Abstract
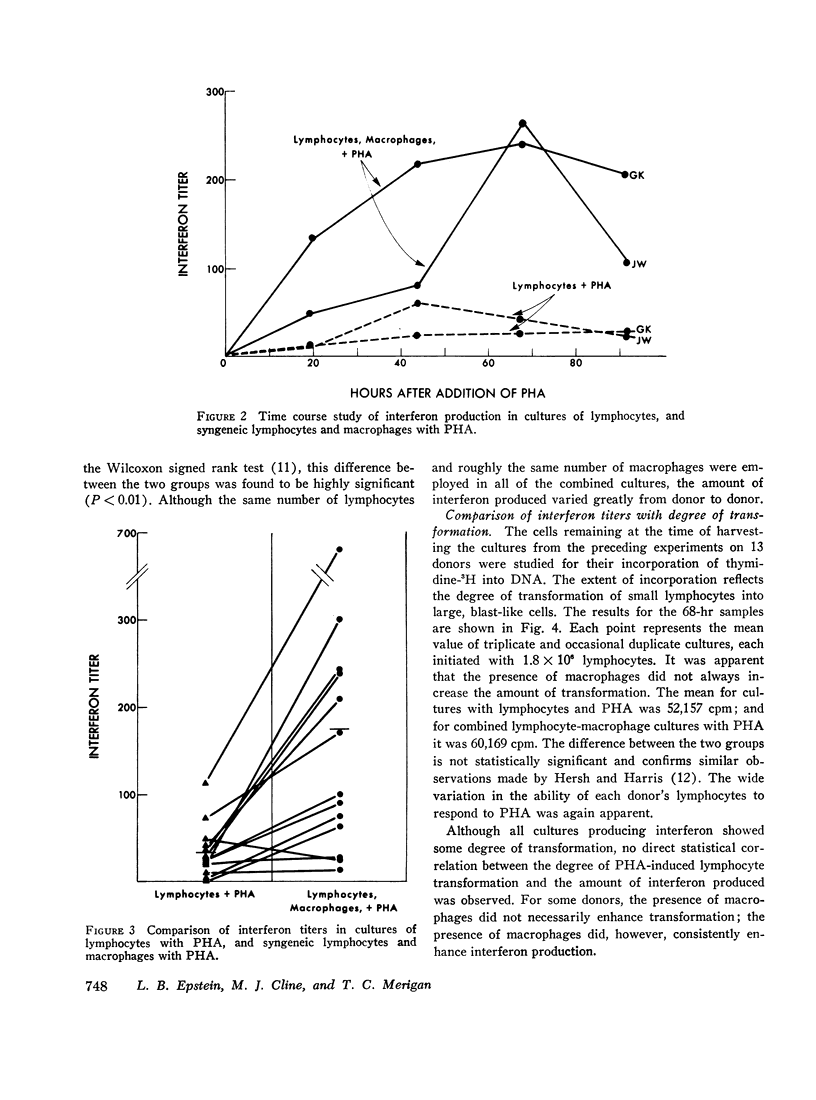
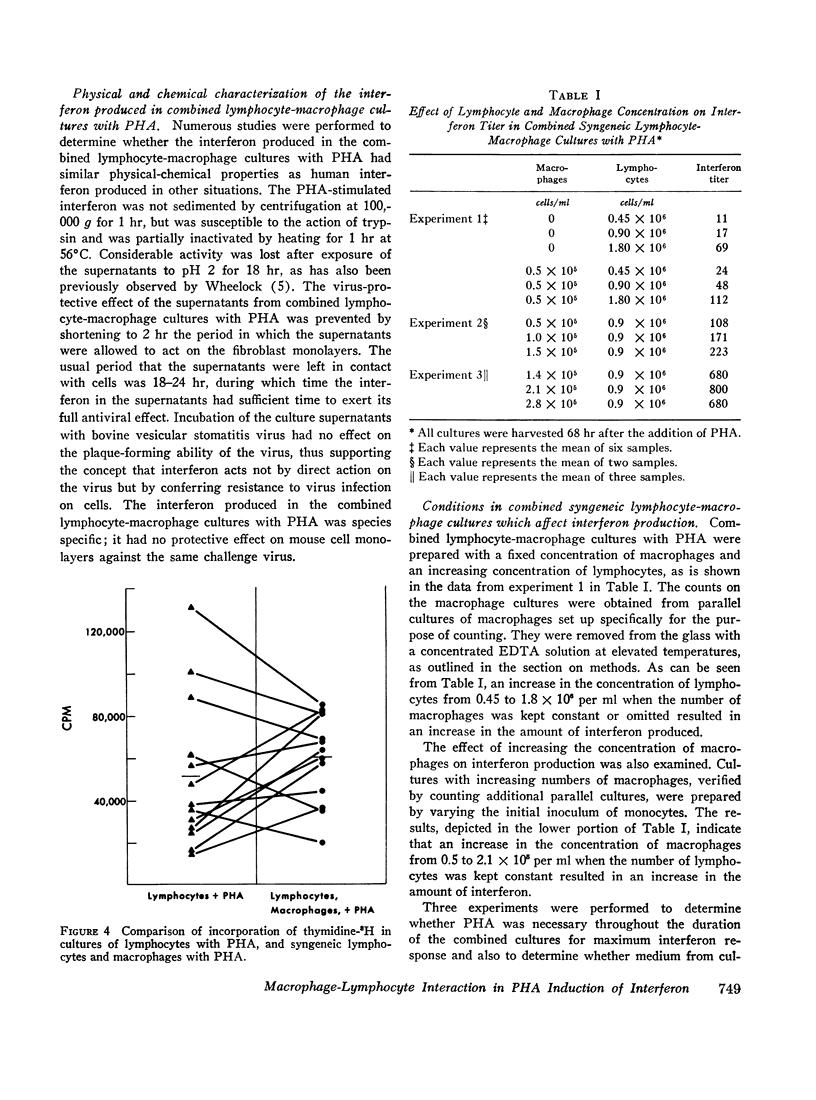
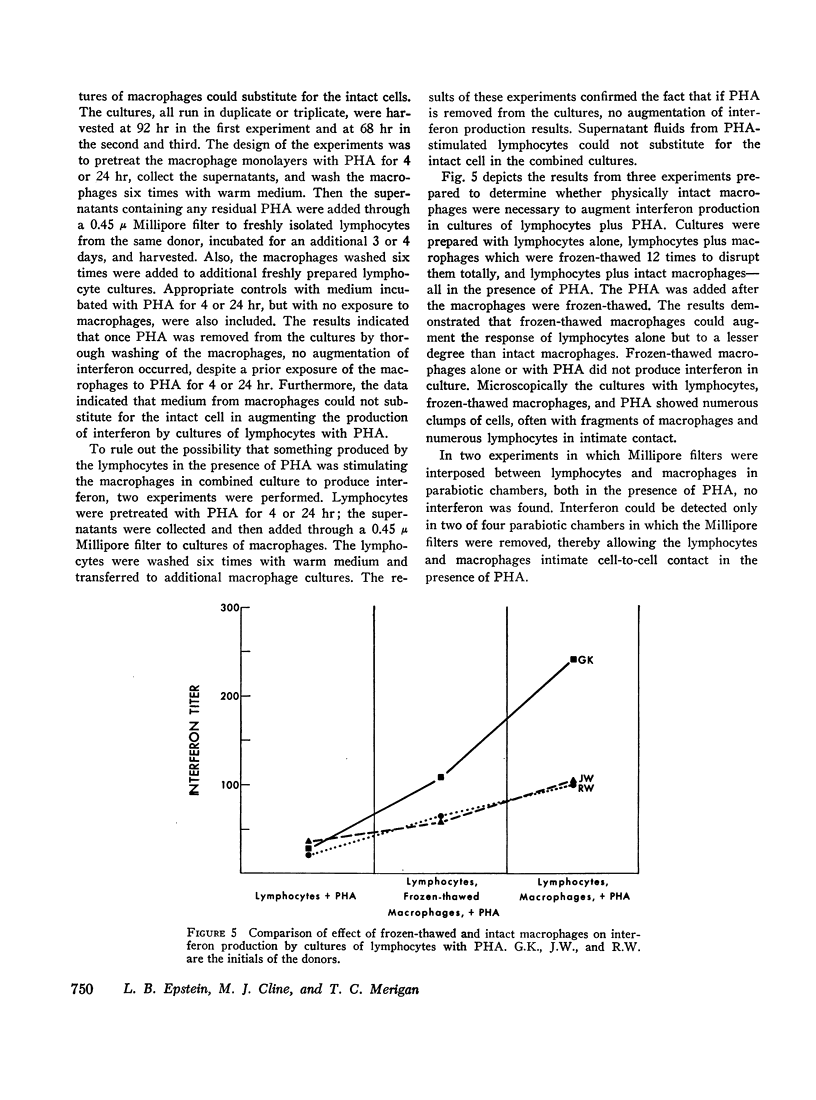
In studies of 13 normal adults to determine the blood cell types responsible for interferon production induced by phytohemagglutinin, the following observations were made. (a) In cultures containing 96-100% pure macrophages derived from blood monocytes, no interferon was detected in either the presence or the absence of phytohemagglutinin for up to 92 hr. (b) In cultures of 99.5-100% pure lymphocytes, low levels of interferon were detected in the presence, but not in the absence, of phytohemagglutinin. (c) An average fivefold increase in interferon titers occurred when pure lymphocytes were combined with the macrophages in culture with phytohemagglutinin. The peak response of interferon occurred at 68 hr after the initiation of the combined cultures. For maximum response, phytohemagglutinin was required for the duration of the culture, and both cell types in association were necessary. Medium from phytohemagglutinin-stimulated macrophages or lymphocytes could not substitute for the corresponding intact cell. However, frozen-thawed macrophages in combination with lymphocytes and phytohemagglutinin produced an intermediate interferon response. An increase in either cell type produced an increased response in the range studied: lymphocytes, 0.45-1.8 × 106 per ml; and macrophages, 0.5-2.1 × 105 per ml. Syngeneic fibroblasts, HeLa cells, or mouse macrophages could not substitute for the human macrophages in the combined cultures with phytohemagglutinin. (d) Although all cultures producing interferon showed some degree of transformation (thymidine-3H incorporation into deoxyribonucleic acid), no direct correlation between the degree of phytohemagglutinin-induced lymphocyte transformation and the interferon titers was observed.
The demonstration of macrophage-lymphocyte interaction in the production of interferon is of interest in view of the known interrelationship of these same cell types in antibody synthesis and cellular immunity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Clercq E., Merigan T. C. Current concepts of interferon and interferon induction. Annu Rev Med. 1970;21:17–46. doi: 10.1146/annurev.me.21.020170.000313. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Smith C. W. The in vivo induction of mouse lymphocyte transformation by phytohemagglutinin. J Immunol. 1968 Feb;100(2):421–435. [PubMed] [Google Scholar]
- Friedman R. M., Cooper H. L. Stimulation of interferon production in human lymphocytes by mitogens. Proc Soc Exp Biol Med. 1967 Jul;125(3):901–905. doi: 10.3181/00379727-125-32235. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966 Jun;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Lev W. R., Robbins J. H. Antigen-induced blastogenesis: the human cell determining the specificity of response in vitro. J Immunol. 1970 May;104(5):1295–1299. [PubMed] [Google Scholar]
- MACKINNEY A. A., Jr, STOHLMAN F., Jr, BRECHER G. The kinetics of cell proliferation in cultures of human peripheral blood. Blood. 1962 Mar;19:349–358. [PubMed] [Google Scholar]
- Merigan T. C., Gregory D. F., Petralli J. K. Physical properties of human interferon prepared in vitro and in vivo. Virology. 1966 Aug;29(4):515–522. doi: 10.1016/0042-6822(66)90276-5. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. Cell interactions in the primary immune response in vitro: a requirement for specific cell clusters. J Exp Med. 1969 Feb 1;129(2):351–362. doi: 10.1084/jem.129.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasanen V. J., Epstein L. B. An appraisal of an autoradiographic technique for enumeration of antibody containing cells in response to Salmonella somatic polysaccharide. Int Arch Allergy Appl Immunol. 1967;32(2):149–163. doi: 10.1159/000229924. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., EPSTEIN L. B., BRECHER G., STOHLMAN F., Jr TRANSFORMATION OF LYMPHOCYTES IN CULTURES OF HUMAN PERIPHERAL BLOOD. Blood. 1963 Nov;22:614–629. [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. [PubMed] [Google Scholar]