Abstract
Background
Although injury to the lateral femoral cutaneous nerve (LFCN) is a known complication of anterior approaches to the hip and pelvis, no study has quantified its’ incidence in anterior arthroplasty procedures.
Questions/purposes
We therefore defined the incidence, functional impact, and natural history of LFCN neuropraxia after an anterior approach for both hip resurfacing (HR) and primary total hip arthroplasty (THA).
Methods
We followed 132 patients who underwent an anterior hip approach (55 THA; 77 HR). We administered self-reported questionnaires for sensory deficits of LFCN, neuropathic pain score (DN4), visual analog scale, as well as SF-12, UCLA, and WOMAC scores at one year postoperatively. A subset of 60 patients (30 THA; 30 HR) was evaluated at two time intervals.
Results
One hundred seven patients (81%) reported LFCN neuropraxia with a mean severity score of 2.32/10 and a mean DN4 score of 2.42/10. Hip resurfacing had a higher incidence of neuropraxia as compared with THA: 91% versus 67%, respectively. No functional limitations were reported on SF-12, WOMAC, or UCLA scores. Of the subset of 60 patients followed over an average of 12 months, 53 (88%) reported neuropraxia at the first followup interval with only three (6%) having complete resolution at second followup. Improvement in DN4 scores was observed over time: 3.6 versus 2.5, respectively.
Conclusions
Although LFCN neuropraxia was a frequent complication after anterior approach THA, it did not lead to functional limitations in our patients. A decrease in symptoms occurred over time but only a small number of patients reported complete resolution.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The soft tissue-preserving nature of the anterior or Hueter hip approach combined with the relatively low risk of dislocation has generated an interest in this exposure for hip arthroplasty over the last decade [47]. The anterior approach is muscle-splitting, and the only true internervous approach to the hip [5, 8]. It leaves the hip abductors and posterior soft tissue envelope intact, and provides the added advantage of preserving femoral head blood supply when performing hip resurfacing [8]. Several large series using this approach for THA, as well as a growing body of literature for hip resurfacing, suggest that this approach produces accurate and reproducible results with regard to component position and leg length restoration, with no increase in dislocation rates [32, 33, 41, 53, 59, 61]. In smaller community hospital settings, concern has been raised regarding trochanteric fractures; Woolson and associates reported a 5.7% incidence [66] in their series of anterior approach THA. However, two larger multicenter studies found this incidence to be only 1% in over 1100 patients [59, 61]. In all three studies, the risk of dislocation was less than 1%. This is critical as other branded “less invasive surgical approaches” have been associated with major complications and no real added benefit in terms of patient function and/or recovery [65].
Despite these promising results, the incidence of injury to the lateral femoral cutaneous nerve (LFCN) in anterior approach arthroplasty procedures is unknown in spite of anecdotal concerns. This nerve is at particular risk with other anterior approaches to the hip, such as pelvic osteotomies and iliac crest bone graft harvesting [9, 19, 28, 32, 42, 43, 67]. Although injury to the LFCN does not represent a major neurologic complication as compared with the potentially catastrophic outcomes of sciatic and femoral nerve palsies [4, 20, 21, 23, 31, 54, 55], its incidence after joint arthroplasty through an anterior approach is not well reported [39, 49, 57, 61].
