Introduction
Over the years, the quiet waters of flow cytometric analysis of circulating micro-particles (MPs) have given way to a deluge of original papers, technical briefs, and critical reviews. However, given the vast scope of work about circulating MPs, it is not possible to cover all the literature. Instead, this review focuses on flow cytometric analysis and applications of fetal-derived MPs. Although other cell-derived MPs such as platelet MPs (PMPs), endothelial MPs (EMPs) and leukocyte MP (LMPs) are not a focus of this review, many of the same principles apply. A brief introduction to MPs is given below.
Historically, the terms “exosomes”, “exosomes-like vesicles”, “micro-particles,” “micro-vesicles,” “membrane-bound particles”, “apoptotic bodies” and “apoptotic micro-particles” have been used interchangeably. However, this terminology is vague at best and does not distinguish between these “entities”. As reviewed by Theory et al., (1) a key difference between these subcellular fragments is the source and mechanism of derivation. Secreted MPs derived from intracellular multivesicular bodies fused with the plasma membrane are termed exosomes and exosomes-like vesicles, whereas MPs released from the surface of plasma membranes are referred to as micro-vesicles, membrane particles, and apoptotic vesicles.
Exosomes are 50 – 100 nm in diameter, contain lipid rafts, RNA and miRNA (2,3), but are devoid of DNA (4). Exosomes are secreted from epithelial cells (5), dendritic cell (6), B cells (7), T cells (8) and platelets (9) and are found in biological fluids such as plasma (10), urine (11), breast milk (12) and saliva (13). Exosome-like vesicles, which have similar characteristics of exosomes such as the “cup-shape” and contain mRNA, are slightly smaller in size (30 – 90 nm) and do not contain lipid rafts (14).
By contrast, micro-vesicles, membrane particles, ectosomes and apoptotic vesicles are typically larger in size (more than 100 nm) than secreted exosomes or exosomes-like vesicles (1). As mentioned above, what differentiates these MPs from exosomes and exosome-like vesicles is the mechanism of release, typically from the surface of blebbing membranes that arise after activation (15) or apoptosis (16). Our lab primarily focuses on apoptotic MPs generated in vitro and MPs (0.5 – 3 µm) found in maternal plasma (10,17).
Materials and Methods
Antibodies and reagents
Rabbit anti-human AT1 (sc-1173), and mouse anti-human AT1 (LS-C20663) were purchased from Santa Cruz Technologies (Santa Cruz, CA) and LifeSpan Biosciences (Seattle, WA), respectively. PE-conjugated goat anti-rabbit (GAR) F(ab’)2 and PE-conjugated goat anti-mouse (GAM) IgG were purchased from Invitrogen (Carlsbad, CA) and Serotec (Raleigh, NC), respectively. Monoclonal antibody (mAb) MEM-G/1 was purchased from Serotec (Raleigh, NC). CD49e–FITC, CD51-FITC, CD14-APC and CD41-Alexafluor-647 were purchased from BioLegend (San Diego, CA). Hoechst 33342 was purchased from Invitrogen.
Cell culture
The human trophoblastic cell line, JEG-3, was obtained from the American Type Culture Collection (ATCC) (Rockville, MD) and cultured as previously described (17). HTR-8/SVneo cells were donated by Dr. Y. Xia (University of Texas - Houston Health Science Center, Department of Biochemistry and Molecular Biology) and cultured as previously described (18).
Assessment of AT1 expression on HTR-8/SVneo cells by Flow Cytometry
HTR-8/SVneo cells were permeabilized and fixed using the BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (BD Biosciences, San Diego, CA) according to manufacturer's instructions. Briefly, after fixation/permeabilization, cells were incubated with anti-angiotensin II type 1 receptor (AT1) Abs: rabbit anti-human AT1 (sc-1173, Santa Cruz, CA) and mouse anti-human AT1 (LS-C20663, LifeSpan Biosciences, Seattle, WA) at various concentrations for 30 min on ice. Cells were then labeled with PE-conjugated GAR or PE-conjugated GAM for 20 min on ice and analyzed using a Beckman Coulter Gallios (Beckman Coulter, Miami Lakes, FL). The number of events was stopped at 10,000 counts. Data collected from the experiments were analyzed using Kaluza (analysis software from Beckman Coulter).
Isolation and Quantification in vitro apoptotic MPs
JEG-3 and HTR-8/SVneo apoptotic MPs were prepared as previously described (17). Isolation and quantification of MPs was performed using double filtered (0.25 µm) PBS (dfPBS). MPs from apoptotic supernatants were separated from detached cells by two centrifugation steps (300 × g, 5 minutes; 800 × g, 5 minutes). MP concentration was determined as previously described (19).
Polychromatic flow cytometric analysis of JEG-3 MPs
To avoid spectral overlap between the four flourochromes (FITC, PE, APC/Alexa Fluor-647 and Hoechst 33342), we used fluorescence minus-one (FMO) controls (20). The FMO control for FITC consisted of a sample labeled with PE, APC/Alexa Fluor-647 and Hoechst 33342, minus FITC. The PE FMO control consisted of FITC, APC/Alexa Fluor-647 and Hoechst 33342, minus PE. The APC/Alexa Fluor-647 FMO control consisted of FITC, PE and Hoechst 33342, minus APC/Alexa Fluor-647. The Hoechst 33342 FMO control consisted of FITC, PE and APC/Alexa Fluor-647, minus Hoechst 33342. One million MPs were centrifuged (10,000 rpm, 10 min) using an Eppendorf Centrifuge 5415c (Eppendorf, Westbury, NY, USA). After centrifugation, MPs were labeled with MEM-G/1 and PE-conjugated GAM as previously described (10). MPs were then labeled with CD49e–FITC, CD51-FITC, CD14-APC on ice for 20 min. After Ab labeling, MPs were countered-stained with Hoechst 33342 as previously described (17) and analyzed using an LSR II (BD Instruments, San Jose, CA). The number of events was stopped at 10,000 counts. Data collected from the experiments were analyzed using Summit V3.1 (analysis software from DAKO).
Assessment of AT1 expression on HTR-8/SVneo MPs by Flow Cytometry
Briefly, 1 × 106 apoptotic HTR-8/SVneo MPs were centrifuged (10,000 rpm, 10 min) using an Eppendorf Centrifuge 5415c (Eppendorf). MPs were permeabilized and fixed using the BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (BD Biosciences) according to manufacturer's instructions. Titration curve of PE-conjugated secondary Abs. After fixation, MPs were incubated on ice for 20 min with PE-conjugated GAR or PE-conjugated GAM and analyzed using a Beckman Coulter Gallios (Beckman Coulter). Data collected from the experiments were analyzed using Kaluza (analysis software from Beckman Coulter). Titration curve of AT1 Abs. After fixation/permeabilization, MPs were incubated with anti-AT1 Abs: rabbit anti-human AT1 (sc-1173) and mouse anti-human AT1 (LS-C20663) at various concentrations for 30 min on ice. MPs were then labeled with PE-conjugated GAR or PE-conjugated GAM for 20 min on ice and analyzed using a Beckman Coulter Gallios (Beckman Coulter). Data collected from the experiments were analyzed using Kaluza (analysis software from Beckman Coulter).
Plasma sample collection
After obtaining Institutional Review Board approval from Baylor College of Medicine and written consent from the human subjects, 5 to 10 ml of peripheral blood was collected in vacutainer tubes containing 1.5 ml of ACD Solution A (trisodium citrate, 22.0 g/L; citric acid, 8.0 g/L; and dextrose 24.5 g/L) and processed within 24 h. Plasma was separated from whole blood by centrifugation at 800 × g for 10 minutes. Recovered plasma was centrifuged for an additional 10 minutes at 1,600 × g to remove residual cells. Finally, cell-free supernatant was removed and stored in −80°C freezer.
Labeling of plasma MPs
MP concentration was determined as previously described by Montes et al. (19). The number of MPs was counted using an LSR II cytometer (BD Instruments). All labeling procedures were done at room temperature.
One million MPs were re-suspended in dfPBS and labeled with anti-AT1 Abs and mixed on a BD ADAMS Nutator (Aria Medical Equipment) for 15 min. MPs were then labeled with secondary PE-conjugated GAR and mixed for another 15 min. Afterwards, MPs were re-suspended in a total volume of 500 µl dfPBS and analyzed by an LSR II flow cytometer. The number of events was stopped at 10,000 counts. Data collected from the experiments were analyzed using Summit V3.1 (analysis software from DAKO).
Statistical Analysis
Comparison between two groups, sc-1173 Abs and LS-c20633 Abs, were performed using the two-sample t-test. P-values less than 0.05 were reported to be statistically significant. All statistical analyses were performed using Microsoft Office Exce®l.
