henk HJ, Luskin AT. A comparison of asthma-related expenditures for patients started on montelukast versus fluticasone propionate as monotherapy. Clin Ther. 2001;23:1589–1600. doi: 10.1016/S0149-2918(01)80130-2. [DOI] [PubMed] [Google Scholar]
- 7.Bulkrishnan R, Nelsen LM, Kulkarni AS, Pleasants RA, Whitmire JT, Schechter MS. Outcomes associated with initiation of different controller therapies in a Medicaid asthmatic population: a retrospective data analysis. J Asthma. 2005;41:35–40. doi: 10.1081/JAS-200044769. [DOI] [PubMed] [Google Scholar]
- 8.Chisholm MA, Lance CE, Williamson GM, Mulloy LL. Development and validation of the immunosuppressant therapy adherence instrument. Nephrol Dial Transplant. 2005;20:181–188. doi: 10.1093/ndt/gfh576. [DOI] [PubMed] [Google Scholar]
- 9.Colwell CW Jr; Annenberg Center for Health Sciences and Quadrant Medical Education. Thromboprophylaxis in orthopaedic surgery. Am J Orthop. 2006;suppl:1–9; quiz 10–11. [PubMed]
- 10.Colwell CW, Jr, Pulido P, Hardwick ME, Morris BA. Patient compliance with outpatient prophylaxis: an observational study. Orthopaedics. 2005;28:143–147. doi: 10.3928/0147-7447-20050201-16. [DOI] [PubMed] [Google Scholar]
- 11.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–3277. doi: 10.1001/jama.261.22.3273. [DOI] [PubMed] [Google Scholar]
- 12.Donovan JL, Blake DR. Patient non-compliance: deviance or reasoned decision-making. Soc Sci Med. 1992;34:507–513. doi: 10.1016/0277-9536(92)90206-6. [DOI] [PubMed] [Google Scholar]
- 13.Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, Ansell J, American College of Chest Physicians The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 suppl):299S–339S. doi: 10.1378/chest.08-0675. [DOI] [PubMed] [Google Scholar]
- 14.Eikelboom J, Baker R. Routine home treatment of deep vein thrombosis. BMJ. 2001;322:1192–1193. doi: 10.1136/bmj.322.7296.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomized trials. Lancet. 2001;358:9–15. doi: 10.1016/S0140-6736(00)05249-1. [DOI] [PubMed] [Google Scholar]
- 16.Elliott RA, Shinogle JA, Peele P, Bhosle M, Hughes DA. Understanding medication compliance and persistence from an economics perspective. Value Health. 2008;11:600–610. doi: 10.1111/j.1524-4733.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson BI, Dahl OE, Büller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kälebo P, Reilly P, BISTRO II Study Group A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–111. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR, RE-NOVATE Study Group Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 20.Friedman RJ, Gallus AS, Cushner FD, Fitzgerald G, Anderson FA, Jr, Global Orthopaedic Registry Investigators Physician compliance with guidelines for deep-vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin. 2008;24:87–97. doi: 10.1185/030079908x242746. [DOI] [PubMed] [Google Scholar]
- 21.Geerts WH, Bergqvist DF, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW, American College of Chest Physicians Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 22.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantel S, Schnee JM, Caprini JA. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 24.Hahn SR, Park J, Skinner EP, Yu-Isenberg KS, Weaver MB, Crawford B, Flowers PW. Development of the ASK-20 adherence barrier survey. Curr Med Res Opin. 2008;24:2127–2138. doi: 10.1185/03007990802174769. [DOI] [PubMed] [Google Scholar]
- 25.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 26.Hasford J, Behrend C, Sangha O. Vergleichende Analyse und Bewertung von Methoden zur Erfassung der Compliance. In: Petermann F, editor. Compliance und Selbstmanagement. Göttingen, Germany: Hogrefe; 1998. pp. 21–45. [Google Scholar]
- 27.Hawkins D. Pharmacoeconomics of thrombosis management. Pharmacotherapy. 2004;24(7 pt 2):95S–99S. doi: 10.1592/phco.24.10.95S.36123. [DOI] [PubMed] [Google Scholar]
- 28.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 29.Haynes RB, McDonald HP, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications (review) Cochrane Database Syst Rev. 2005;4:CD000011. doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 30.Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Baltimore, MD: Johns Hopkins University Press; 1979. pp. 49–62. [Google Scholar]
- 31.Haynes RB, Taylor DW, Sackett DL, Gibson ES, Bernholz CD, Mukherjee J. Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2:757–764. doi: 10.1161/01.hyp.2.6.757. [DOI] [PubMed] [Google Scholar]
- 32.Heuer H, Heuer S, Lennecke K. Compliance in der Arzneitherapie. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft; 1999. pp. 5–20. [Google Scholar]
- 33.Höntzsch D, Scheffer J, Hafner S, Tolle A, Spannagel U. Fully automated injection pen for prevention of thrombosis: tolerance, convenience and patient compliance] [in German. MMW Fortschr Med. 1999;141:50. [PubMed] [Google Scholar]
- 34.Jerant A, DiMatteo R, Arnsten J, Moore-Hill M, Franks P. Self-report adherence measures in chronic illness: retest reliability and predictive validity. Med Care. 2008;46:1134–1139. doi: 10.1097/MLR.0b013e31817924e4. [DOI] [PubMed] [Google Scholar]