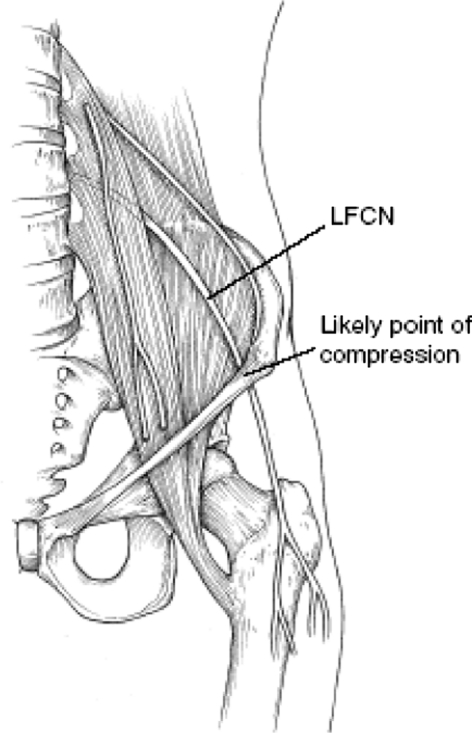
Patients with injuries to the LFCN typically report numbness and/or a burning sensation on the anterolateral thigh and, in worst cases, of dysesthesias. The common involvement of the LFCN with anterior approaches to the iliac wing or to the hip is believed to be the result of the wide variability in its regional anatomy [16, 28, 37, 42, 46, 48, 55]. The nerve is particularly variable at the level of the anteroposterior iliac spine and the inguinal ligament [14, 16, 22, 28, 48, 60]. It is also vulnerable distally as it emerges from the sartorius muscle or through the interval between sartorius and the tensor fascia lata [5, 8, 38] and then arborizes into the anterior and posterior branches, which supply the cutaneous area of the anterolateral thigh [5, 21, 43, 46]. Consequently, not only can the nerve be injured at various levels, but also through various mechanisms, including stretching, compression, laceration, and involvement in scar tissue formation [15, 16, 22, 28, 30, 36, 44, 55, 67] (Fig. 1). In addition, the risk of permanent or temporary neurologic impairment to the LFCN could range from a temporary neuropraxia to a painful neuroma with symptoms analogous to meralgia paresthetica.
Fig. 1.
The anatomic course of the LFCN is shown. (Reprinted with permission. Adapted by American Academy of Orthopaedic Surgeons from Mirovsky Y, Neuwirth M: Injuries to the lateral femoral cutaneous nerve during spine surgery. Spine 2000;25:1266–1269.)
The objectives of this study are to (1) define the incidence of LFCN neuropraxia after THA, including both stem-type direct anterior approach (DAA) THA and hip resurfacing, (2) identify risk factors associated with this complication; (3) determine the impact of LFCN neuropraxia on patient function; and (4) describe the natural history of LFCN neuropraxia.
Patients and Methods
We followed 132 patients who underwent DAA hip arthroplasty between September 2006 and January 2008. We included all patients during this interval who were (1) skeletally mature; (2) had primary THA or resurfacing arthroplasty; and (3) had no prior hip surgery. There were 70 males and 62 females with a mean age at surgery of 55.5 years (range, 29.9–88.7 years). Over 80% of patients had a diagnosis of osteoarthritis. Fifty-five patients, or 41.7%, underwent THA and 77 patients, or 58.3%, had hip resurfacing. Fifty-five procedures involved the left hip, 77 involved the right hip, and 15 patients had bilateral procedures. The mean body mass index (BMI) was 27.6 kg/m2 (range, 18–44 kg/m2). The mean followup for this group was 13.4 months (range, 8.4–26.5 months). Approval was sought and obtained from the hospital Institutional Review Board, and the research was performed in accordance with the 1964 Declaration of Helsinki’s ethical standards. Before surgery, we obtained informed consent for study participation.
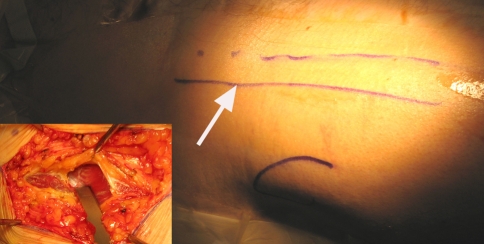
All surgery was performed by two surgeons (PB, PK) experienced in adult hip reconstruction. Patients were placed either supine on a specialized positioning table or on a standard orthopaedic operating table based on surgeon preference. The incision began 2 cm lateral and distal to the anterosuperior iliac spine and proceeded distally for up to 10 cm along a line angling toward the fibular head, centered over the greater trochanter [10]. The fascial sheath over the tensor was incised with the muscle belly retracted laterally (Fig. 2). The rectus femoris was then mobilized medially after releasing the fascia. An anterior and lateral capsulectomy was then performed [10].
Fig. 2.
Location of incision for anterior approach being centered over the tip of the greater trochanter and 1–2 cm lateral to anterosuperior iliac spine. Inset shows dissection performed within the tensor fascial sheath.