Flow Cytometric Analysis of MPs
Preparation of MPs from biological fluids and cell/tissue cultures
Prior to analysis of MPs by flow cytometry, the first step is to remove contaminating/unwanted cells by centrifugation. Typically, both in vitro and in vivo MPs are isolated by differential centrifugation (Table 1). Although a standardized centrifugation protocol has not been established, the first and slower centrifugation speed generally eliminates cells and debris, whereas the second and third centrifugation speeds are faster and are adjusted depending on the MPs of interest (exosomes vs. plasma membrane-derived MPs). To remove contaminating/unwanted cells from cell/tissue media or bodily fluids, an initial centrifugation speed between 300 – 500 × g (5 – 20 minutes) is required. The cell-free supernatant is then transferred to a new tube. To pellet in vitro apoptotic MPs/bodies, cell-free supernatants undergo a final centrifugation between 25,000 – 100,000 × g. Cell-free plasma samples, undergo a further two-step centrifugation to obtain platelet free plasma (PFP). First, platelet poor plasma (PPP) is obtained by centrifugation speeds between 1,200 – 1,500 × g for 10 – 20 minutes, followed by centrifugation speeds between 10,000 – 13,000 × g for 30 minutes; the remaining supernatant contains MPs and is platelet free. However, this PFP method is limiting for those who are interested in circulating red cell MPs (RMPs) because their forward scatter is located within the platelet region and these are pelleted along with platelets (21). To pellet smaller MPs (exosomes and exosomes like-vesicles), samples undergo final ultracentrifugation speeds of 100,000 × g for 60 – 90 minutes.
Table 1.
Micro-particle centrifugation speeds vary between cell/tissue cultures and biological fluids.
| Type of MP |
Cell/Tissue of Origin |
Media/ Biological Fluid |
Centrifugation Speed |
Time (min) |
Reference | |
|---|---|---|---|---|---|---|
|
In vitro MPs |
Apoptotic MPs |
Jurkat T cells | RPMI 1640 | 2 × 400 × g
35,000 rpm |
5 min 20 min |
[Reich, 2009 #788] |
| Apoptotic bodies |
PBMCs | RPMI 1640 | 2 × 500 × g
100,000 × g |
5 min 30 min |
[Schiller, 2008 #235] |
|
| Apoptotic MPs |
JEG-3 cells | MEM | 300 × g
800 × g 25,000 × g |
5 min 5 min 60 min |
[Orozco, 2008 #717] |
|
| Apoptotic MPs |
Jurkat T cells | DMEM | 1,500 × g
100,000 × g |
5 min 20 min |
[Distler, 2005 #789] |
|
| ELVs | PBMCs | RPMI 1640 | 300 × g
1,200 × g 10,000 × g 100,000 × g |
5 min 20 min 30 min 60 min |
[Caby, 2005 #790] |
|
| STBMs | Placenta villous tissue |
PBS | 1,000 × g
10,000 × g 70,000 × g |
10 min 10 min 90 min |
[Gupta, 2004 #231] |
|
| Exosomes | D1 Dendritic Cells |
IMDM | 300 × g
1,200 × g 10,000 × g 100,000 × g |
5 min 20 min 30 min 60 min |
[Thery, 2001 #237] |
|
|
In vivo MPs |
mpDNA | Placenta | Maternal plasma | 800 × g
1,200 × g |
10 min 10 min |
[Orozco, 2009 #740] |
| PMPs LMPs |
Platelets Leukocytes |
Maternal plasma | 1,560 × g
18,890 × g |
20 min 30 min |
[Lok, 2008 #708] |
|
| Exosomes | Melanoma cells | Plasma | 400 × g
1,200 × g 10,000 × g 100,000 × g |
20 min 20 min 30 min 60 min |
[Logozzi, 2009 #791] |
|
| Exosomes | N/A | Breast milk | 300 × g
3,000 × g 10,000 × g 100,000 × g |
20 min 20 min 30 min 70 – 90 min |
[Admyre, 2007 #792] |
|
| EMPs | Endothelial cells |
Plasma | 160 × g
1,000 × g |
10 min 10 min |
[Esposito, 2006 #793] |
|
| RMPs | Red Blood Cells |
Whole blood | N/A | N/A | [Pattanapa nyasat, 2004 #751] |
|
| PMPs | Platelets | Plasma | 1,500 × g
13,000 × g |
10 min 10 min |
[Shet, 2003 #782] |
PBMCs: Peripheral blood mononuclear cells
RPMI 1640: Royal Park Memorial Institute 1640
DMEM: Dulbecco’s modified Eagle’s medium
MEM: Minimum essential medium
ELVs: Exosome-like vesicles
STMBs: Syncytiotrophoblast-derived micro-particles
PBS: Phosphate buffered saline
IMDM: Iscove's Modified Dulbecco's Media
mpDNA: MPs containing DNA
LMPs: Leukocyte MPs
EMPs: Endothelial MPs
RMPs: Red Blood Cell MPs
PMPs: Platelet MPs
Because our laboratory was initially interested in obtaining circulating cell-free DNA (cfDNA) from maternal plasma for prenatal genetic diagnosis, our main concern was to eliminate unwanted maternal cells and not contaminating platelets, which are enucleate blood cells (non-DNA containing) that originate from the cytoplasm of megakaryocytes (22). Therefore, we used slower centrifugation speeds previously established for isolating cfDNA, an initial centrifugation speed of 800 × g for 10 minutes, followed by a second centrifugation speed of 1,600 × g for 10 minutes (23,24).
Light scatter analysis of MPs
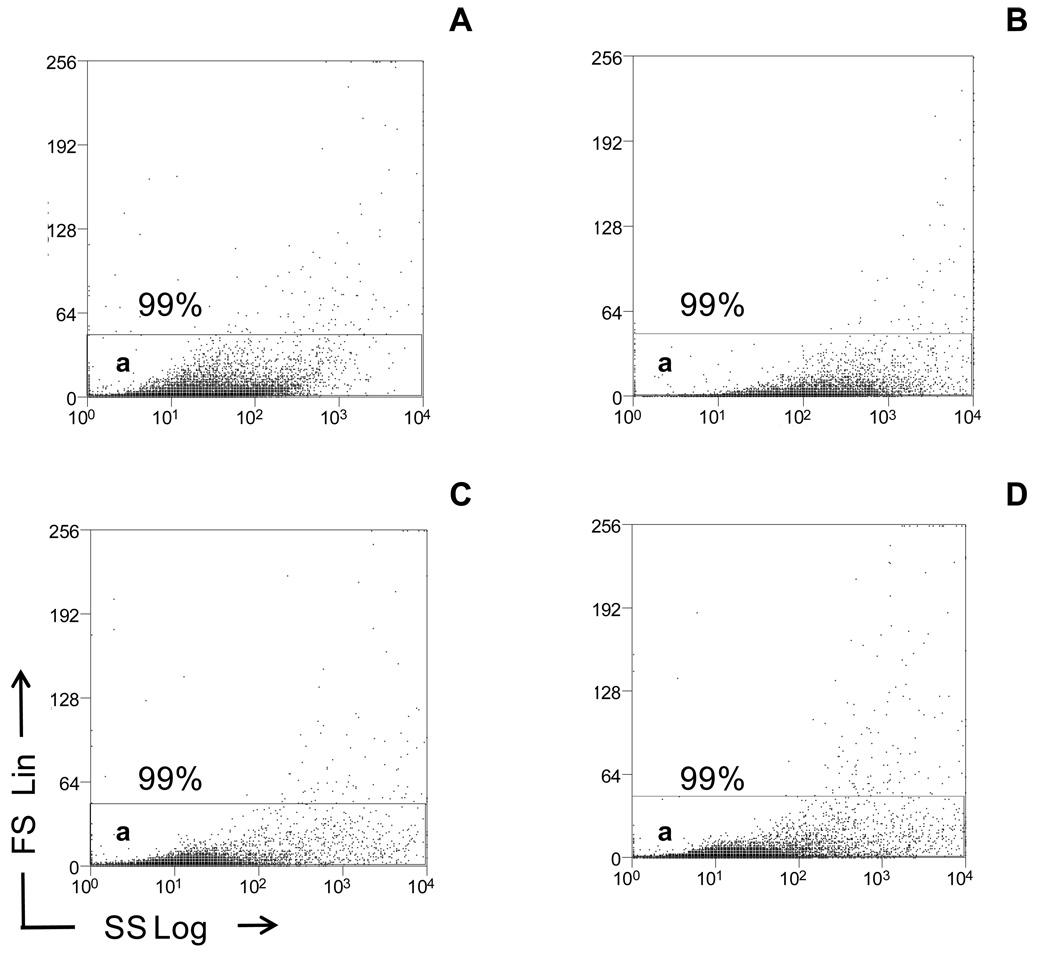
Most characterization of circulating MPs (i.e. lipid membranes, cell surface markers, and DNA association) is done by flow cytometry. MPs are initially studied by relative size (forward scatter light, FS) and relative granularity (side scatter light, SS). We prefer to analyze the size and granularity of MPs generated both in vitro and in vivo by FS set to a linear scale (FS Lin, y-axis) and SS set to a logarithmic scale (SS Log, x-axis), respectively (Figure 1). Because there is no current consensus on the threshold setting (which determines the smallest size MPs analyzed) for analysis of MPs by flow cytometry, our threshold level is based on the number of background “noise”/events per second when double filtered (0.2 µm) phosphate buffered saline (PBS) is passed through the machine. Generally, we accept a background “noise” of 25 – 50 events per second at high speed (60 µL/min) using the BD LSR II, when set to a threshold of 500 (0 – 262,140 channels, FS Lin). We also use double filtered PBS as an instrument quality control for background “noise”. Depending on the MP size of interest, standard fluorescent beads can be used to set the electronic gates. For example, although the threshold is set to identify MPs < 1 µm in size, an electronic gate can be set at ≥ 1 µm. This allows the user to reproducibly analyze MPs ≥ 1 µm. If examination of MPs < 1 µm is needed, the electronic gate can be expanded. However, one might take caution in analyzing MPs < 1 µm as several labs have reported that 0.5 µm is the cut off value for accurately identifying MPs (25). This may change with newer instruments.