- 35.Karliński M, Stolarczyk A, Siuda M, Ziólkowski M. Compliance with low molecular weight heparin in ambulatory orthopaedic patients. Ortop Traumatol Rehabil. 2006;8:633–638. [PubMed] [Google Scholar]
- 36.Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22:313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- 37.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2789. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 38.Le Gall C, Jacques E, Medjebeur C, Darques L, Briand F, Haddad J, Bleichner G. Low molecular weight heparin self-injection training: assessment of feasibility, tolerance and economic analysis in emergency departments. Eur J Emerg Med. 2006;13:264–269. doi: 10.1097/00063110-200610000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Lipp HP, Goldinger A, Haussmann W, Krämer I, Pfaff A, Schweitzer E. Antithrombotika zur Therapie und Prophylaxe venöser Thromboembolien. Krankenhauspharmazie. 2004;25:195–210. [Google Scholar]
- 40.Liu H, Miller HG, Hays RD, Golin CE, Zhao H, Wenger NS, Kaplan AH. A comprehensive evaluation of survey questions for adherence to antiretroviral medications and exploratory analyses for identifying optimal sets of survey questions. AIDS Patient Care STDs. 2006;20:760–772. doi: 10.1089/apc.2006.20.760. [DOI] [PubMed] [Google Scholar]
- 41.Mazor KM, Baril J, Dugan E, Spencer F, Burgwinkle P, Gurwitz JH. Patient education about anticoagulant medication: is narrative evidence or statistical evidence more effective? Patient Educ Couns. 2007;69:145–157. doi: 10.1016/j.pec.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 42.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 43.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hyperten (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 46.Paes AH, Bakker A, Soe-Agnie CJ. Measurement of patient compliance. Pharm World Sci. 1998;20:73–77. doi: 10.1023/A:1008663215166. [DOI] [PubMed] [Google Scholar]
- 47.Petrie KJ, Weinman J. Why illness perceptions matter. CME Psychiatry. 2006;6:536–539. doi: 10.7861/clinmedicine.6-6-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolley JX, Davidson PM, Dennison CR, Ong A, Everett B, Salamonson Y. Medication adherence self-reported instruments: implications for practice and research. J Cardiovasc Nurs. 2008;23:497–505. doi: 10.1097/01.JCN.0000338931.96834.16. [DOI] [PubMed] [Google Scholar]
- 49.Spahn G. Compliance with self administration of heparin injections in outpatients. Eur J Trauma. 2002;2:104–109. doi: 10.1007/s00068-002-1176-1. [DOI] [Google Scholar]
- 50.Stannard JP, Lopez-Ben RR, Volgas DA, Anderson ER, Busbee M, Karr DK, McGwin GR, Jr, Alonso JE. Prophylaxis against deep-vein thrombosis following trauma: a prospective, randomized comparison of mechanical and pharmacological prophylaxis. J Bone Joint Surg Am. 2006;88:261–266. doi: 10.2106/JBJS.D.02932. [DOI] [PubMed] [Google Scholar]
- 51.Statistisches Bundesamt (Federal Bureau of Statistics Germany), Fachserie 12, Reihe 6.4, year 2008.
- 52.Aken H, Bode C, Darius H, Diehm C, Encke A, Gulba DC, Haas S, Hacke W, Puhl W, Quante M, Riess H, Scharf R, Schellong S, Schrör T, Schulte KL, Tebbe U. Anticoagulation: the present and future. Clin Appl Thromb Hemost. 2001;7:195–204. doi: 10.1177/107602960100700303. [DOI] [PubMed] [Google Scholar]
- 53.Warwick D, Friedman RJ, Agnelli G, Gil-Garay E, Johnson K, FitzGerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared to the time course of thromboembolic events. J Bone Joint Surg Br. 2007;89:799–807. doi: 10.1302/0301-620X.89B6.18844. [DOI] [PubMed] [Google Scholar]
- 54.Watts AC, Howie CR, Simpson AH. Assessment of a self-administration protocol for extended subcutaneous thromboprophylaxis in lower limb arthroplasty. J Bone Joint Surg Br. 2006;88:107–110. doi: 10.1302/0301-620X.88B1.17003. [DOI] [PubMed] [Google Scholar]
- 55.Wilke T. Patient preferences for an oral anticoagulant after major orthopaedic surgery: results of a German survey. The Patient. 2009;2:39–49. doi: 10.2165/01312067-200902010-00005. [DOI] [PubMed] [Google Scholar]
- 56.Wilke T, Neumann K, Klapper U, Messer I, Werner A, Seidel U, Röleke D. [Oral anticoagulation after major hip or knee replacement surgery: a process-driven managerial pharmacoeconomic analysis in German hospitals] [in German] Orthopäde. 2008;37:448–456. doi: 10.1007/s00132-008-1254-0. [DOI] [PubMed] [Google Scholar]
- 57.Winnick S, Lucas DO, Hartman AL, Toll D. How do you improve compliance? Pediatrics. 2005;115:e718–e724. doi: 10.1542/peds.2004-1133. [DOI] [PubMed] [Google Scholar]
- 58.Adherence to Long Term Therapies: Evidence for Action. Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 59.Zeller A, Ramseier E, Teagtmeyer A, Battegay E. Patients’ self-reported adherence to Cardiovascular medication using electronic monitors as comparators. Hypertens Res. 2008;31:2037–2043. doi: 10.1291/hypres.31.2037. [DOI] [PubMed] [Google Scholar]