Of the 132 patients, we retrospectively evaluated a subsample of 60 patients with followup at two time intervals to assess the natural history of LFCN neuropraxia, which we defined as the presence of any one of three symptoms: numbness, tingling, or burning in the anterolateral thigh. These patients were chosen based on having completed all questionnaires and functional outcome scores at two separate time intervals. The mean followup was 5.7 months (range, 2.6–9.0 months) at the first assessment and 12.3 months (range, 8.4–20.8 months) at the second assessment. The average length of time between interval followups was 6.6 months (SD, 2.48). Thirty patients (50%) underwent THA and 30 patients had a resurfacing procedure. Participants in this subset included 30 females and 30 males with a mean age at surgery of 58.1 years (range, 38.4–77.4 years) and a mean BMI of 28.3 kg/m2 (range, 19–44 kg/m2).
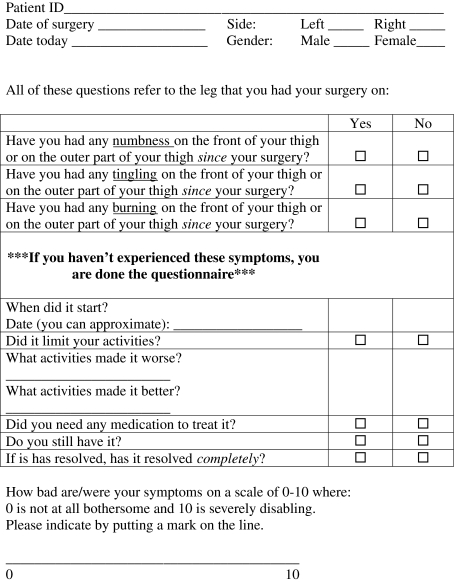
In the absence of a validated diagnostic tool for LFCN neuropraxia, patients responded to a standardized, in-house, self-reported questionnaire documenting sensory disturbances in the LFCN distribution at the first and second postoperative visit (see Appendix 1). Patients were asked if they had experienced any symptoms (numbness, tingling, burning) on the front or outer area of the thigh since the time of their surgery, the date it started, and whether those symptoms had resolved. Severity of the neuropraxia was assessed by (1) a visual analog scale, in which 0 indicates that the symptoms are “not at all bothersome” and 10 indicates “severely disabling: symptoms; and (2) the DN4 score, a two-part interview and physical examination scored out of 10, which documents sensory changes on clinical history and correlates this to dysesthesias to touch and painful stimuli in the affected area as assessed by a clinician. The DN4 is a validated diagnostic tool to estimate the probability of neuropathic pain, in which a score of 4 or greater is considered a positive test (sensitivity, 82.9%; specificity, 89.9%) [13]. The tool was developed by the French Neuropathic Pain group, who identified discriminating properties of neuropathic pain in a group of 160 patients with known neurological or somatic lesions, in order to formulate a reproducible questionnaire with the psychometric properties to diagnose neuropathic pain [13].
Lastly, we obtained a WOMAC Osteoarthritis Index (Pain, Stiffness, and Function subscales) [7], SF-12 (Mental and Physical Component subscales) [64], and the UCLA Activity Rating Scale [6] functional outcome scores for all patients. Higher scores reflect a higher level of functioning.
We used logistic regression to determine whether age, procedure, BMI, or gender were associated with LFCN neuropraxia. To assess the impact of LFCN neuropraxia on patient function, we used Mann-Whitney U tests to compare differences in the three functional scores between patients reporting neuropraxia versus those with no symptoms. We assessed the correlation between DN4 scores and functional scores for those patients who reported LFCN neuropraxia using a Pearson correlation. In evaluating the natural history of LFCN neuropraxia, a chi square analysis was performed on the subsample of 60 patients to determine the proportion of patients who reported neuropraxia at the early followup interval and had resolution of symptoms at the second interval. We used Wilcoxon signed rank tests to assess the change in symptom severity and the DN4 score between the two testing intervals. All statistical analyses were performed using SPSS® 15.0 for Windows® (SPSS Inc, Chicago, IL).
Results
One hundred seven of 132 patients (81%) reported sensory deficits in the anterolateral thigh area with a mean severity score of 2.32 out of 10 (SD, 2.11; range, 0–10) and a mean DN4 score of 2.42 out of 10 (SD, 2.37; range, 0–9).
Hip resurfacing was associated with a higher incidence of neuropraxia as compared with THA; 70 of the 77 patients who underwent resurfacing (91%) reported LFCN neuropraxia as compared with 37 of the 55 patients (67%) who had THA (p = 0.02). However, we observed no differences in the severity of symptoms as measured by the visual analog scale (p = 0.32) or the DN4 (p = 0.96) between resurfacing and THA patients. We found no association between LFCN neuropraxia and age (p = 0.96), BMI (p = 0.59), or gender (p = 0.62).