Figure 1.
Light scatter analysis of in vitro and in vivo MPs by flow cytometry. In vitro MPs were centrifuged at 300 × g to remove cells, followed by a second 800 × g centrifugation. The MP supernatants were then diluted in PBS. Frozen plasma samples were thawed and also diluted in PBS. Light scatter properties of in vitro and in vivo plasma MPs were then compared by flow cytometry and displayed as dot plots (x-axis, SS Log; y-axis, FS Lin). A total of 10,000 events were taken. (A) In vitro apoptotic HTR-8SVneo MPs were re-suspended in PBS and shown in Gate a. (B) In vitro apoptotic JEG-3 MPs were re-suspended in PBS and shown in Gate a. (C) Maternal plasma samples were re-suspended in PBS and shown in Gate a. (D) Non-pregnant plasma samples were re-suspended in PBS and shown in Gate a. Dot plots are representative of three independent experiments.
MP quantitation for fluorescent staining (in vitro and in vivo)
Higher levels of specific cell-derived MPs are associated with various diseases, including thrombosis (platelet MPs)(26), congestive heart failure (endothelial MPs) (27), breast cancer patients (leukocyte MPs) (28) and women with preeclampsia (29). This suggests the number of total MPs and/or a subset of cell-specific MPs might be used to predict or monitor such pathologic conditions.
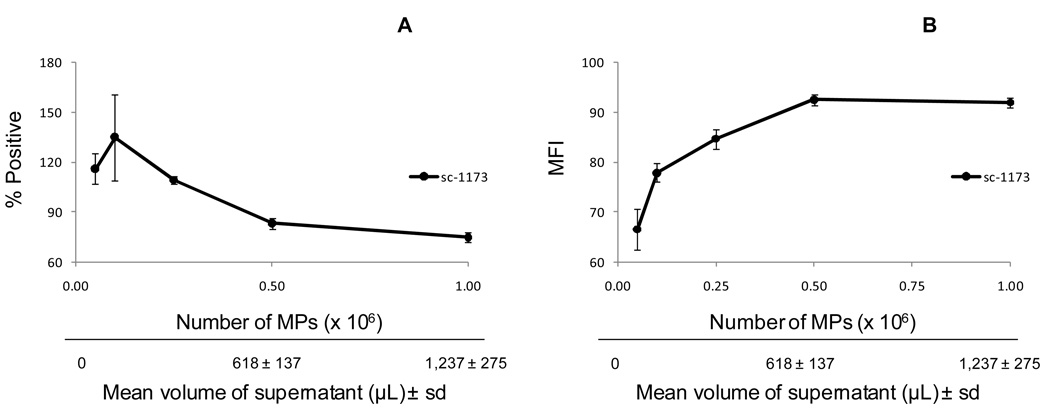
When labeling cells with antibodies (Abs) (i.e. CD3 (30), CD4 (31)), DNA dyes (i.e. Hoechst 33342 (32), propidium iodide(17), 7-aminoactinomycin-D (7-AAD) (30)) or lipid membrane dyes/probes (i.e. annexin v (33), CFSE (34), PKH26 (35), or Cell Tracker CM-DiI (lipophilic carbocyanine dye containing 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate) (36) for flow cytometry, a known amount of cells is used so that the experiment can be reproduced. Although it might seem appropriate to label MPs with Abs or annexin v and then analyze/quantitate MPs, this erroneous procedure is common practice among researchers who use flow cytometry to analyze MPs. Given the inter-variation in centrifugation protocols between labs (Table 1) and variation in the number of MPs between genders, within the same genders (menstruating vs. non-menstruating women (37) and pregnant vs. non-pregnant women (10,29) and between physiological and pathological conditions (vasculitis (38), diabetic retinopathy (39) and cancer (40), an accurate quantitation of total and/or sub-sets of MPs are required for optimal and reproducible staining of MPs (i.e. Abs and fluorescent dyes). In our hands, MPs are first quantitated using a known concentration of fluorescent beads and then a known amount of MPs are fluorescently labeled, rather than adding Abs/membrane fluorescent dyes to a known volume of plasma or supernatant with an unknown amount of MPs. For example, apoptotic MPs released from HTR-8/SVneo cells (trophoblast cell line that expresses the angiotensin II receptor type 1 (AT1) were first quantitated using a fluorescent bead-count assay (19). Then known amounts of apoptotic MPs were titrated, labeled with two different concentrations of the anti-AT1 Abs and analyzed by flow cytometry. Figure 2a shows a 1.6-fold increase in AT1 mean fluorescence intensity (MFI) between 0.1 ×106 MPs and 0.5 ×106 MPs labeled with AT1 Abs, respectively, suggesting that the optimal number of MPs labeled by anti-AT1 Abs is 0.1 ×106 MPs. However, it appears that labeling low amounts of MPs (0.05 – 0.1 ×106 MPs) with anti-AT1 Abs is associated with higher variability (MFI) compared to labeling higher amounts of MPs between 0.25 – 1.0 × 106 MPs, suggesting that the lower limit of detection for the number of MPs is around 0.25 – 0.5 × 106 MPs. Although it seems counterintuitive for an Ab to bind fewer antigens (Ag) at a high Ab to Ag ratio, this could be explained by Ab saturation. For example, bivalent Abs have high avidity at low concentration and low avidity at high concentrations. When the Ag binding sites become more saturated due to the increase in Ab concentration, the Ab undergoes monovalent binding, which has less avidity for the Ag and therefore dissociates more rapidly. This suggests at low MP numbers (0.05 – 0.1 ×106 MPs), the Ag binding sites become more saturated, resulting in higher frequency of low avidity binding Abs as shown in Figure 2b (66.6 ± 4.1% and 77.9 ± 1.8%, respectively). Furthermore, at higher MP numbers (0.25 – 0.5 × 106 MPs) there are more Ag binding sites to allow for higher avidity binding Abs as also shown in Figure 2b (84.7 ± 1.9% and 92.5 ± 1.2%). In addition to Ab saturation, the amount of MPs used is necessary for reproducibility of an experiment. For example, if the number of MPs used in Figure 2 was not determined, then one might conclude that 618 ± 137 µL is the optimal volume to label MPs in future experiments. However, Figure 3 clearly shows that the concentration of plasma MPs (MPs per µL) can vary between individuals. Therefore, if the amount of plasma used for labeling MPs with anti-AT1 Abs was determined solely by the volume shown in Figure 2 (618 ± 137 µL) rather than an the optimal number of MPs (0.25– 0.5 ×105 MPs), then suboptimal amounts of MPs (6, 13 and 24 × 105 MPs) would be labeled with AT1 Abs rather than the optimal number of MPs.
Figure 2.
Quantitation of MPs necessary for optimization of Ab labeling. In vitro HTR-8/SVneo MPs were centrifuged at 300 × g to remove cells, followed by a second 800 × g centrifugation. MPs were quantitated by flow cytometry using a fluorescent bead assay described in the MP quantitation for fluorescent staining (in vitro and in vivo) section. For detection of AT1 MPs, supernatants were indirectly labeled with rabbit anti-human AT1 Abs, followed by secondary labeling using PE-conjugated goat anti-rabbit F(ab')2 fragments. A total of 10,000 events were taken. The results are shown as the mean ± standard deviation. (A) The line graph represents the percentage of AT1+ MPs (n = 3). (B) The line graph represents the mean fluorescence intensity (MFI) of AT1 MPs (n = 3).