We observed no difference between patients who reported neuropraxia versus those with no symptoms on the following functional outcome measures: UCLA (p = 0.31), WOMAC Pain (p = 0.58), WOMAC Stiffness (p = 0.14), WOMAC Function (p = 0.74), SF-12 Physical Component Subscale (p = 0.71), or SF-12 Mental Component Subscale (p = 0.81) (Table 1). Furthermore, there was no association between DN4 scores and functional outcome as measured by UCLA (p = 0.89), WOMAC Pain (p = 0.52), WOMAC Stiffness (p = 0.74), WOMAC Function (p = 0.67), SF-12 Physical Component Subscale (p = 0.66), or SF-12 Mental Component Subscale (p = 0.94).
Table 1.
Functional outcomes in asymptomatic patients versus patients with LFCN neuropraxia
| Functional outcome | Asymptomatic group mean (95% CI) | Neuropraxia group mean (95% CI) |
|---|---|---|
| WOMAC pain | 89.7 (82.31, 97.06) | 90.1 (86.67, 93.46) |
| WOMAC stiffness | 85.2 (75.99, 94.33) | 78.0 (73.51, 82.41) |
| WOMAC function | 90.1 (83.31, 96.84) | 87.6 (84.17, 91.09) |
| UCLA | 6.8 (5.79, 7.86) | 7.2 (6.68, 7.71) |
| SF-12 Physical component | 48.6 (43.93, 53.21) | 47.7 (45.59, 49.78) |
| SF-12 Mental component | 56.1 (53.12, 59.08) | 55.2 (53.51, 56.97) |
In the subset of 60 patients assessed at two time intervals, 53 of the 60 patients, or 88.3%, reported neuropraxia at the early followup (mean, 5.3 months). At the second interval (mean, 12.3 months), only three of these 53 patients (5.7%) reported complete resolution of symptoms. Patients who reported neuropraxia at both testing intervals showed no change in symptom severity (2.83 and 2.64 out of 10 at first and second assessments, respectively, p = 0.85) but did report an improvement in DN4 scores, 3.6 to 2.5 out of 10 at first and second assessments respectively (p = 0.02).
Discussion
In contrast to other surgical approaches to the hip, the anterior approach follows a well-delineated internervous plane and provides direct access to the hip with minimal muscle retraction. However, the LFCN and its branches are within the field of dissection and at risk of injury. The primary purpose of our study was to document the incidence and risk factors associated with LFCN neuropraxia after DAA THA and HR, to evaluate its impact on functional outcomes as well as to determine its natural history.
Several study limitations have been identified. First, the study is limited by patient reporting, which relies on patients’ recollections of their own symptoms and can be difficult to quantify. In the absence of a validated standardized tool for documenting injuries of the LFCN, our questionnaire captured an array of symptoms, and correlated these to the timing of the surgical procedure. Furthermore, the inclusion of the DN4 collates clinical symptoms with physicians’ findings, and is validated to identify neuropathic pain [13]. Second, no imaging was performed to rule out other causes of neuropathic pain, such as MRI [12, 25, 28, 43, 48, 67] or ultrasonography, to characterize the nature of the nerve injury [62]. Other studies have included electrophysiology, nerve conduction studies, and somatosensory evoked potentials [16, 18, 20, 30, 36, 58]. We identified only patients who developed symptoms immediately postoperatively, pointing to a more likely involvement of the LFCN considering proximity of the skin incision to the path of the nerve. Third, variability in followup creates a challenge in making definitive statements about natural history; 45% of the cohort completed all study parameters at both followups, and these intervals had wide ranges secondary to patient compliance. A selection bias may have been introduced, as patients with neuropraxia may have self-selected to more consistently return for followup at set intervals. Further, patients with shorter followup intervals may not have had sufficient time to improve as compared to those who were assessed at a longer followup interval.