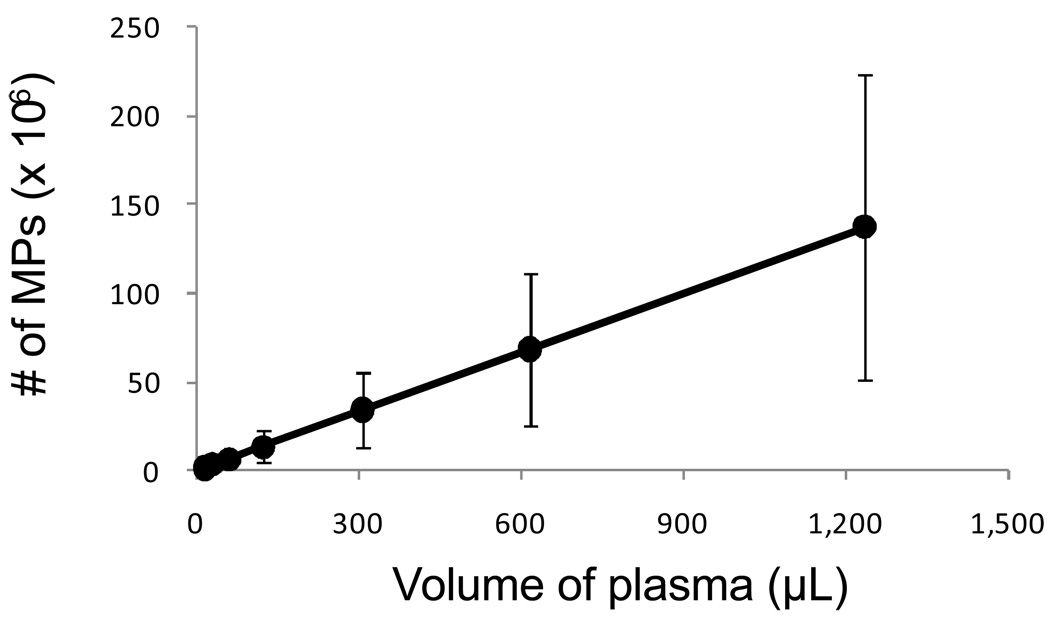
Figure 3.
Amount of plasma MPs varies between individuals. Frozen plasma samples, which underwent a two-step centrifugation (800 × g for 10 minutes, followed by 1,600 × g for additional 10 minutes), were thawed, diluted in PBS and quantitated by flow cytometry using a fluorescent bead assay described in the MP quantitation for fluorescent staining (in vitro and in vivo) section. The results are shown as the mean ± standard deviation. Line graph represents the concentration (number of MPs/µL) of plasma MPs from three individuals (n = 3). A standard curve was generated by plotting the plasma volume (µL; x-axis) versus number of MPs (y-axis).
Two general methods used to enumerate circulating MPs are the fluorescent bead count-based and flow rate-based method. The bead count-based method allows for quantitation of MPs based on the addition of known quantities of standardized fluorescent beads to the samples. The method is suitable for reproducible and accurate measurements of MP numbers (21). However, because TrucountTM beads are added to each sample, this method has the potential of becoming labor intensive and expensive depending on the number of samples analyzed. By contrast, the flow rate method is less expensive. The method uses a derived calibration factor to convert cytometer events into absolute counts (41). Although this technique requires the use of TrucountTM beads to calibrate the machine only before and after samples, it also can become cumbersome and impractical when biological samples with different viscosities are used because calibrations are needed before and after each different biological sample. Therefore the flow rate method is more beneficial for clinical flow cytometry that routinely analyze a single biological sample such as blood or plasma.
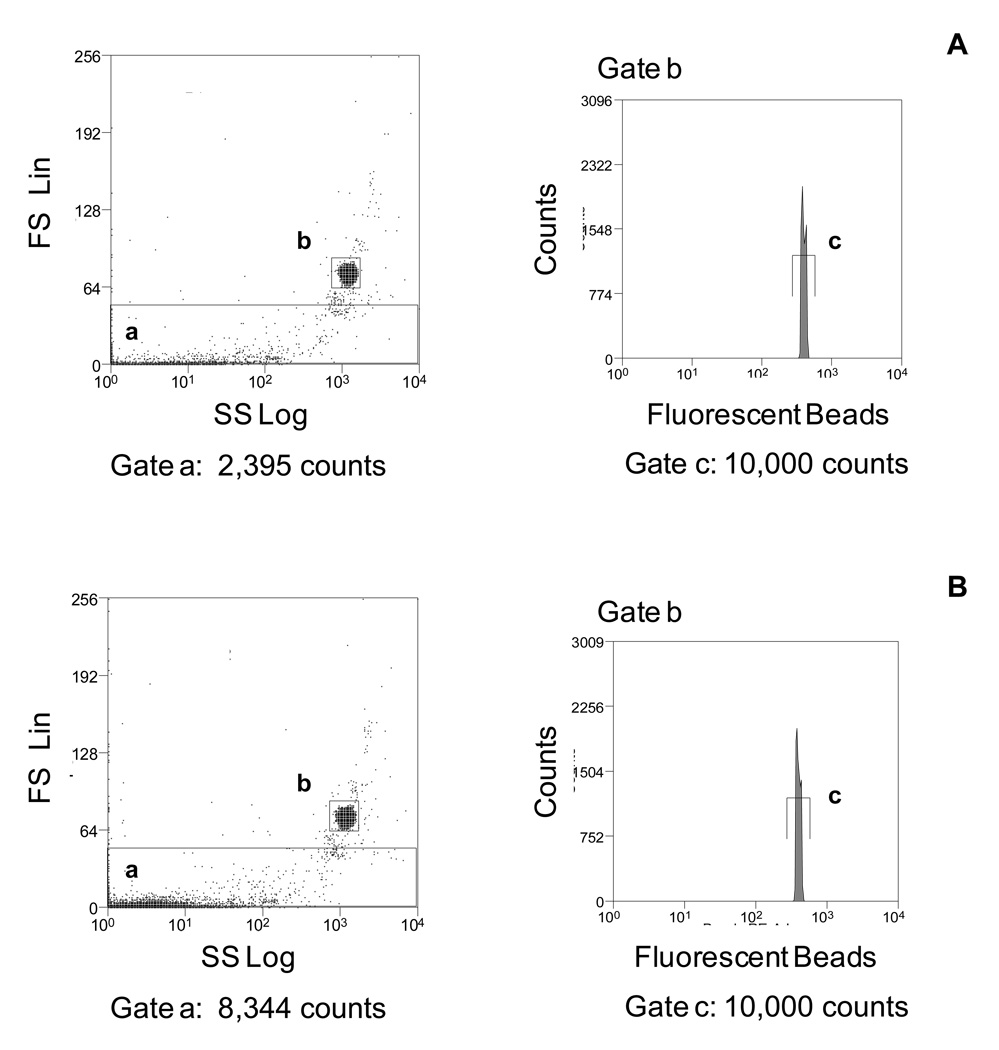
Alternatively, we use a less expensive fluorescent bead count-based assay termed single bead-enhanced cytofluorimetry to quantitate the number of MPs (19) in Figures 1 and 2. This procedure uses Flow-Check™ Fluorospheres (10 µm average diameter), does not require calibration of flow rates or specialized flow cytometric analytic software and can be applied to all flow cytometers. Furthermore, 10 µm sized beads are easily differentiated from the MPs and background “events” when analyzed by light scatter and allows for a separate gate to count beads based on fluorescence (Figure 4). We initially applied this strategy to determine the total number of MPs released into the supernatant in our in vitro model culture system for analysis of apoptotic MPs derived from JEG-3 cells, an extravillous trophoblastic cell line, undergoing various methods of cell death: chemically-induced hypoxia and heat stress (necrosis like)-induced cell death (17). Furthermore, to accurately compare the amount of DNA and lipid membranes present in apoptotic and necrotic MPs (Table 2), the number of MPs was quantitated using this fluorescent bead-count method and equal numbers of MPs were labeled with DNA and membrane lipid dyes. We also applied this fluorescent bead-count method to quantitate the number of in vivo MPs in maternal and non-maternal plasma prior to labeling the plasma MPs with control isotype, anti-human leukocyte antigen-G (HLA-G), placental alkaline phosphatase (PLAP) Abs and counter-staining with a DNA dye, PicoGreen (10) (Table 2).
Figure 4.
10µm sized beads are easily differentiated from MPs and background “noise”. Frozen plasma samples, which had undergone a two-step centrifugation process (800 × g for 10 minutes and 1,600 × g for 10 minutes) were thawed and the number of MPs was quantitated by flow cytometry using a fluorescent bead count assay. (A) The scattergram shows the events detected in Gate a from samples with PBS + 10µm beads. (B) The scattergram shows the events detected in Gate a from samples with MPs + 10µm beads. The number of beads analyzed is obtained from the corresponding bead histogram Gate c in the PE-fluorescence channel 2. Histograms are representative of three independent experiments.
Table 2.