Injury to the lateral femoral cutaneous nerve (LFCN) has most commonly been associated with iliac crest harvest for bone grafting, acetabular fixation, and pelvic osteotomies [28], with reported rates of 4.5% to 37% [2, 3, 17, 24, 26, 27, 32, 35, 68]. The literature is sparse with respect to its investigation in anterior arthroplasty procedures. A multicenter observational study reported 13 neurological complications in 1,152 DAA THA patients: 7 LFCN palsies, one anterolateral thigh paresthesia and five cases of lateral thigh numbness [61]. Seng reported two transient LFCN paresthesias in 182 anterior THA, both of which resolved [57]. In a recent prospective randomized controlled trial comparing lateral to anterior approach THA, Restrepo reported a 2% rate of LFCN neuropraxia in 50 DAA THA [49]. The incidence reported in our series was 81%, higher than in other series. We suspect LFCN neuropraxia is underreported. Being aware of the issue we specifically asked each patient in great detail about peri-incisional and anterolateral thigh sensory changes. The majority of our patients did not volunteer this information, and only admitted to a presence of findings when asked specifically. Similarly, Lovell reported that the majority of patients had some numbness in the lateral and distal aspect of the incision after anterior approach total hip arthroplasty [39]. The ATHAC investigators distinguished between LFCN and anterolateral thigh paresthesias [61]; perhaps LFCN neuropraxia is overrepresented in our sample due to the confounder of peri-incisional sensory changes, however, differentiating the two is difficult. A clearer definition of this nerve lesion clinically and by imaging will provide us with a better understanding of its' etiology as well as natural history. In the future, it may be worth comparing rates of LFCN neuropraxia in anterior approach with peri-incisional numbness from other hip approaches. One factor that may have also influenced the presence of LFCN neuropraxia is how lateral the incision was to the ASIS (Fig. 2). Unfortunately, we did not assess the variability of the placement of our incision with respect to the ASIS but did do the dissection in every case within the tensor fascial sheath.
Hip resurfacing was identified as a risk factor for LFCN neuropraxia, and we postulate the longer incision over arborized branches of the LFCN, in addition to the increased retraction needed for femoral head-neck preparation may be culprits. The etiology of LFCN neuropraxia is likely multifactorial [8, 21, 28]. Intraoperative modifications to avoid injury include lateralizing the skin incision, strict subfascial dissection within TFL [38] and avoidance of medial subcutaneous fat pad dissection, and rigorous retraction of the rectus femoris during component implantation [5, 8, 28].
Meralgia paresthetica was first described as a spontaneous entrapment of the main trunk of the LFCN [9, 16, 28, 48, 51]; it is now loosely applied to any involvement of the LFCN [48]. Our findings suggest that LFCN neuropraxia is not analogous with meralgia paresthetica; this is comparable to Lovell’s series wherein less than 1% of his cases of neuropraxia resulted in meralgia paresthetica [39]. Our patients’ pain scores were minimal and no functional impairments were discernable; in fact the functional scores at both followups were comparable to those in a large series of patients undergoing anterior approach THA [61]. Peri-incisional dysesthesias after DAA THA may in fact be analogous to injury of the infrapatellar branch of the saphenous nerve (IPBSN) [29] in ACL reconstruction and TKA, in which numbness around the surgical wound is neither uncommon nor functionally impairing. Incidence ranges from 4% after TKA to up 88% after ACL reconstruction [1, 11, 34, 40, 45, 52]. Similar to our findings, the clinical ramifications of an injury to the IPBSN, a cutaneous nerve, are few [1, 52].
The incidence of LFCN neuropraxia did not decrease substantially over time in our subsample, with only 3 patients going on to full resolution. In Seng’s series, the authors reported resolution of LFCN neuropraxia in both of their patients, however, it was not clear how the neuropraxia was identified or followed [57]. Our findings, in conjunction with a large body of literature on the resolution of meralgia paresthetica [9, 14, 18, 21, 37, 42, 43, 46, 48, 50, 51, 56, 63, 67] suggest that DAA associated LFCN neuropraxia may be a transient sensory disturbance which is slow to resolve. Regardless, patients should be advised preoperatively of the incidence of LFCN neuropraxia. In future, larger numbers and longer, less variable followup is needed to better delineate the natural history of this neuropraxia as well as ways to minimize its incidence.
Acknowledgments
We thank Heather Belanger RN, BScN, and Gillian Parker, BSc (Ottawa Hospital–General Campus, Ottawa, ON, Canada) for their assistance with data collection and the coordination of this research project.
Appendix 1: Self-reported antero-lateral thigh numbness
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abbott LC, Carpenter WF. Surgical approaches to the knee joint. J Bone Joint Surg Am. 1945;27:277–310. [Google Scholar]
- 2.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84:716–720. doi: 10.1302/0301-620X.84B5.12571. [DOI] [PubMed] [Google Scholar]
- 3.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Barrack RL. Neurovascular injury: avoiding catastrophe. J Arthroplasty. 2004;19(Suppl 1):104–107. doi: 10.1016/j.arth.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Barton C, Kim PR. Complications of the direct anterior approach following total hip arthroplasty. Orthop Clin N Am. 2009;40:371–375. doi: 10.1016/j.ocl.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Beaule PE, Dorey FJ, LeDuff M, Amstutz HC. Validation of activity level in the assessment of the clinical outcome of total hip arthroplasty. J Bone Joint Surg Br. 2005;87(Suppl 3):360. [Google Scholar]
- 7.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 8.Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin N Am. 2009;40:321–328. doi: 10.1016/j.ocl.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Benezis I, Boutaud B, Leclerc J, Fabre T, Durandeau A. Lateral femoral cutaneous neuropathy and its surgical treatment: a report of 167 cases. Muscle Nerve. 2007;36:659–663. doi: 10.1002/mus.20868. [DOI] [PubMed] [Google Scholar]
- 10.Benoit B, Gofton W, Beaule PE. Hueter anterior approach for hip resurfacing: Assessment of the learning curve. Orthop Clin N Am. 2009;40:357–363. doi: 10.1016/j.ocl.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Bhave A, Mont M, Tennis S, Nickey M, Starr R, Etienne G. Functional problems and treatment solutions after total hip and knee joint arthroplasty. J Bone Joint Surg Am. 2005;87:9–21. doi: 10.2106/JBJS.E.00628. [DOI] [PubMed] [Google Scholar]
- 12.Brown TE, Larson B, Shen F, Moskal JT. Thigh pain after cementless total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2002;10:385–392. doi: 10.5435/00124635-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Buch KA, Campbell J. Acute onset meralgia paresthetica after fracture of the anterior superior iliac spine. Injury. 1993;24:569–570. doi: 10.1016/0020-1383(93)90043-6. [DOI] [PubMed] [Google Scholar]
- 15.Capdevila X, Macaire P, Dadure C, Choquet O, Biboulet P, Ryckwaert Y, D’Athis F. Continuous psoas compartment block for postoperative analgesia after total hip arthroplasty: new landmarks, technical guidelines, and clinical evaluation. Anesth Analg. 2002;94:1606–1613. doi: 10.1097/00000539-200206000-00045. [DOI] [PubMed] [Google Scholar]
- 16.Coert JH, Dellon AL. Documenting neuropathy of the lateral femoral cutaneous nerve using the pressure specific sensory testing device. Ann Plast Surg. 2003;50:373–377. doi: 10.1097/01.SAP.0000041483.93122.58. [DOI] [PubMed] [Google Scholar]
- 17.Colterjohn NR, Bednar DA. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J Bone Joint Surg Am. 1997;79:756–759. doi: 10.2106/00004623-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Cordato DJ, Yiannikas C, Stroud J, Halpern JP, Schwartz RS, Akbunar M, Cook M. Evoked potentials elicited by stimulation of the lateral and anterior femoral cutaneous nerves in meralgia paresthetica. Muscle Nerve. 2004;29:139–142. doi: 10.1002/mus.10515. [DOI] [PubMed] [Google Scholar]
- 19.Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:33–37. doi: 10.1097/00003086-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 20.DeHart MM, Riley LH., Jr Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg. 1999;7:101–111. doi: 10.5435/00124635-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Dellon AL, Mont M, Ducic I. Involvement of the lateral femoral cutaneous nerve as source of persistent pain after total hip arthroplasty. J Arthroplasty. 2008;23:480–485. doi: 10.1016/j.arth.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Dibenedetto LM, Lei Q, Gilroy AM, Hermey DC, Marks SC, Jr, Page DW. Variations in the inferior pelvic pathway of the lateral femoral cutaneous nerve: implications for laparoscopic hernia repair. Clin Anat. 1996;9:232–236. doi: 10.1002/(SICI)1098-2353(1996)9:4<232::AID-CA3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop Relat Res. 1987;218:136–141. [PubMed] [Google Scholar]