Fluorescent staining of MPs in vitro and in vivo.
| Tissue Culture | ||||||
|---|---|---|---|---|---|---|
| Fluorescent Dye |
Number of MPs | (×106) | Percentage of MPs positive (%) |
Mean fluorescence intensity (MFI) |
||
|
Apoptotic
MPs |
Necrotic
MPs |
Apoptotic
MPs |
Necrotic
MPs |
Apoptotic MPs |
Necrotic MPs |
|
| N/A | *6.7 ± 0.1 | 2.7 ± 0.1 | - | - | - | - |
| Ho 33342 | - | - | - | - | 91 ± 11 | 82 ± 8 |
| Br-dUTP | - | - | *20 ± 6 | 5 ± 2 | *45 ± 3 | 27 ± 6 |
| PKH26 | - | - | - | - | *66 ± 10 | 45 ± 9 |
| Ho 33342+/ PKH26+ |
- | - | 92 ± 9 | 93 ± 5 | - | - |
| CTB | - | - | - | - | *45 ± 6 | 33 ± 4 |
| Ho 33342+/ CTB+ |
- | - | 48 ± 2 | 50 ± 5 | - | - |
| Maternal Plasma | ||||||
|---|---|---|---|---|---|---|
| Fluorescent Dye |
Number of MPs/mL (×106) |
Percentage of MPs positive (%) |
Mean fluorescence intensity (MFI) |
|||
| NP | PE | NP | PE | NP | PE | |
| N/A | 68.7 (29.1 – 160.5) |
*119.5 (39.0 – 256.6) |
- | - | - | - |
| Ho 33342+/ HLA-G+ |
3.5 (0.2 – 10.8) |
3.5 (0.8 – 45.2) |
- | - | 30.0 (15.0 – 78.0) |
*55.5 (24.0 – 128.0) |
| 3.5 (1.2 – 10.8) |
5.0 (1.0 – 28.7) |
- | - | 293.0 (220.0 – 421.0) |
280.0 (92.0 – 453.0) |
|
p < 0.05
Tissue culture data: shown as mean ± standard deviation
Maternal plasma data: shown as median (range)
N/A: Not applicable
Ho 33342 (Hoechst 33342): DNA intercalating dye
Br-dUTP (Bromodeoxyuridine triphosphate): DNA binding dye
PKH26: Lipid membrane binding dye
CTB (Cholera Toxin B): Lipid raft binding dye
NP: Normotensive pregnancies
PE: Preeclamptic Pregnancies
Measuring fluorescence intensity of MPs (in vitro and in vivo)
In addition to determining the total number of plasma MPs and the concentration/percentage of sub-populations of plasma MPs, mean fluorescence intensity (MFI) can be used to qualitatively analyze individual differences between MP samples.
We tested for the presence of DNA by labeling a predetermined amount of in vitro MPs with DNA dye, Hoechst 33352 and evaluated the DNA content using the MFI. Although Halicka et al., (42) showed that DNA and RNA are packaged separately in apoptotic bodies, this is the first time that flow cytometric analysis has been used to demonstrate the relative level of DNA content per individual particle (as determined by the MFI).
Apoptotic DNA contains a range of sizes with fragments cut into sizes of 180 bp (nucleosomes) and multiplicity of 180 bp, which produces a characteristic ladder on gel electrophoresis. To determine the molecular form of mpDNA, DNA fragmentation was tested by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. MPs were labeled with Bromodeoxyuridine triphosphate (Br-dUTP), counterstained with propidium iodide, and analyzed by flow cytometry. Our data showed significantly more Br-dUTP+PI+ apoptotic MPs than necrotic MPs (Table 2). In addition, gel electrophoresis showed that DNA from apoptotic MPs displayed a disrupted DNA ladder pattern, similar to apoptotic cellular DNA, whereas necrotic MPs has a random and general cleavage pattern. Not only was our data the first to show that oligonucleosomal/fragmented DNA is preserved and released with apoptotic MPs, but the use of flow cytometry showed that Br-dUTP MFI was significantly higher in apoptotic MPs than in necrotic MPs, suggesting that apoptotic MPs contained more fragmented DNA than necrotic MPs.
To analyze lipid membranes of these MP, we performed flow cytometry after labeling MPs with PKH26 (membrane dye) and Hoechst 33342 (DNA dye). Although no statistically significant difference in the percentage of PKH26+ Hoechst 33342+ MPs between apoptosis and necrosis was detected, PKH26 MFI of apoptotic MPs was significantly greater than the PKH26 MFI of necrotic MPs (no difference in the Hoechst 33342 MFI between apoptotic and necrotic MPs was detected). To determine whether MPs contained lipid rafts, the MPs were labeled with the β subunit of cholera toxin (CTB), which binds to ganglioside GM1 lipids found on the cell surface (lipid rafts), and Hoechst 33342. Similarly, CTB MFI apoptotic MPs was significantly higher than CTB MFI of necrotic MPs (no significant difference in the percentage of CTB+ Hoechst 33342+ MPs or Hoechst MFI between apoptotic and necrotic MPs). Our data show that apoptotic mpDNA have more material that stains with membrane specific dyes than necrotic mpDNA, suggesting that apoptotic MPs contain more membranous material than necrotic MPs. Without labeling equal numbers of MPs, there could be no comparison of the MFI between samples.
Polychromatic flow cytometric measurements of MPs
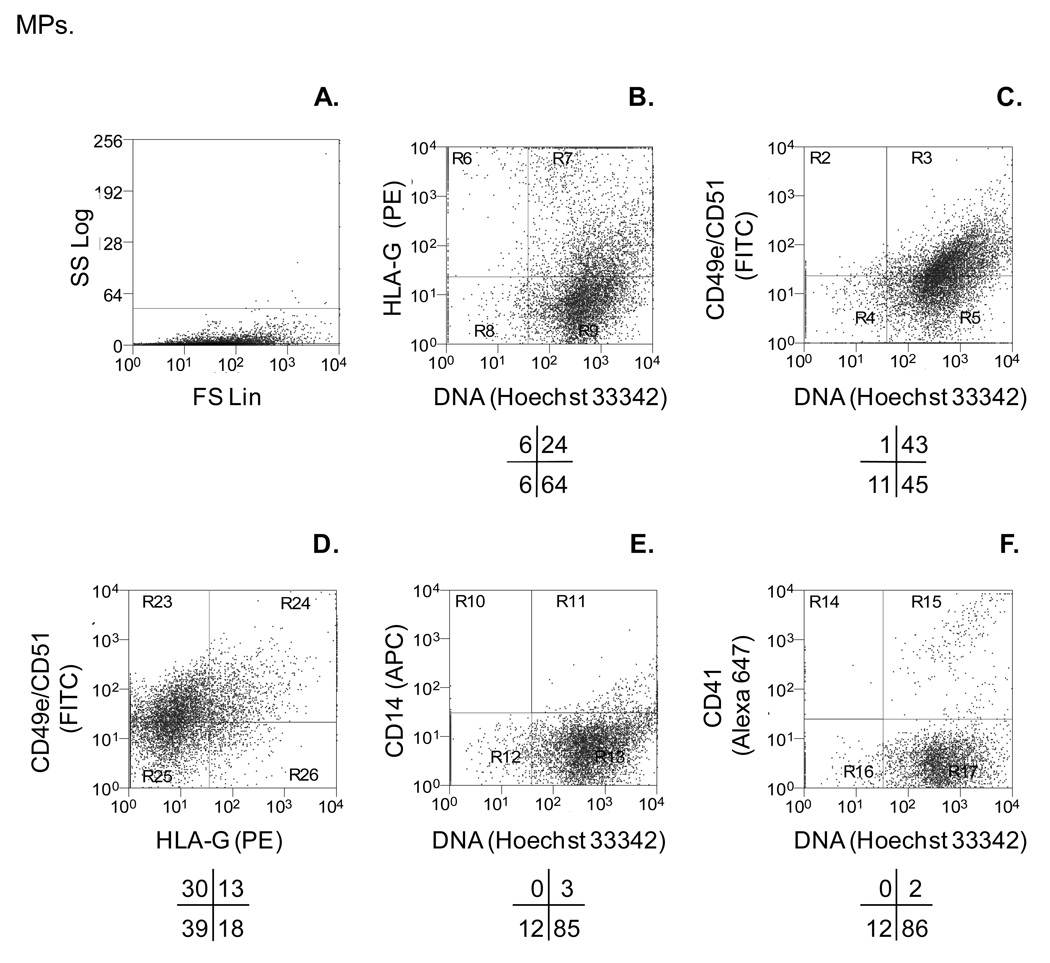
As mentioned above, our lab is interested in plasma mpDNA derived from placental cells in preeclamptic women. Preeclampsia is a multi-system disorder of unknown cause and is the second leading cause of maternal mortality worldwide. (43) Preeclampsia occurs in approximately 5 – 7% of all pregnancies worldwide. The etiology of preeclampsia and pathogenesis is unclear. However, the literature suggests that a main causation of preeclampsia is shallow extravillous cytotrophoblast invasion into the uterus. Invasive extravillous cytotrophoblast express surface markers such as HLA-G, integrin α5 (CD49e), and integrin αv (CD51), whereas proliferating extravillous cytotrophoblast HLA-G, integrin α2 (CD49b), and integrin α6 (CD49f). Given that 90% of circulating plasma MPs are platelet-derived (CD41+) and approximately 8% are HLA-G+ mpDNA, it might be difficult to quantitate statistically significant changes in the level of MPs derived from invasive extravillous cytotrophoblasts. One strategy is to use polychromatic flow cytometric analysis of MPs, which labels MPs simultaneously with multiple fluorescently-labeled Abs and -dyes. Therefore, we first used our established in vitro apoptotic MP system to optimally label apoptotic MPs derived from JEG-3 cells (extravillous cytotrophoblast cell line), which express integrin α5 (CD49e) and integrin αv (CD51). To avoid spectral overlap and reduce fluorescence compensation between the four flourochromes, we used fluorescence minus-one (FMO) controls.(20) FMO controls consist of all the fluorochromes used to label a sample, minus the fluorochrome (i.e. FITC) detected in its channel (FL1). Therefore, our FMO control for FITC would consist of a sample labeled with PE, APC and Hoechst 33342, minus FITC. Figure 5 shows JEG-3 derived MPs are integrin α5 + and integrin αv + and contain DNA. As expected, Abs against non-trophoblast markers (CD14, monocytes; CD41, platelets) do not bind to trophoblast MPs. We thus have a method to differentiate between MPs derived from invasive extravillous cytotrophoblasts (HLA-G+, CD49e+, CD51+) and maternally derived MPs (CD14+, CD41+).
Figure 5.
Polychromatic flow cytometric assay detects subpopulations of trophoblastic MPs. In vitro MPs were centrifuged at 300 × g to remove cells, followed by a second 800 × g centrifugation. Trophoblastic MPs were quantitated using a fluorescent bead assay. MPs were simultaneously labeled with monoclonal Abs against HLA-G (MEM-G/1-unconjugated), PE-conjugated secondary goat anti-mouse IgG, and monoclonal Abs against integrin α5 (CD49e–FITC), integrin αv (CD51-FITC), monocyte marker CD14 (CD14-APC), and platelet marker integrin alpha 2b (CD41-Alexa 647). After Ab labeling, MPs were counter-stained with Hoechst 33342 (dsDNA dye) and analyzed by flow cytometry. (A) Light scatter characteristics of apoptotic MPs. (B) HLA-G+ and DNA+ MPs. (C) CD49e/CD51+ and DNA+ MPs. (D) CD49e/CD51+ and HLA-G+ MPs. (E) CD14+ and DNA+ MPs. (F) CD41+ and DNA+ MPs. Dot plots are representative of three independent experiments.
Think outside the flow cytometric box
Nearly all investigators have analyzed (in vitro and in vivo) MPs by flow cytometry using Abs applicable to flow cytometry. However, some “Abs of interest” are traditionally used for only Western blot analysis, immunohistochemical studies or ELISA-based assays. For example, our laboratory focuses on AT1+ MPs derived from placental cells known as extravillous cytotrophoblasts. Although we are interested in quantitative and qualitative analysis/studies of AT1+ MPs by flow cytometry, commercially available anti- AT1 Abs are for Western blot analysis: human gastric cancer cell lines and gastric cancer tissues (44); human prostate cancer cell lines (45); rat mesangial cells (46) and immunohistochemistry: guinea pig enteric neurons(47); smooth muscle cell layer and endothelial cell layer (48); and Human epithelial ovarian tumor tissues (49). Therefore, a flow cytometric protocol to detect AT1 with the commercially available Abs was needed.
To test whether Abs used for Western blot analysis are applicable for flow cytometric analysis, a three-step experimentation process should be performed. The first step is to test whether the Abs can detect antigens expressed on a cell line that is most physiologically relevant to the parental cell releasing the MPs into circulation. If the Abs cannot recognize and bind to fixed cells, then there is very little chance the Abs will recognize and bind to in vitro or in vivo MPs. The second step is to test whether the Abs can detect antigens expressed on MPs derived from the cell line. This second step is critical because it allows for quantitation of MPs to label the optimal number of MPs with Abs or fluorescence dyes (as discussed above) as well as determining the optimal concentration of Abs (i.e. primary and secondary labeling) and fluorescence dyes. The final step is to apply the protocol used for the in vitro MPs to the in vivo MPs.
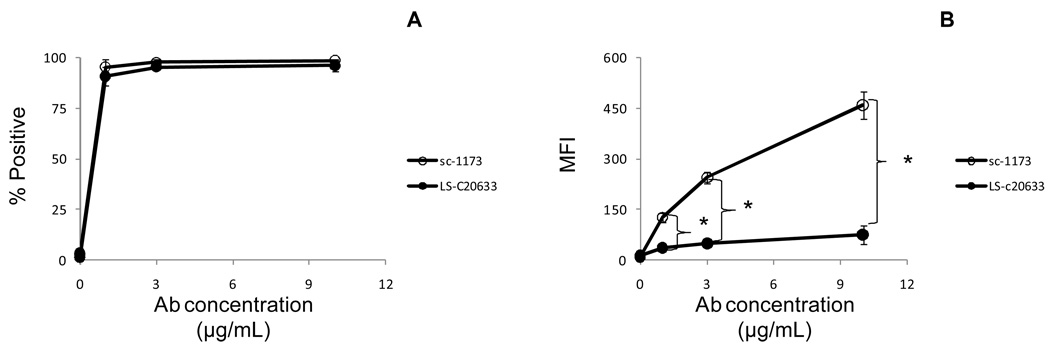
In this example, we used HTR-8/SVneo cells (trophoblastic cell line), which express AT1. HTR-8/SVneo cells were permeabilized, labeled intracellularly with unconjugated anti- AT1 Abs, followed by a secondary labeling with conjugated fragment antigen binding (Fab) Abs and analyzed by flow cytometry. We prefer to use Fab Abs, which do not have the Fc (fragment crystallizable) region, to reduce background fluorescence when secondary Ab labeling is needed for intracellular staining. Figure 6 shows a titration curve of two anti- AT1 Abs, which recognize different epitopes. Although both anti- AT1 Abs bound to HTR-8/SVneo cells, the sc-1173 had greater reactivity than the LS-C20633 Ab as determined by MFI (Figure 6a–b).
Figure 6.
Abs used for Western blot analysis can be used for flow cytometry. After cell fixation/permeabilization, 0.5 × 106 HTR-8/SVneo cells were indirectly labeled with rabbit anti-human AT1 Abs (sc-1171), followed by secondary labeling using PE-conjugated goat anti-rabbit F(ab')2 fragments or indirectly labeled with mouse anti-human AT1 Abs (LS-C20633), followed by secondary labeling using PE-conjugated goat anti-mouse Abs and analyzed by flow cytometry. The results are shown as the mean ± standard deviation. (A) The line graph represents the percentage of AT1+ HTR-8/SVneo cells (n = 3). (B) The line graph represents the mean fluorescence intensity (MFI) of AT1 HTR-8/SVneo cells (n = 3). (*) indicates significant difference (P < 0.05) compared to LS-C20633.
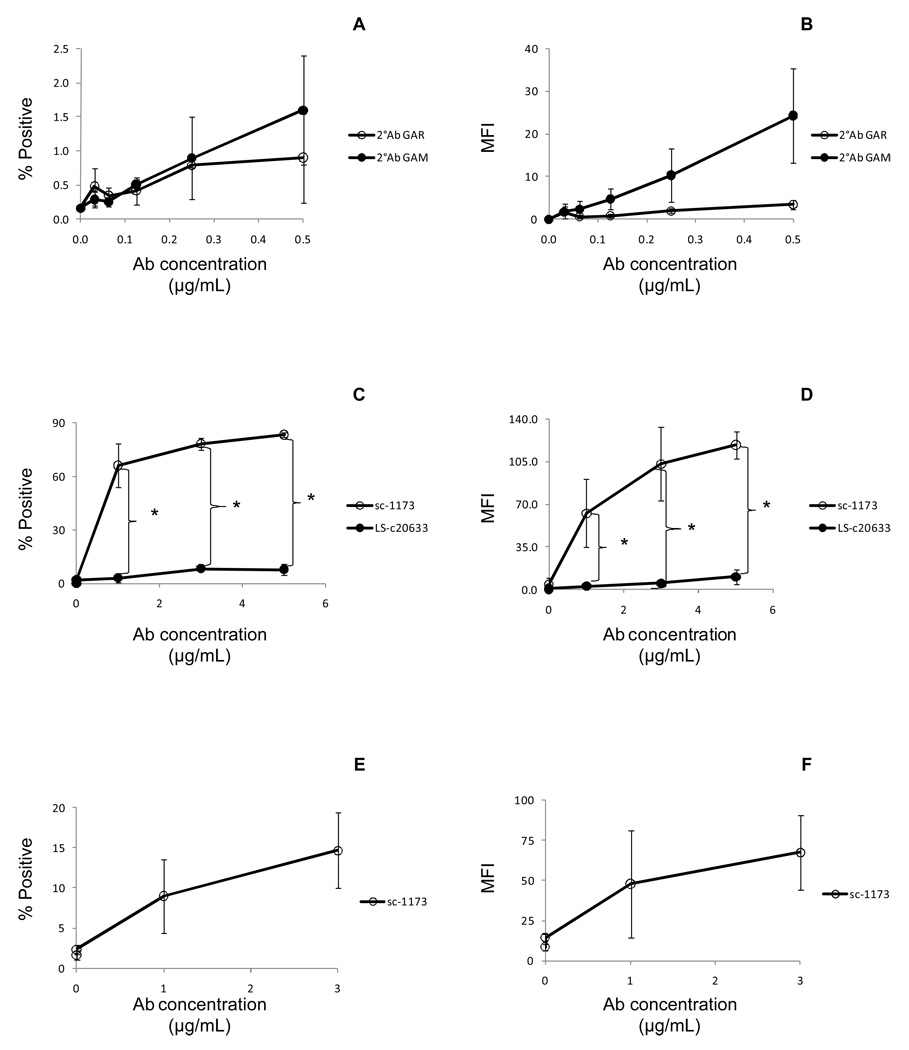
After determining that the “Ab of interest” binds to intact cells, the next step is to test whether the Abs will recognize the epitope expressed on/in MPs released from activated or apoptotic cells. Because we are interested in apoptotic plasma MPs released from dying placental cells, we used our in vitro apoptotic MP model system (17) to generate apoptotic MPs derived from HTR-8/SVneo cells. Like most Abs used for Western blot analysis, the Abs are unconjugated. Therefore, a phycoerythrin-conjugated secondary Ab titration was performed to minimize the amount of fluorescence background (Figure 7a–b) prior to labeling HTR-8/SVneo MPs with anti- AT1 Abs. Titration of conjugated secondary Abs is important because a high fluorescence background can overshadow a low fluorescent signal from indirect labeling of MPs. Next, apoptotic MPs were labeled with anti- AT1 Abs, followed by a secondary labeling with conjugated Fab Ab and analyzed by flow cytometry. Figure 7 shows an Ab titration curve of sc-1173 and LS-C20633. Similar to the results showed in Figure 4, sc-1173 bound more to HTR-8/SVneo MPs than LS-C20633 as determined by MFI (Figure 7c–d). The last step is to label plasma MPs according to the protocol designed from the in vitro MPs. Figures 7e–f show that sc-1173 Abs bound to plasma MPs. A fourth step can be performed prior to labeling plasma MPs of interest by labeling plasma MPs from normal control subjects. Together the three to four step processes helps to design optimal conditions for labeling plasma MPs, which might be a limiting resource.