- 24.Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Flowers RS. Meralgia paresthetica. A clue to a retroperitoneal malignant tumour. Am J Surg. 1968;116:89–92. doi: 10.1016/0002-9610(68)90423-6. [DOI] [PubMed] [Google Scholar]
- 26.Fowler BL, Dall BE, Rowe DE. Complications associated with harvesting autogenous iliac bone graft. Am J Orthop. 1995;24:895–903. [PubMed] [Google Scholar]
- 27.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336–344. doi: 10.5435/00124635-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Hunter LY, Louis DS, Ricciardi JR, O’Connor GA. The saphenous nerve: Its course and importance in medial arthrotomy. Am J Sports Med. 1979;7:227–230. doi: 10.1177/036354657900700403. [DOI] [PubMed] [Google Scholar]
- 30.Jablecki CK. Postoperative lateral femoral cutaneous neuropathy. Muscle Nerve. 1999;22:1129–1131. doi: 10.1002/(SICI)1097-4598(199908)22:8<1129::AID-MUS19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Johanson NA, Pellicci PM, Tsairis P, Salvati EA. Nerve injury in total hip arthroplasty. Clin. Orthop Relat Res. 1983;179:214–222. doi: 10.1097/00003086-198310000-00034. [DOI] [PubMed] [Google Scholar]
- 32.Keller EE, Triplett WW. Iliac bone graft: review of 160 consecutive cases. J Oral Maxillofac Surg. 1987;45:11–14. doi: 10.1016/0278-2391(87)90079-6. [DOI] [PubMed] [Google Scholar]
- 33.Kennon RE, Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85(Suppl 4):39–48. doi: 10.2106/00004623-200300004-00005. [DOI] [PubMed] [Google Scholar]
- 34.Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring autograft. Int J Sports Med. 2008;29:507–511. doi: 10.1055/s-2008-1038338. [DOI] [PubMed] [Google Scholar]
- 35.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Kushnir M, Klein C, Kimiagar Y, Pollak L, Rabey JM. Distal lesion of the lateral femoral cutaneous nerve. Muscle Nerve. 2008;37:101–103. doi: 10.1002/mus.20876. [DOI] [PubMed] [Google Scholar]
- 37.Lee CH, Dellon AL. Surgical management of groin pain of neural origin. J Am Coll Surg. 2000;191:137–142. doi: 10.1016/S1072-7515(00)00319-7. [DOI] [PubMed] [Google Scholar]
- 38.Letournel E. Acetabular fractures: classification and management. Clin Orthop Relat Res. 1980;151:81–106. [PubMed] [Google Scholar]
- 39.Lovell TP. Single incision direct anterior approach for total hip arthroplasty using a standard orthopaedic table. J Arthroplasty. 2008;23(7): Suppl 1:64–68. [DOI] [PubMed]
- 40.Luo H, Yu JK, Ao YF, Yu CL, Peng LB, Lin CY, Zhang JY, Fu X. Relationship between different skin incisions and the injury of the infrapatellar branch of the saphenous nerve during anterior cruciate ligament reconstruction. Chin Med J (Engl) 2007;120:1127–1130. [PubMed] [Google Scholar]
- 41.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2004;441:115–124. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 42.Murata Y, Takahashi K, Yamagata M, Shimada Y, Moriya H. The anatomy of the lateral femoral cutaneous nerve, with special reference to the harvesting of iliac crest bone graft. J Bone Joint Surg Am. 2000;82:746–747. doi: 10.2106/00004623-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Nahabedian MY, Dellon AL. Meralgia paresthetica: etiology, diagnosis, and outcome of surgical decompression. Ann Plast Surg. 1995;35:590–594. doi: 10.1097/00000637-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Park JW, Kim DH, Hwang M, Bun HR. Meralgia paresthetica caused by hip-huggers in a patient with aberrant course of the lateral femoral cutaneous nerve. Muscle Nerve. 2007;35:678–680. doi: 10.1002/mus.20721. [DOI] [PubMed] [Google Scholar]
- 45.Portland GH, Martin D, Keene G, Menz T. Injury to the infrapatellar branch of the saphenous nerve in anterior cruciate ligament reconstruction: comparison of horizontal versus vertical harvest site incisions. Arthroscopy. 2005;21:281–285. doi: 10.1016/j.arthro.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Puig L, Alegro M, Moragas JM. Treatment of meralgia paraesthetica. Dermatology. 1995;191:73–74. doi: 10.1159/000246496. [DOI] [PubMed] [Google Scholar]