Figure 7.
Labeling of HTR-8/SVneo and plasma MPs with anti- AT1 Abs. MPs were quantitated by flow cytometry using a fluorescent bead assay described in the MP quantitation for fluorescent staining (in vitro and in vivo) section. (A–B) Half a million HTR-8/SVneo MPs were directly labeled with PE-conjugated secondary goat anti-rabbit (GAR) F(ab')2 fragments only or directly labeled with PE-conjugated secondary goat anti-mouse (GAM) Abs only. The results are shown as the mean ± standard deviation. (A) The line graph represents the percentage of PE positive MPs (n = 3). (B) The line graph represents the mean fluorescence intensity (MFI) of PE positive MPs (n = 3). (C–D) Half a million HTR-8/SVneo MPs were indirectly labeled with rabbit anti-human AT1 Abs (sc-1173), followed by secondary labeling using PE-conjugated GAR F(ab')2 fragments or indirectly labeled with mouse anti-human AT1 Abs (LS-c20633), followed by secondary labeling using PE-conjugated GAM Abs and analyzed by flow cytometry. The results are shown as the mean ± standard deviation. (C) The line graph represents the percentage of AT1+ MPs labeled with sc-1173 or LS-c20633 Abs (n = 3). (D) The line graph represents the mean fluorescence intensity (MFI) of AT1 MPs (n = 3). (*) indicates significant difference (P < 0.05) compared to LS-C20633. (E–F) Frozen plasma samples were thawed and quantitated using the fluorescence bead-based method. One million plasma MPs were indirectly labeled with rabbit anti-human AT1 Abs (sc-1173), followed by secondary labeling using PE-conjugated GAR F(ab')2 fragments and analyzed by flow cytometry. The results are shown as the mean ± standard deviation. (E) The line graph represents the percentage of AT1+ plasma MPs (n = 3). (F) The line graph represents the mean fluorescence intensity (MFI) of AT1 plasma MPs (n = 3).
In some situations, there are Abs applicable to flow cytometry that recognize and bind to surface molecules expressed on intact cells, but they do not recognize the epitope expressed on apoptotic MPs. For example, our laboratory also focuses on HLA-G+ MPs derived from derived from extravillous cytotrophoblasts. To detect and measure levels of circulating HLA-G+ MPs in maternal plasma, we first identified useful HLA-G Abs. Monoclonal Abs MEM-G/9, G233, and 87G recognize the heavy chain of HLA-G class I molecules associated with beta-2-microglobulin (β2m). By contrast, 4H84 and MEM-G/1 bind to (different) linear epitopes on the α1 domain of unfolded HLA-G free heavy chains dissociated from β2m. All five mAbs reacted to HLA-G expressed on JEG-3 trophoblastic cells (Table 3) as measured by flow cytometry and not to Jurkat T cells, which do not express HLA-G (data not shown). Surprisingly, none of the four Abs bound to JEG-3 apoptotic MPs (Table 3). However, after mild acid treatment of JEG-3 apoptotic MPs, which dissociates beta-2-microglobulin and allows HLA-G to unfold in the denatured form (50), both 4H84 and MEM-G/1 bound to JEG-3 apoptotic MPs (Table 3). When tested with maternal plasma, however, MEM-G/1 was the only mAb that bound to MPs (Table 3). These results imply that MEM-G/1 reacts to partially unfolded HLA-G and that the epitope recognized by 4H84, which recognizes another linear determinant, could be blocked or hidden.
Table 3.
Detection of HLA-G MPs in maternal plasma requires MEM-G/1 Abs.
| Anti-HLA-G Monoclonal Antibodies | ||||||
|---|---|---|---|---|---|---|
| Native | Denatured | |||||
| Samples | MEM-G/9 | MEM-G/9 | MEM-G/9 | MEM-G/9 | MEM-G/1 | |
| Untreated JEG-3 cells | + | + | + | − | − | |
| Acid-treated JEG-3 cells | − | + | + | |||
| Untreated JEG-3 cells | − | − | nt | − | − | |
| Acid-treated JEG-3 cells | − | − | nt | + | + | |
| Maternalplasma | − | − | − | − | + | |
Isotype antibodies do not bind
nt = not tested
n = 3
Conclusion
In conclusion, we have examined several key variables that are important in the study of MPs. The centrifugation speed is critical and must be optimized for the MPs of interest. Standardization in the field is necessary. Next, to stain MPs for flow cytometry, MPs should be counted first before staining, much as is required for staining of cells before Abs are added. In addition to the number and/or percentage of cell-specific MPs, the MFI of fluorescently-labeled Abs or fluorescent dyes reveal the amount of surface markers or DNA content between samples. To distinguish smaller sub-populations of MPs, polychromatic flow cytometric analysis is recommened. The use of polyclonal Abs designed for Western blotting requires careful titration and a control Ab. Because these Abs are primarily unconjugated, titration of a conjugated secondary Ab is also required. Consideration of the epitope recognized is also of importance as in the case of the anti-HLA-G Abs. In addition, titration of Abs on a model cell line may not translate to normal cells of that same lineage; another titration is required. In summary, study of MPs is promising in many areas of science, but the variables should be standardized to be able to allow comparison of compare flow cytometric data between studies.
Acknowledgments
This study was supported by grants, National Institute of Health/National Institute of Child Health and Human Developments (NIH/NICHD) HDO46623 and T32AI007495.
References
- 1.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 3.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A. Human Villous Trophoblasts Express and Secrete Placenta-Specific MicroRNAs into Maternal Circulation via Exosomes. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.075481. and others. [DOI] [PubMed] [Google Scholar]
- 4.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 5.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–74. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 6.Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, Wang Z, Wang H, Ravikumar TS, Tracey KJ. Immature Dendritic Cell-Derived Exosomes Rescue Septic Animals Via Milk Fat Globule Epidermal Growth Factor VIII. J Immunol. 2009 doi: 10.4049/jimmunol.0802994. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunderson SC, Schuberth PC, Dunn AC, Miller L, Hock BD, MacKay PA, Koch N, Jack RW, McLellan AD. Induction of exosome release in primary B cells stimulated via CD40 and the IL-4 receptor. J Immunol. 2008;180:8146–8152. doi: 10.4049/jimmunol.180.12.8146. [DOI] [PubMed] [Google Scholar]
- 8.Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang X, Cheng Z, Liu C, Wang J, Zhang L. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellam J, Proulle V, Jungel A, Ittah M, Miceli-Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis, and relation with disease activity. Arthritis Res Ther. 2009;11:R156. doi: 10.1186/ar2833. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orozco AF, Jorgez CJ, Ramos-Perez WD, Popek EJ, Yu X, Kozinetz CA, Bischoff FZ, Lewis DE. Placental Release of Distinct DNA-associated Micro-particles into Maternal Circulation: Reflective of Gestation Time and Preeclampsia. Placenta. 2009 doi: 10.1016/j.placenta.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z, Clayton A. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7:4. doi: 10.1186/1479-5876-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 13.Houali K, Wang X, Shimizu Y, Djennaoui D, Nicholls J, Fiorini S, Bouguermouh A, Ooka T. A new diagnostic marker for secreted Epstein-Barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:4993–5000. doi: 10.1158/1078-0432.CCR-06-2945. [DOI] [PubMed] [Google Scholar]
- 14.Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JB, Jauch EC, Lindsell CJ, Campos B. Endothelial microparticle levels are similar in acute ischemic stroke and stroke mimics due to activation and not apoptosis/necrosis. Acad Emerg Med. 2007;14:685–690. doi: 10.1197/j.aem.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Nusbaum P, Laine C, Seveau S, Lesavre P, Halbwachs-Mecarelli L. Early membrane events in polymorphonuclear cell (PMN) apoptosis: membrane blebbing and vesicle release, CD43 and CD16 down-regulation and phosphatidylserine externalization. Biochem Soc Trans. 2004;32:477–479. doi: 10.1042/BST0320477. [DOI] [PubMed] [Google Scholar]
- 17.Orozco AF, Jorgez CJ, Horne C, Marquez-Do DA, Chapman MR, Rodgers JR, Bischoff FZ, Lewis DE. Membrane protected apoptotic trophoblast microparticles contain nucleic acids: relevance to preeclampsia. Am J Pathol. 2008;173:1595–1608. doi: 10.2353/ajpath.2008.080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem. 2002;277:24601–24608. doi: 10.1074/jbc.M201369200. [DOI] [PubMed] [Google Scholar]
- 19.Montes M, Jaensson EA, Orozco AF, Lewis DE, Corry DB. A general method for bead-enhanced quantitation by flow cytometry. J Immunol Methods. 2006;317:45–55. doi: 10.1016/j.jim.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tung JW, Parks DR, Moore WA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Pattanapanyasat K, Noulsri E, Fucharoen S, Lerdwana S, Lamchiagdhase P, Siritanaratkul N, Webster HK. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom. 2004;57:23–31. doi: 10.1002/cyto.b.10064. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 23.Jorgez CJ, Simpson JL, Bischoff FZ. Recovery and amplification of placental RNA from dried maternal blood spots: utility for non-invasive prenatal diagnosis. Reprod Biomed Online. 2006;13:558–561. doi: 10.1016/s1472-6483(10)60645-1. [DOI] [PubMed] [Google Scholar]
- 24.Jorgez CJ, Dang DD, Simpson JL, Lewis DE, Bischoff FZ. Quantity versus quality: optimal methods for cell-free DNA isolation from plasma of pregnant women. Genet Med. 2006;8:615–619. doi: 10.1097/01.gim.0000241904.32039.6f. [DOI] [PubMed] [Google Scholar]
- 25.Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, Sampol J, Dignat-George F. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–197. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 26.Jy W, Tiede M, Bidot CJ, Horstman LL, Jimenez JJ, Chirinos J, Ahn YS. Platelet activation rather than endothelial injury identifies risk of thrombosis in subjects positive for antiphospholipid antibodies. Thromb Res. 2007;121:319–325. doi: 10.1016/j.thromres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Garcia S, Chirinos J, Jimenez J, Del Carpio Munoz F, Canoniero M, Jy W, Jimenez J, Horstman L, Ahn Y. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Heart Lung Transplant. 2005;24:2184–2189. doi: 10.1016/j.healun.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Toth B, Nieuwland R, Liebhardt S, Ditsch N, Steinig K, Stieber P, Rank A, Gohring P, Thaler CJ, Friese K. Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res. 2008;28:1107–1112. and others. [PubMed] [Google Scholar]
- 29.Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, Nieuwland R. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:344–360. doi: 10.1080/10641950801955733. [DOI] [PubMed] [Google Scholar]
- 30.Le Roy C, Varin-Blank N, Ajchenbaum-Cymbalista F, Letestu R. Flow cytometry APC-tandem dyes are degraded through a cell-dependent mechanism. Cytometry A. 2009;75A:882–890. doi: 10.1002/cyto.a.20774. [DOI] [PubMed] [Google Scholar]
- 31.Salameire D, Le Bris Y, Fabre B, Fauconnier J, Solly F, Pernollet M, Bonnefoix T, Leroux D, Plumas J, Jacob MC. Efficient characterization of the TCR repertoire in lymph nodes by flow cytometry. Cytometry A. 2009;75A:743–751. doi: 10.1002/cyto.a.20767. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Traganos F, Dobrucki J, Wlodkowic D, Darzynkiewicz Z. Induction of DNA damage response by the supravital probes of nucleic acids. Cytometry A. 2009;75A:510–519. doi: 10.1002/cyto.a.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratosin D, Tcacenco L, Sidoroff M, Cotoraci C, Slomianny C, Estaquier J, Montreuil J. Active caspases-8 and −3 in circulating human erythrocytes purified on immobilized annexin-V: a cytometric demonstration. Cytometry A. 2009;75A:236–244. doi: 10.1002/cyto.a.20693. [DOI] [PubMed] [Google Scholar]
- 34.Wallace PK, Tario JD, Jr, Fisher JL, Wallace SS, Ernstoff MS, Muirhead KA. Tracking antigen-driven responses by flow cytometry: monitoring proliferation by dye dilution. Cytometry A. 2008;73A:1019–1034. doi: 10.1002/cyto.a.20619. [DOI] [PubMed] [Google Scholar]
- 35.Rimaniol AC, Garcia G, Till SJ, Capel F, Gras G, Balabanian K, Emilie D, Humbert M. Evaluation of CD4+ T cells proliferating to grass pollen in seasonal allergic subjects by flow cytometry. Clin Exp Immunol. 2003;132:76–80. doi: 10.1046/j.1365-2249.2003.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daubeuf S, Bordier C, Hudrisier D, Joly E. Suitability of various membrane lipophilic probes for the detection of trogocytosis by flow cytometry. Cytometry A. 2009;75A:380–389. doi: 10.1002/cyto.a.20679. [DOI] [PubMed] [Google Scholar]
- 37.Toth B, Nikolajek K, Rank A, Nieuwland R, Lohse P, Pihusch V, Friese K, Thaler CJ. Gender-specific and menstrual cycle dependent differences in circulating microparticles. Platelets. 2007;18:515–521. doi: 10.1080/09537100701525843. [DOI] [PubMed] [Google Scholar]
- 38.Daniel L, Fakhouri F, Joly D, Mouthon L, Nusbaum P, Grunfeld JP, Schifferli J, Guillevin L, Lesavre P, Halbwachs-Mecarelli L. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 2006;69:1416–1423. doi: 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]
- 39.Ogata N, Nomura S, Shouzu A, Imaizumi M, Arichi M, Matsumura M. Elevation of monocyte-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2006;73:241–248. doi: 10.1016/j.diabres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Toth B, Liebhardt S, Steinig K, Ditsch N, Rank A, Bauerfeind I, Spannagl M, Friese K, Reininger AJ. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb Haemost. 2008;100:663–669. [PubMed] [Google Scholar]
- 41.Nantakomol D, Chimma P, Day NP, Dondorp AM, Combes V, Krudsood S, Looareesuwan S, White NJ, Pattanapanyasat K, Chotivanich K. Quantitation of cell-derived microparticles in plasma using flow rate based calibration. Southeast Asian J Trop Med Public Health. 2008;39:146–153. [PubMed] [Google Scholar]
- 42.Halicka HD, Bedner E, Darzynkiewicz Z. Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp Cell Res. 2000;260:248–256. doi: 10.1006/excr.2000.5027. [DOI] [PubMed] [Google Scholar]
- 43.Walker MC, Murphy KE, Pan S, Yang Q, Wen SW. Adverse maternal outcomes in multifetal pregnancies. Bjog. 2004;111:1294–1296. doi: 10.1111/j.1471-0528.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- 44.Huang W, Yu LF, Zhong J, Qiao MM, Jiang FX, Du F, Tian XL, Wu YL. Angiotensin II type 1 receptor expression in human gastric cancer and induces MMP2 and MMP9 expression in MKN-28 cells. Dig Dis Sci. 2008;53:163–168. doi: 10.1007/s10620-007-9838-9. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka T, Miyajima A, Shirotake S, Kikuchi E, Hasegawa M, Mikami S, Oya M. Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate. 2009 doi: 10.1002/pros.21049. [DOI] [PubMed] [Google Scholar]
- 46.Xu ZG, Miao LN, Cui YC, Jia Y, Yuan H, Wu M. Angiotensin II type 1 receptor expression is increased via 12-lipoxygenase in high glucose-stimulated glomerular cells and type 2 diabetic glomeruli. Nephrol Dial Transplant. 2009;24:1744–1752. doi: 10.1093/ndt/gfn703. [DOI] [PubMed] [Google Scholar]
- 47.Wang GD, Wang XY, Hu HZ, Fang XC, Liu S, Gao N, Xia Y, Wood JD. Angiotensin receptors and actions in guinea pig enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G614–G626. doi: 10.1152/ajpgi.00119.2005. [DOI] [PubMed] [Google Scholar]
- 48.Dimitrijevic I, Malmsjo M, Andersson C, Rissler P, Edvinsson L. Increased angiotensin II type 1 receptor expression in temporal arteries from patients with giant cell arteritis. Ophthalmology. 2009;116:990–996. doi: 10.1016/j.ophtha.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Suganuma T, Ino K, Shibata K, Kajiyama H, Nagasaka T, Mizutani S, Kikkawa F. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005;11:2686–2694. doi: 10.1158/1078-0432.CCR-04-1946. [DOI] [PubMed] [Google Scholar]
- 50.Polakova K, Bennink JR, Yewdell JW, Bystricka M, Bandzuchova E, Russ G. Mild acid treatment induces cross-reactivity of 4H84 monoclonal antibody specific to nonclassical HLA-G antigen with classical HLA class I molecules. Hum Immunol. 2003;64:256–264. doi: 10.1016/s0198-8859(02)00777-2. [DOI] [PubMed] [Google Scholar]