- 47.Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin N Am. 2009;40:311–320. doi: 10.1016/j.ocl.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Reid V, Cros D. Proximal sensory neuropathies of the leg. Neurol Clin. 1999;3:655–667. doi: 10.1016/S0733-8619(05)70157-2. [DOI] [PubMed] [Google Scholar]
- 49.Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of 2 surgical approaches in total hip arthroplasty. J Arthroplasty. 2010 April 7. [Epub ahead of print]. [DOI] [PubMed]
- 50.Rosenquist RW, Lederhaas G. Femoral and lateral femoral cutaneous nerve block. Reg Anesth Pain Manag. 1999;3:33–38. doi: 10.1016/S1084-208X(99)80020-2. [DOI] [Google Scholar]
- 51.Roth WK. Meralgia Paresthetica. Berlin, Germany: S Karque; 1895. [Google Scholar]
- 52.Sanders B, Rolf R, McClelland W, Xerogeanes J. Prevalence of saphenous nerve injury after autogenous hamstring harvest: an anatomic and clinical study of sartorial branch injury. Arthroscopy. 2007;23:956–963. doi: 10.1016/j.arthro.2007.03.099. [DOI] [PubMed] [Google Scholar]
- 53.Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23:266–272. doi: 10.1016/j.arth.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Schmalzried TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. Risk factors and prognosis. J Bone Joint Surg Am. 1991;73:1074–1080. [PubMed] [Google Scholar]
- 55.Schnatz P, Wax JR, Steinfeld JD, Ingardia CJ. Meralgia paresthetica: an usual complication of post-cesarean analgesia. J Clin Anesth. 1999;11:416–418. doi: 10.1016/S0952-8180(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 56.Seddon HJ. Surgical Disorders of the Peripheral Nerves. 2. Edinburgh, Scotland: Churchill Livingstone; 1975. [Google Scholar]
- 57.Seng BE, Berend KR, Ajluni AF, Lombardi AV. Anterior-supine minimally invasive total hip arthroplasty: Defining the curve. Orthop Clin N Am. 2009;40:343–350. doi: 10.1016/j.ocl.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Shin YB, Park JH, Kwon DR, Park BK. Variability in conduction of the lateral femoral cutaneous nerve. Muscle Nerve. 2006;33:645–649. doi: 10.1002/mus.20505. [DOI] [PubMed] [Google Scholar]
- 59.Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res. 2004;426:164–173. doi: 10.1097/01.blo.0000136651.21191.9f. [DOI] [PubMed] [Google Scholar]
- 60.Surucu HS, Tanyeli E, Sargon MF, Karahan ST. An anatomic study of the lateral femoral cutaneous nerve. Surg Radiol Anat. 1997;19:307–310. doi: 10.1007/BF01637599. [DOI] [PubMed] [Google Scholar]
- 61.The Anterior Total Hip Arthroplasty Collaborative (ATHAC) Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin N Am. 2009;40:329–342. [DOI] [PubMed]
- 62.Toros T, Karabay N, Ozaksar N, Sugun TS, Kayalar M, Bal E. Evaluation of peripheral nerves of the upper extremity with ultrasonography: a comparison of ultrasonographic examination and the intra-operative findings. J Bone Joint Surg Br. 2009;91:762–765. doi: 10.1302/0301-620X.91B6.22284. [DOI] [PubMed] [Google Scholar]
- 63.Wall PD, Devor M. Physiology of sensation after peripheral nerve injury, regeneration, and neuroma formation. In: Waxman G, editor. Physiology and Pathohistology of Axons. New York, NY: Raven Press; 1978. pp. 377–388. [Google Scholar]
- 64.Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Woolson ST, Mow CS, Syquia JF, Lannin JV, Schurman DJ. Comparison of primary total hip replacements performed with a standard incision or a mini-incision. J Bone Joint Surg Am. 2004;86:1353–1358. doi: 10.2106/00004623-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Woolson ST, Pouliot BA, Huddlestone JI. Primary total hip arthroplasty using an anterior approach and a fracture table. J Arthroplasty. 2009;24:999–1005. doi: 10.1016/j.arth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spinal surgery: incidence, risk factors, and clinical outcomes. Spine. 2005;30:E547–E550. doi: 10.1097/01.brs.0000178821.14102.9d. [DOI] [PubMed] [Google Scholar]
- 68.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]